Research Article Open Access
The Topography of White Mater Peri-Ventricular Hyperintensities on Brain Magnetic Resonance Imagings (MRI) Among Cameroonians
| Uduma FU1,5*, Ongolo CP2,5, Okoye IJ3 and Muna W4,5 | |
| 1Department of Radiology, Faculty of Clinical Sciences, College of Health Sciences, University of Uyo, Uyo, Nigeria | |
| 2Department of Radiology, University of Yaounde, Yaounde, Cameroon | |
| 3Department of Radiation Medicine, Faculty of Health Sciences and Technology, College of Medicine, University of Nigeria, Enugu, Nigeria | |
| 4Department of Medicine, University of Yaounde, Yaounde, Cameroon | |
| 5Polyclinic Bonanjo, Douala, Cameroon | |
| Corresponding Author : | Uduma FU Department of Radiology Faculty of Clinical Sciences College of Health Sciences University of Uyo, Uyo, Nigeria E-mail: felixuduma@yahoo.com |
| Received May 29, 2013; Accepted July 25, 2013; Published August 03, 2013 | |
| Citation: Uduma FU, Ongolo CP, Okoye IJ, Muna W (2013) The Topography of White Mater Peri-Ventricular Hyperintensities on Brain Magnetic Resonance Imagings (MRI) Among Cameroonians. OMICS J Radiology 2:136. doi: 10.4172/2167-7964.1000136 | |
| Copyright: © 2013 Uduma FU, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Radiology
Abstract
Background: Peri-Ventricular Hyperintensity (PVH) refers to bright signal adjacent to lateral ventricles on T2 weighted (T2W) and Fluid Attenuated Inversion Recovery (FLAIR) brain MR images. It is a qualitative evaluation of white mater lesions. Aim: Evaluating the incidence and topographic distribution of cerebral periventricular hyperintensities among Cameroonians using FLAIR and T2W brain MR images. Materials and methods: Prospective study of patients who came for brain MRI from June 2009 to Febuary 2010. Patients were scanned after data documentation with AIRIS 11 0.3 Tesla imager starting from medullo-cervical cord junction. Axial T2W and FLAIR images were acquired using 6-8mm slice tissue thickness and 0.5-1 mm intersection gap. Images were evaluated qualitatively into absent, type 1-3 PVH. Type 1-peri-ventricular frontal capping, rimming or thin smooth halo. Type 2-PVH extends into deep white mater. Type 3-PVH extends into sub-cortical white mater. Inclusion criteria included reportable quality images. Results were analysed with Computer statistical package SPSS 13 Results: Ninety seven patients with 60 (61.86%), males and 37 (38.14%) females were studied. Age range was 0- 89 with mean of 44.5. 75.26% of all cases, 70% of all males and 83.78% of all females had absent PVH. 24.74% (n=24, M: F=3:1) of studied population had PVH, shared into 33.33% type 1 PVH, 58.33% type 2 and 8.33% type 3. Peaks of type 2 PVH in males and females were 60-69 and 70-79 respectively. The commonest association of type 2 PVH in males and females were CVD (62%) and cerebral atrophy (50%) respectively. Conclusion: The incidence rate of PVH among Cameroonians without population bias is 24.74% with male earlier onset and predominance. PVH is common and severer among elderly (50 years and above). The predominant type of PVH in both sexes is type 2 with commonest association in males being CVD but cerebral atrophy in females.
| Keywords |
| Periventricular hyperintensity; MRI; FLAIR; White mater |
| Introduction |
| Peri-Ventricular Hyperintensity (PVH) as the name connotes refers to homogenous high signals contiguous to lateral ventricles on T2 weighted (T2W), Fluid Attenuated Inversion Recovery (FLAIR), and Proton Density (PD) images, without prominent hypointensity on T1 weighted (T1W) MR images [1-5]. They are differentiated from infarcts as they are not usually evident on T1W MR images and they do not follow specific vascular territories [2]. PVH is a qualitative evaluation of white mater lesions in brain Magnetic Resonance Imaging (MRI) [1]. |
| Regional localizations to peri-ventricular areas equate to white mater which is known to contain nerve fibres. White mater is dichotomized into deep (central) and subcortical (peripheral) white maters of either cerebral or cerebellar hemisphere [6,7]. This accounts for 60% of the total brain volume [6]. Major commissural tracts, cortical association fibres and cortical afferent/ efferent fibres are the constituents of the deep white mater. These nerve fibres consist of neuronal axons with myelin envelopes [6]. Others are supporting cells, interstitial spaces and vascular structures [6]. The supporting cells (neuroglia) are either oligo-dendrocytes or astrocytes [6]. Oligodendrocytes produce and maintain myelin which functions as axonal insulators and facilitates rapid impulse transmission [6]. |
| The presence of normal myelin confers characteristic MRI intensity on normal brain MR images. Myelin has relatively short T2 and T1 relaxation times primarily owing to its lipid content. Normal myelin is therefore hypointense on T2W and hyperintense on T1W. If a disease decreases the myelin content, the white mater becomes less hydrophobic and takes on more water causing prolongation of T1 and T2 relaxation times. This leads to high signal on T2W, FLAIR and low signal on T1W as seen in PVH [6]. |
| T2W and FLAIR MRI pulse sequences have been exploited in segmentation and anatomical mappings of white mater hyperintensities [7,8]. There are different such mapping systems by different authors. But for the purpose of this study, only five will be considered. |
| De Carli et al. identified two categories of white mater hyperintensity based on qualitative MRI anatomical localization with attendant pathophysiological and behavioural sequellae [7]. These two categories are peri-ventricular white mater hyperintensity (PVWMH) and deep white mater hyperintensity (DWMH). The PVWMH about the cerebral ventricles and measures <1 cm from the ventricular surface [7]. While DWMH are patchy areas of white mater hyperintensity in subcortical white mater [7]. |
| Fukuda and Kitani [9] used a five point scale to classify PVH into grade O-absent PVH, grade 1-caps only on anterior horns of the lateral ventricle at the level of basal ganglia. Grade 2-thin lining, smooth halo or irregular PVH within an inner half area of the white mater at the level of the body of lateral ventricle. Grade 3-PVH have extended into the outer half area of the white mater at any region around the lateral ventricle. Grade 4 - when the PVH cover the entire white mater. Score of 2 or below was considered mild but severe if greater than or equals to 3 [9]. |
| Kobayashi et al. simply redefined the above five point scale of PVH. Grade 0, no PVH; grade 1, a small localized focus of PVH at the frontal horn; grade 2, thin PVH halo, grade 3, thick PVH halo and grade 4, marked diffuse PVH [10]. |
| The above 0-4 PVH grading was accepted by Kario and Matsuo et al. but with modification of definitions [11]. Here Grade 1 is defined as no abnormality or minimal peri-ventricular hyperintensity in the form of caps confined exclusively to the anterior horns or rims lining the ventricle. Grade 2-caps in both the anterior and posterior horns of lateral ventricles or peri-ventricular unifocal patches. Grade 3-multiple peri-ventricular hyperintense punctuated lesions with early confluent stages. Grade 4-multiple areas of high signal that reached confluence in the peri-ventricular region [11]. |
| A semi-quantitative four-point separate rating scale for PVWMLs and DWMLs was devised by Fazekas et al. [2,12,13]. For DWMH - absent (grade 0), punctate (grade 1), nearly coalescent (grade 2), or confluent (grade 3). For PVWH, absent (grade 0), pencil thin lines (grade 1), caps or bands (grade 2), or confluent (grade 3) [2,12,13]. |
| In this study, for ease of qualitative simplicity, we have adopted a three type PVH classification system. |
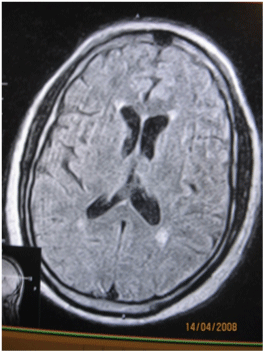
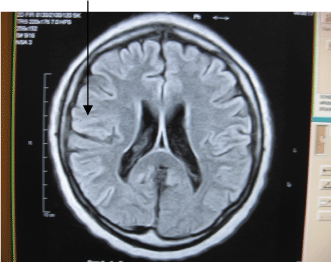
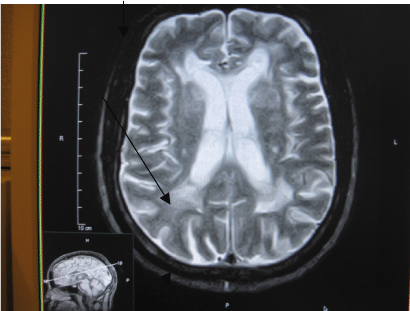
| Type 1 includes frontal capping, peri-ventricular rimming or thin smooth halo of PVH. Type 2 is PVH extending into the deep white mater. |
| Type 3 is when the PVH extends into the sub-cortical white mater. |
| PVH aetiologies´ are multi-factorial and include the followings: [2] |
| (1) Small vessel Ischaemia like Hypertension, Diabetus mellitus, aging. |
| (2) Geriatric disorders like cerebrovascular diseases (CVD), cardiovascular diseases and Dementia, [4]. |
| (3) Central nervous system infections–Tuberculosis, neurosyphilis, neurocysticercosis, Human T cell leukaemia virus infections, Herpes viral encephalitis, Diffuse cytomegaloviral encephalitis, HIV encephalopathy, Epstein-Barr virus encephalitis, Varicella-zoster encephalitis, and Congenital rubella [5,14]. |
| (4) Inherited metabolic white mater disease (Leukodystrophies or dysmyelinations)-Lysosomal disorders (Metachromatic Leukodystrophy, Krabbe´s Leukodystrophy), Peroxisomal disorders (Adreno-leukodystrophy, Zellweger´s Syndrome), Mitochondrial dysfunctions (Leigh´s disease, MELAS, Kearns-Sayre Syndrome,); Amino and organic acidopathy (Canavans disease, Maple Syrup Urine Disease), Others (Pelizaeus-Merzbacher disease, Alexander´s disease) [16]. |
| (5) Inflammatory Demyelinating Diseases–Multiple Sclerosis (MS), Progressive multifocal leukoencephalopathy (PML), Acute disseminated encephalomyelitis (ADEM), Cystic Inflammatory lesions like Lyme´s disease, Neuro-myelitis optica |
| (6) Degenerative Disorders–Biswanger´s disease, Spino-cerebellar degenerations |
| (7) Metabolics-Wernicke´s encephalopathy, Osmotic demyelination syndrome, Vitamin B12 deficiency, Phenylketonuria [5]. |
| (8) CNS malignancy–Lymphoma, Glioma |
| (9) Vasculopathies-Paraneoplastic, Leucocytoclastic Vasculitis, Isolated angitis of central nervous system, Collagen diseases [14]. |
| Miscellaneous |
| Leukoaraiosis, Systemic lupus erthyromatosis, Schizophrenia, CADASIL (Cerebral autosomal dorminant arteriopathy with subcortical infarcts and Leukoencphalopathy), Sneddon´s syndrome, Fabrey´s disease, Bone marrow transplantations and Toxic leukoencephalopathy, Encephalomalacia, Normal pressure hydrocephalus, subcortical arteriosclerotic angiopathy (Binswanger´s disease), Picks disease, Cerebral atrophy, Alzheimer´s disease and Lewy body dementia, Vaccinations, Chemotherapy, cerebral palsy, seizures like pyridoxine dependent seizures neuromotor abnormalities [3-5,9,11,17-19,20-26]. Neuropathologically, PVH has been reported to represent demyelination, gliosis, and spongiosis [19]. It could constitute a potentially useful intermediate or surrogate marker for the identification of new risk factors for stroke, small vessel diseases and dementia [1,10,27-29]. This potentiality and PVH ubiquitousness among older individuals make scientific evaluation of PVH an important and much needed avenue of research [34,30]. More so, since no such PVH documentation has been ever done in Cameroon. |
| AIMS |
| To evaluate the incidence, sex distribution and topographic distribution of cerebral periventricular hyperintensities among Cameroonians using FLAIR and T2W brain MR images. |
| Materials and Methods |
| Settings |
| Polyclinic Bonanjo, Douala, Cameroon. |
| Patient selection |
| A prospective MRI studies of patients who underwent brain MRI from June 2009 to February 2010 were done. |
| Mr imaging protocols, parameters and scoring system |
| Patients were scanned consecutively after recording their clinical and dermographic data. MR imaging was performed in an orbitomeatal plane using a multipolarization radiofrequency head coil receiver on AIRIS 11 0.3 Tesla imager (Toshiba, Japan) Scans began at the medullocervical cord junction and extended superiorly to the inner table of the skull. The parameters for T2W Signal images were (TR 2240-3000, 90 mseconds) and axial FLAIR (7130-8130, 94 mseconds) Multi-slice and Multi-echo acquisitions were done using 6-8mm slice tissue thickness and 0.5-1 mm intersection gap. Total acquisition time was 6 minutes 7 seconds Acquisition matrix is 256×192 or 256×256 and field of view is 240×192 or 230×230. Acquisitions used were principally axial and coronal. Intravenous contrast medium Gadolinium meglumine dipentate at 0.1 mmol/Kg was only given in some patients to further elucidate any associated pathology like masses. |
| FLAIR and T2W MR images were evaluated qualitatively into absent, type 1-3. As previously described. Type 1-frontal capping, rimming or thin smooth halo of PVH. Type 2-PVH extending into the deep white mater. Type 3-when the PVH extends into the subcortical white mater. Any MRI study that yielded borderline results was automatically assigned to the lower grade (Figures 1-3). |
| Inclusion criteria of the study included all patients with T2W and FLAIR reportable quality images. MR images with artifacts and lacking sufficient quality to permit evaluation of the PVH typing were excluded. Punctate hyperintensities in the deep white matter without PVH were also excluded. Cases of extreme hydrocephalus, hydrancephaly or non-detection of frontal horn and atria of lateral ventricles for whatever reasons were also excluded. |
| Results were analysed statistically using computer package SPSS version 13.0. |
| Results |
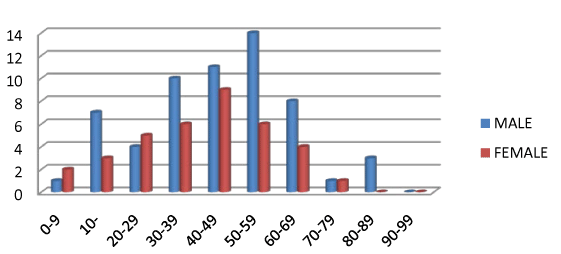
| Ninety-seven patients consisting of 60 (61.86%) males and 37 (38.14%) females were studied. Age range was 0-89 with mean age of 44.5 years. The highest number of subjects was 20 (20.62%) in 40-49 and 50-59 years. The least number of subjects was 3 (3.10%) seen in 0-9 and 80-89 years. The highest number of males studied was 14 (23.33%) in 50-59. While the highest number of females was 9 (24.32%) in 40- 49. 73 cases (75.26%) of studied population had absent PVH. Of this, 42 (57.53%) were males and 31 (42.47%) were females. This implies 70% of all males and 83.78% of all females studied had absent PVH. Subjects with PVH were 24 constituting 24.74% of studied population. Males were 18 (75%) and females were 6 (25%). Of these, 8 cases (33.33%) of all positive PVH had Type 1 PVH with male to female ratio of 3:1. Type 2 were 14 cases forming 58.33% of all PVH. Males were 10 (71.43%) and females were 4 (28.57%) with male to female ratio of 2.5:1. |
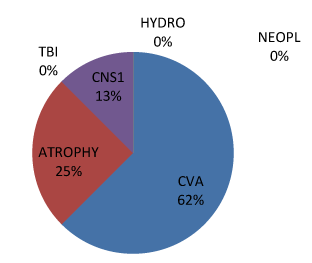
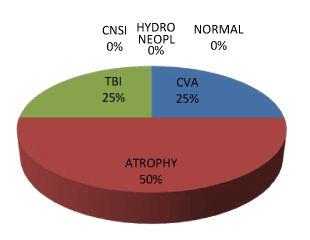
| Type 3 PVH were 2 (8.33%) of all PVH and were all males. 50% of all males with type 1 PVH had associated diffuse cerebral atrophy, whereas 33% and 17% had associated CVD and Meningo-encephalitis respectively. The highest number of males with Type 1 PVH (33.33%) was seen in the 60-69 age range whereas the age ranges of 30-39 and 40-49 of females shared type 1 PVH with 50% each. Males with type 2 PVH were highest in 60-69 (40%) while type 2 PVH females were highest in 70-79 (50%). The commonest association of type 2 PVH in males was CVD (62%), then cerebral Atrophy 25%, meningoencephalitis 13% whereas type 2 females had commonest associations of cerebral atrophy (50%) and meningo-encephalitis (25%) and CVD (25%). Type 3 PVH was seen only in males older than 70 and no associated abnormal radiological features. Type 1 and 2 PVH were first noticed in the first decade of life in males. But third and 5th decades respectively in females. Subcortical PVH were 2 (8.33%) of all PVH (Figures 4-6). |
| Discussion |
| The choice of T2 weighted and FLAIR sequences in this study was predicated on their inherent sensitivity in detecting water content alteration in white mater disease evaluations [22,31]. The attendant hydrophilicity and macromolecular degeneration in white mater lesions (WMLs) cause differences in relaxation rates unlike healthy white mater leading to higher signals in T2W, PD and FLAIR sequences [4]. FLAIR sequence is especially sensitive to WMLs as the inversion pulse can be chosen to null the signal from CSF, thus facilitating easy detection of periventricular region, and decreasing the signal from gray mater permitting optimization of lesion contrast with surrounding brain [4,32]. Fujikawa et al. noted in their study 85% detection of peri-ventricular abnormal intensities with FLAIR, 60% on contrast enhanced T1W images, 55% in diffusion weighted images and 30% in T2W images. FLAIR sensitivity and specificity reported for periventricular white mater lesions (PVWMLs) was 95% (87-99%) and 71% (44-90%), and those for (deep white mater lesions (DWMLs) were 86% (79-93%) and 80% (72-88%) [4]. |
| However, disadvantages of FLAIR are lesion over-estimation and insensitivity to posterior fossa [4,33] Complementary utility of T2W and FLAIR as we have done increases the certainty of the lesion delineation and false positives reductions [4]. |
| In this study, the incidence of peri-ventricular hyperintensity is only 24.74%. Though this is within a reported value of 5.3% to 100%, [4] but our incidence is still lower than some reported values [25,34]. This could be explicable by the wider age spectrum of our studied population (0-89 years). Lack of population bias with inclusion of younger age groups who are known to have lower occurrence of PVH [2,35]. Invariably diluted our PVH incidence. A community based study in US enrolled patients aged 55-72 years (mean 62 years) and found an overall prevalence rate of 86% [25]. In consonance to the above, our study showed 75% of subjects with PVH were in ages of 50-89 years. PVH are common neuroradiological findings in the elderly people [4,10,25]. This brings to mind the possible risk factors for PVH. Some of these risk factors are increasing age, hypertension, diabetes mellitus, carotid artery disease, coronary heart disease, lower education/income, artherosclerosis, haemodynamic dysregulation, impaired cerebral vasomotor reactivity auto-regulation, genetics transient ischemic attack, intracranial malignancy, deep white mater ischemia, hypoxia, female sex and smoking [2,6,9,13,25,30,35-37]. But Fukuda and Kitani in their study pointed out that cigarette smoking was a weak but significant positive predictor of the PVH score and that lipid abnormalities are not related to the PVH score [9,29]. Indeed, age and hypertension are the main predictors of white matter hyperintensities [1,9,10,19,30]. In hypertensives, significant extensive PVH exist in hypertensive receiving no or irregular treatment than those receiving regular treatment [9]. Also subjects with abnormally low or high levels of diastolic blood pressure have more white matter lesions than do subjects with normal diastolics [2]. The triggering effects of above risk factors is understood since PVH is based on deep white mater ischemia. Peri-ventricular and subcortical white mater are located in arterial border zones called watershed areas which are prone to ischemic injury [25]. The deep white mater receives its blood supply from long, small calibre arteries that penetrate the cerebral cortex and traverse the superficial white mater fibre tracts. The white mater does not have as generous blood supply as the grey mater and more prone to ischemia. The nutrient arteries become narrowed by arteriosclerosis and lipohyaline deposits within the vessel walls and the white mater becomes ischaemic [4,7]. This shows pathologically as increased peri-vascular interstitial fluid especially at the arteriolar level. Progression leads to atrophy of axons and myelin resulting in myelin pallor and gliosis. These features are common in the aged [4,25]. In Diabetes mellitus, metabolic perturbations, accumulation of end products of advanced cerebral glycation, micro-vascular ischemic occlusion, elevated erythrocyte aggregation and increased bloodbrain barrier permeability are recognized risk factors for PVH [2]. |
| Concisely, the most important pathogenetic mechanisms in PVH are peri-ventricular fluid dynamics alterations, myelin loss/myelin pallor, tissue rarefaction, gliosis, lipohyalinosis and vasculopathy [1,3, 20,34,31]. |
| PVH and cardiovascular disease like CVD share the same risk factors since both involve small vessel disease [4,10,14]. It is not out of place for CVD to be co-existent with higher grade of PVH as seen in type 2 PVH among males in this study followed by cerebral atrophy. The reversal of trend in females with cerebral atrophy being the commonest associations with type 2 PVH may not be unconnected with the lesser incidence of CVD among females [38,39]. Nevertheless, this might still be a continuum of same spectrum as the neuropathological denominator in ischemic CVD and cerebral atrophy still remains occlusive small vessel disease [1,4] The covert form of this disease will signpost as brain atrophy and the ominous one as CVD. |
| The commonest PVH in this study was type 2 and this is ascribed to the unintended bias in population recruitment as our peak population was middle age (40-59). High prevalence of white mater hyperintensity (WMHs) in mid-adult life has been seen in a study on detailed topographic analysis of WMHs in a large representative middle-aged sample [40]. Type 3 PVH is the severest and mostly seen in advanced age (from 7th decade in this study) since avanlanche of risk factors like CVD, diabetes mellitus, hypertension, and hyperlipidaemia exit in these age extremes. It is also noteworthy that 15 (93.75%) subjects of the 16 subjects who had type 2 and 3 PVH were from 50 years and above. This shows that the severity of PVH increases with age. Age has consistently been the most powerful predictor of PVH. Sarpel et al. concluded in their own study that PVH among white men is high and increases with age [35]. |
| Type 2 and 3 or subcortical PVH are regarded as severe PVH [41]. Involvement of the sub-cortical fibres corresponding to type 3 PVH or DWMH is seen in advanced stages because of their protective compact myelin than white mater [34]. Also, subcortical or U-fibers (a strip of 3 to 4 mm wide cerebral white matter located immediately beneath the cerebral cortex) are spared from early and mild white mater lesions due to rich vascularisation [4]. Their irrigation is not only from the long penetrating medullary vessels but also by shorter vessels that straddle both the white matter and the adjacent cortex [4]. |
| It is reckonable that PVH spares no age group as in this study and others [4]. This is because of known variable paediatric causes like hydrocephalus, dysmyelinating diseases or even in-utero aetiologies like acqueductal stenosis. The earliest PVH we saw was in the 10-19 age range and attributable to meningo-encephalitis. The observed PVH in this age range was the milder form in keeping with PVH congruence with age. |
| PVH process tends to be multi-focal in appearance and their location and shape become the basis of their classification such as triangular caps around the frontal horns, halos, partially confluent or confluent PVH and subcortical multiple punctuate lesions. [4,32]. One such pattern-symmetric capping hyperintensity immediately anterior to the frontal horns of the lateral ventricles was present on all our scans with PVH just like other studies [35]. Periventricular caps seem to present first, since the area that has to be drained of interstitial water will be greatest for the tips of the ventricles especially the frontal and occipital horns [4-6]. Periventricular caps and smooth halo constitute areas of demyelination associated with subependymal gliosis and ependymal lining discontinuity, which are non-ischemic in nature whereas DWMLs and irregular PVWMLs are ischemic [3]. The margin of PVH can also aid in distinguishing the origin of PVH. In transependymal resorptions, PVH peripheral margins are irregular and blunted and do not extend to cortico-medullary junction whereas in demyelination disease, PVH is more sharply angled and extend to cortico-medullary junctions [31]. The hyperintense band of white mater ischaemia has slight heterogenous texture and irregular outer margins distinguishing it from the phenomenon of transependymal flow or peri-ventricular halo of obstructive hydrocephalus [6]. |
| Simple dichotomization of white mater hyperintensites into PVH and DWMH based on anatomical localization yielded 33.33% of our positive cases being purely PVWML. Fazekas et al. were first to rate PVWML and DWML separately based on ‘continuity [to ventricle] rule’ [4]. There is a general consensus that PVH correspond to a single vascular white mater watershed area extending 3 mm to 13 mm from the ventricular surface [7]. They are more likely to be hemodynamically determined since this area is supplied by no collateralizing ventriculofugal vessels arising from subependymal arteries [4]. These branches originate either from the choroidal arteries or from terminal branches of the rami striate [4]. Irregular PVWMLs or PVWMLs larger than 13 mm were classified as DWMLs [4]. The deep white matter region is supplied by the deep perforators of the anterior choroidal and the lenticulostriate branches of the middle cerebral arteries [19]. DWML may be more attributed to small vessel disease (SVD) [4]. The subtype of ischemic strokes has been found to be differentially associated with the type of WML [4]. PVWML is a predictor for the border-zone infarcts especially around the posterior horns, whereas DWML is a predictor for lacunar infarcts [4]. |
| PVH alone may be non-specific except when corroborated with other ancillary MRI brain features that are seemingly specific to a disease entity. For instance, in adrenoleukodystrophy, PVH is predominantly around the atria of lateral ventricles [16,22]. In Leigh disease, apart from PVH, T2W hyperintense signals are seen in deep grey matter nuclei, tegmentum thalami, peri-aqueductal region and dentate nuclei [22]. |
| Where as in Alexander´s disease 5 MRI features are (a) extensive white mater abnormalities with frontal predominance, (b) signal changes in basal ganglia, thalamus and brain stem. (c) Ventricular dilatation with cyst in third ventricle (d) Megaloencephaly (e) Enhancement of optic radiations, caudate nucleus and periventricular white mater [16,22,42]. Krabbe´s disease, an autosomal deficiency of galactosyl ceramide B-galactosidase deficiency presents in the first 6months of life and early childhood death. MRI features are symmetrical abnormalities in the posterior white mater of the optic radiation, centrum semiovale, thalamus, caudate nucleus with late severe cerebral atrophy [16,43,44]. Micro-vascular incomplete white mater infarction, typically sparing the short, arcuate fibres in a pattern presenting as diffuse peri-ventricular white mater lesions in the elderly is called Binswanger disease [45]. |
| The clinical significance of PVH or DWMH is controversial [34]. Mild PVH are often not indicative of brain disease as they are seen in healthy individuals with no evidence of neuropsychological impairment [19,20]. More extensive and severe PVH are usually associated with intra-cerebral pathology [19]. However, the disease related specificity is yet undetermined [35]. But Baum et al. in their study found that the progression in PVH volume paralleled the decline in mental processing speed [41]. However it is correlated with aging, decreasing memory, visuo-perceptual skills, cognitive impairment and neuro-psychiatric implications [1,2,34]. |
| Heterogeneity in our population sample recruitment is a limitation to this study as it invariably downplayed our PVH incidence since PVH is known to increase with age. Also, this study has a selection bias as it was not a population-based study but patients who visited the Radiology department for brain MRI. We also made no scientific valid attempt to link PVHs to disease pathoaetiogenesis [45]. Another snag is that this study is purely a visual qualitative assessment of PVH instead of automated quantitative or visual semi-quantitative measurement of white matter hyperintensity volume, Future research aimed at correlating specific neuromotor abnormalities with volumetric data will be important in characterizing which patterns of peri-ventricular hyperintensity are clinically important and likely to result in neuromotor abnormalities (Tables 1 and 2). |
| Conclusion |
| The incidence rate of PVH among Cameroonian without population bias is 24.74% with male earlier onset and predominance. Earliest onset of PVH is first decade. PVH is common and severer among elderly (50 years and above). The predominant type of PVH in both sexes was type 2 with commonest association in males being CVD but cerebral atrophy in females. |
References |
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 18301
- [From(publication date):
September-2013 - Jul 06, 2025] - Breakdown by view type
- HTML page views : 13611
- PDF downloads : 4690
