Research Article Open Access
The Study of Heterotrophic and Crude Oil-utilizing Soil Fungi in Crude OilContaminated Regions
| Vida Dawoodi1*, Mahbobeh Madani2, Arezoo Tahmourespour3 and Zeynab Golshani1 | |
| 1Young researchers and Elite Club, Falavarjan Branch, Islamic Azad University, Isfahan, Iran | |
| 2Department of Microbiology, Falavarjan Branch, Islamic Azad University, Isfahan, Iran | |
| 3Basic medical sciences department, islamic azad university, Khorasgan branch, Iran | |
| Corresponding Author : | Vida Dawoodi Young researchers and Elite Club, Falavarjan Branch Islamic Azad University, Isfahan, Iran Tel: +989391534433 E-mail: vdawoodi@yahoo.com |
| Received October 30, 2014; Accepted January 28, 2015; Published January 30, 2015 | |
| Citation: Dawoodi V, Madani M, Tahmourespour A, Golshani Z (2015) The Study of Heterotrophic and Crude Oil-utilizing Soil Fungi in Crude Oil Contaminated Regions. J Bioremed Biodeg 6:270. doi:10.4172/2155-6199.1000270 | |
| Copyright: © 2015 Dawoodi V, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
In the present study, the population of heterotrophic and oil-utilizing fungi was investigated in soils with different oil contamination from Khuzestan, Iran. Diversity and distribution of soil population were determined in relation to a number of environmental factors such as pH, electrical conductivity and soil organic matter. In the soil samples, counts of the total heterotrophic fungi ranged from 0.41 ± 0.16 to 3333.33 ± 288.00 × 102 CFU/g soil while counts of crude oil-utilizing fungi ranged from 0.26 ± 0.10 to 2860.00 ± 163.20 × 102 CFU/g soil. In soil samples 3 and 5, population of oil-utilizing fungi was more than heterotrophic fungi. Moreover, sixty species belonging to thirteen fungal genera were isolated from oil-contaminated soils. The thirteen isolated fungal genera included Aspergillus, Penicillium, Fusarium, Acremonium, Candida, Rhodotorula, Mucor, Aureobasidium, Cunninghamella, Rhizopus, Alternaria, Beauveria and Paecilomyces, among which Beauveria, Paecilomyces and species of Aspergillus were isolated only from mineral salts medium (MSM). Investigation of the isolation of fungi from oil-contaminated environments showed that abundance and fungal diversity in different stations were significantly different.
| Keywords |
| Fungal population; Crude oil-utilizers; Heterotrophic soil fungi |
| Introduction |
| Oil products are continually used as a main source of energy in industry and daily life [1]. Release of hydrocarbons into the environment, whether accidentally or due to anthropogenic activities, is a major cause of water and soil contamination [2]. As dependency to oil is increasing, the allied problems are becoming more and more cumbersome [3]. Soils which are contaminated by hydrocarbons expose an extensive damage to local ecosystems as accumulation of pollutants in animals and plants tissues can cause death or mutation [4]. We need to prevent from expansion of contamination and also clean up the contaminated areas. Various methods, including bioremediation, can be used for this purpose [5]. The ability of microorganisms to utilize hydrocarbons in oil-contaminated environments has been documented [6-8] .Several fungi have been found to exhibit greater hydrocarbon biodegradability than bacteria [9]. The advantages associated with fungal bioremediation lay primarily in versatility of the technology and its cost efficiency as compared to other remediation technologies such as incineration, thermal desorption and extraction [10]. The use of fungi is expected to be relatively economical as they can be grown on a number of inexpensive agricultural or forest wastes such as corncobs and sawdust. Moreover, their utilization is a gentle non-aggressive approach [11]. Species of many fugal genera are known to metabolize hydrocarbons and thrive in oil-contaminated sites and the most common fungi which have been recorded as biodegraders belong to the genera: Alternaria, Amorphoteca, Aspergillus, Candida, Cephalosporium, Cladosporium, Fusarium, Geotrichum, Graphium, Mucor, Paecilomyces, Penicillium, Rhizopus, Rhodotorula, Sphaeropsidales, Talaromyces and Trichoderma [12-16]. |
| Despite many studies focused on isolation of microorganisms in oil fields, no research yet has been conducted on indigenous fungi of Khuzestan province in Iran. The objectives of this study were, therefore, to investigate population of soil heterotrophic and oil-utilizing fungi in Khuzestan oil fields with different oil contaminations and to identify the heterotrophic and hydrocarbon-degrading fungi isolated from these regions. |
| Materials and Methods |
| Sample collection |
| During April and May 2012 soil samples were collected from several oil-contaminated areas of Khuzestan province, Iran, including Ahvaz (soil sample1), Haftkel (soil sample 2), Kupal Oilfield (soil sample 3), Maroon (soil sample 4), Omidiyeh (soil sample 5), Tembi (soil sample 6), Masjed Soleyman (soil sample 7) and a non-contaminated soil from Masjed Soleyman (soil sample 8). Each soil sample was collected from the surface (0-15 cm depth) of each site using a stainless steel sampling probe, and was air-dried and sieved (< 2 mm mesh) in the laboratory. |
| Primary soil analysis |
| Soil analysis tests were done with the help of Lamottes' soil testing outfit, phosphorus, pH, etc., were estimated as outlined in Piper's book [17]. Organic carbon was determined using Walkley and Black method [18]. |
| Cultivation, counting and isolation of indigenous fungi |
| The enumeration of fungi was done using the dilution plate technique [19]. Potato dextrose agar (PDA) medium was used for isolating and enumerating of heterotrophic soil fungi, and mineral salts medium (MSM) supplemented with 1% crude oil was used for isolation, enumeration and preliminary identification of petroleumutilizing fungi of soil samples. Four replicates were made. The MSM medium was composed of NaCl of 10.0 g; MgSO4. 7H2O of 0.42 g; KCl of 0.29 g; KH2PO4 of 0.83 g; Na2HPO4 of 1.25 g; NaNO3 of 0.42 g; agar of 15 g; distilled water of 1 L and final pH of 7.2. Streptomycin antibiotic was added to the media to prevent bacterial growth [20,21]. Then the fungi that grew on these media were considered as oil-degrading ones and subcultured on PDA and identified according to macroscopic and microscopic characteristics [22-24]. |
| Statistical analysis |
| Data were analyzed by statistical SPSS-14 software. To study the significant difference, variance analysis and the difference were calculated in a significant level (p ≤ 0.05). |
| Result and Discussion |
| Analysis of soil and population of soil fungi |
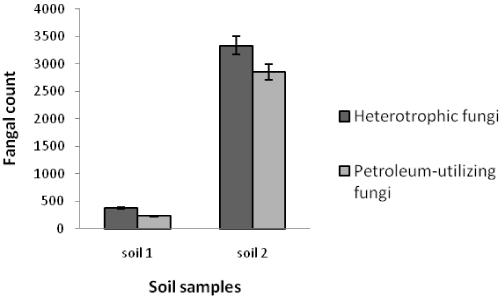
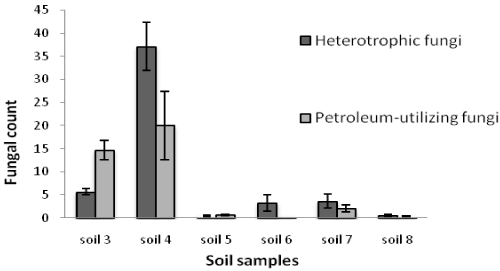
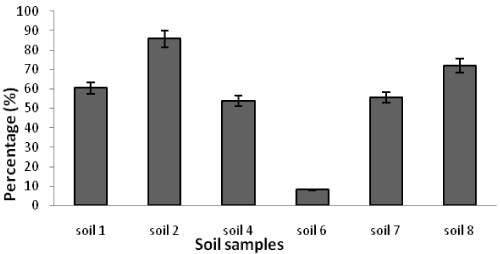
| Table 1 shows the results of soil analysis as well as population of total heterotrophic and oil-utilizing fungi. After enumeration of the indigenous fungi, the average counts of total heterotrophic fungi on PDA media and the average counts of crude oil-utilizing fungi in oil agar media were expressed as ×102 CFU/g soil. In the soil samples, the counts of total heterotrophic fungi ranged from 0.41 ± 0.16 to 3333.33 ± 288.00 while the counts of crude oil-utilizing fungi ranged from 0.26 ± 0.10 to 2860.00 ± 163.20. Figures 1 and 2 show the population of heterotrophic and crude oil-utilizing fungi. Due to the significant difference in fungal population of soils 1 and 2, the corresponding population graphs are presented separately. The highest populations of heterotrophic fungi (3333.33 ± 288.00) and crude oil-utilizing fungi (2860.00 ± 163.20) were related to soil 2. The least total fungal populations of 0.41 ± 0.16 and 0.50 ± 0.10 belonged to soils 5 and 8 respectively, and the least amount of crude oil-utilizing fungi of 0.26 ± 0.10 was related to soil 6. According to the results, population of crude oil-utilizing fungi was more than heterotrophic fungi in soils 3 and 5 (Figure 2). Figure 3 shows the ratio (%) of oil-degrader fungi to heterotrophic fungi. The highest amount of these fungi belonged to soil 2, whereas soil 6 had the least. |
| Isolation and identification of indigenous fungi |
| In the present study, sixty indigenous fungi species of thirteen fungal genera were isolated from different soil samples. The identified fungal genera included Aspergillus, Penicillium, Fusarium, Acremonium, Candida, Rhodotorula, Mucor, Aureobasidium, Cunninghamella, Rhizopus, Alternaria, Beauveria and Paecilomyces. Of these, Beauveria, Paecilomyces and species of Aspergillus were isolated only from mineral salts medium (MSM). |
| In this study, the total heterotrophic fungal and crude oil-utilizing fungal counts varied significantly in different stations, considering the amount of oil contamination and the time of spill (Figures 1 and 2). Soils 2 and 1 had the highest population of both fungal groups, respectively. The study area had been polluted for a long time, and a higher organic carbon was the reason for greater population in soil 2 (Table 1). Organic carbon of soil 2 was 20.51% whereas that of soil 1 was 11.86%. Since increase in entrance of crude oil into the environment increased the amount organic carbon in soil and owing to the desirable amounts of phosphorus and neutral pH, population of crude oilutilizing fungi was increased. However, soil 1 had a higher pH than soil 2 which reduced the population of oil-degraders. Westlake et al. [25] also reported that specific microbes show increase in population because of using petroleum hydrocarbons as a nutrient [25]. |
| Soils 4 and 5 had organic carbon of 18.35% and 12.22% respectively, and neutral pH. They also had the same phosphorus amounts as soils 1 and 2; however, their fungal population was very low which could be attributed to the short time exposure to oil spill. Soil 5, due to shorter time of exposure to the oil spill, also had a higher electrical conductivity which was a limiting factor for fungal growth (Table 1). An excess of dissolved salts (anions and cations) in the soil is detected by electrical conductivity [26]. Electrical conductivity is one of the characteristics that promote anti-microbial activity. Excessive heavy metal concentration in the soils was reported to cause decrease in microbial population [27]. Most metal ions have the ability to create oxygen radicals, thus forming molecular oxygen which is highly toxic to bacteria and fungi [28]. |
 |
| The electrical conductivity of soils varies depending on the amount of moisture held by soil particles. |
| Increasing soil solution salinity (EC) will decrease available water. This will have a negative effect on crop yield and microorganisms such as fungi, also may affect their survival [29,30]. |
| Sudden discharge of large quantities of oil to these areas has affected most of the microorganisms and has reduced the microbial population. Because of a short time exposure to oil spill, the remaining microorganisms did not have enough time to adapt to the environment. Low population of soils 4 and 5 can be explained by the fact that primary reduction in fungal population occurs whenever the crude oil is added to the environment, probably because of crude oil toxicity; then, some organisms are killed or controlled by toxic components of crude oil while other oil degrading heterotrophic organisms are increased in number [31]. In soils 6, 7 and 8, a lower fungi population was observed because of low amount of organic carbon. It seems that the percent of oil hydrocarbon consumers can be an index for existence of hydrocarbons in the environment. In heavily polluted environments, immediate harmful effects will be observed in biological forms [21]. The effect of petroleum pollution depends on chemical composition of oil product and the species of microorganisms [25]. These results are in agreement with findings of Obire and Anyanwu that treated soil with different concentrations of crude oil and counted fungal populations within eighteen weeks. Fungal population was decreased in control soil (without oil), a primary reduction and then considerable increase were observed in high concentrations of oil [21]. The toxicity of crude oil or petroleum products varies widely, depending on their composition and concentration. Moreover, the scale of pollution depends on the amount of oil and the damage done to the environment [31]. An interesting observation in this study was a significant increase in oil-utilizing population in soil 3 in comparition to heterotrophic population which was recognized to be Aspergillus species (Figure 2). This species have been never observed previously on PDA medium at the time of counting and isolation. |
| In studies conducted by Chaudhry et al. [32] population of heterotrophic fungi in all samples was more than diesel utilizing fungi [32]. In this study the same results were observed in soils 1, 2, 4, 6, 7 and 8, and the opposite results were observed in soils 3 and 5. |
| Although, in this study, the heterotrophic fungal population was expected to be greater than oil-utilizing fungal population due to use of enrichment medium (PDA), opposite results were observed in two areas. This could be attributed to adaptation of oil-utilizing fungi to the amount of hydrocarbon in the environment and there by their increase in two regions. However, it should be noted that nutrients found in the oil can also, stimulate the growth of fungi. |
| Shaw found that organisms break down hydrocarbons and use the energy to synthesize cellular components. After being completely broken down, the reaction releases carbon (IV) oxide, water and energy used to create cellular biomass [33]. The results of studying heterotrophic fungi and petroleum-utilizing fungi in some soil samples suggest that the petroleum-utilizing fungi were adapted to the quantity of hydrocarbons in the environment, and thereby increased the number of petroleum-utilizing fungi in polluted areas. |
| In soil 8, despite the lack of crude oil pollution, the ratio of degrading fungi to heterotrophic fungi was 72.0% showing isolation of oil-degrading microorganisms from irrelevant environments. Microbes which can use hydrocarbons are found naturally in the environment. However, they increase after oil spill because an additional carbon resource (hydrocarbons) is being prepared [34]. |
| Soil 2, despite of having the greatest population and the largest amount of organic carbon, showed a low fungal diversity composed of a species of Penicillium (dominant soil population) and two species of Fusarium and Aspergillus terreus (both heterotrophic and crude oilutilizing fungi). The highest fungal diversity belonged to soil 8 which had the least organic carbon. |
| Obire and Anyanwu reported that reduction in diversity of species (fungal genus) with an increase in concentration of added crude oil was an index for environmental stresses of oil hydrocarbons [21]. |
| Nkwelang et al. [35] showed that the main active fungal genera of contaminated soils were Aspergillus, Penicillium and mucor [35]. |
| In the present study sixty fungal isolates (13 genera including Aspergillus, Penicillium, Fusarium, Acremonium, Candida, Rhodotorula, Mucor, Aureobasidium, Cunninghamella, Rhizopus, Alternaria, Beauveria and Paecilomyces) were isolated from MSM and PDA media, with genera Aspergillus and Penicillium being dominant. |
| According to Chaillan et al. [13] Aspergillus and Penicillium are the most commonly found fungi in tropical soils, which are able to degrade hydrocarbons [13]. Conceicao et al. [36] concluded that these genera from a group of microorganisms definitely possess mechanisms to resist adverse environmental conditions, and some of them had the ability to degrade oil residues [36]. Bento et al. [37] have isolated Aspergillus species from diesel samples from refineries, storage tanks, as well as from fuel and injection pumps of vehicles [37]. |
| Also Obire et al. [38] in another study, reported that Aspergillus, Candida, Cephalosporium, Cladosporium, Fusarium, Geotrichum, Mucor, Penicillium, Rhodotorula and Trichoderma genera could grew in oil-contaminated places [38]. As filamentous fungi can grow on hydrocarbons, Aspergillus and Penicillium genera have been also frequently reported in this regard [13,39]. |
| Lemos et al. [40] also isolated filamentous fungi including Aspergillus, Penicillium, Fusarium and Paecilomyces from the Brazilian soils (Guararema) [40]. |
| Conclusion |
| Studies on isolation of fungi in oil containing environments showed that, abundance and fungal diversity in different stations significantly were different. According to the structure and physicochemical properties of different polluted soils, various fungi were found in this study. Even in two adjacent places, low similarities in fungi types were observed. |
| The increase in the number of fungi in crude oil soils shows the probability of degradation and consumption of oil contaminated by fungi. The studied species which grew in presence of oil were found to be applicable as a contamination indicator or a means for better understanding of decomposition mechanism. Finally, the study microorganisms could be proposed to be potentially used as the environmental cleaning up techniques. |
| Acknowledgements |
| The authors wish to sincerely appreciate the Falavarjan Branch, Islamic Azad University, Isfahan, Iran, which assisted the authors and supported this research. |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 16193
- [From(publication date):
March-2015 - Jul 02, 2025] - Breakdown by view type
- HTML page views : 11289
- PDF downloads : 4904
