Review Article Open Access
The Status of Histopathology in the Diagnosis of Gastroesophageal Reflux Disease Time for Reappraisal?
Nora I. Schneider and Cord Langner*
Institute of Pathology, Medical University, Graz, Austria
- *Corresponding Author:
- Cord Langner, MD
Institute of Pathology, Medical University of Graz
Auenbruggerplatz 25
8036 Graz, Austria
Tel: +43 (0)316 385 13665
Fax: +43 (0)316 385 13432
E-mail: cord.langner@medunigraz.at
Received date: November 05, 2015 Accepted date: November 07, 2015, Published date: November 14, 2015
Citation: Schneider NI, Langner C (2015) The Status of Histopathology in the Diagnosis of Gastroesophageal Reflux Disease – Time for Reappraisal?. J Gastrointest Dig Syst 5:355. doi:10.4172/2161-069X.1000355
Copyright: © 2015 Schneider NI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
The histological diagnosis of gastroesophageal reflux disease is generally believed to be a tool of limited value. Recent data, however, indicate that histology may be useful for the management of patients with non-erosive reflux disease, who account for up to 60% of all patients with reflux symptoms. Early diagnosis of gastroesophageal reflux disease is crucial because chronic reflux esophagitis is a key risk factor for the development of Barrett´s esophagus, which predisposes to esophageal adenocarcinoma. Histologically, reflux esophagitis is characterized by basal cell hyperplasia, papillary elongation, dilation of intercellular spaces, and inflammatory infiltration. These reflux-induced changes of the squamous epithelium are mainly related to the diagnosis of acute and/or active reflux. The chronic consequences of gastroesophageal reflux disease are mainly characterized by metaplatic mucosal replacement. The origin and significance of cardiac mucosa at the gastroesophageal junction are still controversial However, evidence is accumulating that injury and repair related to gastroesophageal reflux disease contribute to its development and/or expansion. Multilayered epithelium, defined as hybrid epithelium with characteristics of both squamous and columnar epithelium has been identified as a new sensitive marker of gastroesophageal reflux disease. This epithelium may be the precursor of metaplastic cardiac mucosa, and ultimately Barrett’s esophagus.
Keywords
Gastroesophageal reflux disease; Gastroesophageal junction; Cardiac mucosa; Multilayered epithelium; Esohisto criteria; Intestinal metaplasia; Barrett’s esophagus; Histology; Histopathology
Introduction
Gastroesophageal reflux disease (GERD) is a common disease. The incidence of GERD is rising worldwide, with the highest prevalence in the Western world (Europe 23.7% and USA 28.8%) and increasing prevalence even in Asia (East Asia 2.5-9.4%, Mid Asia 7.6-19.4%, and Western Asia 12.5-27.6%) where GERD has not traditionally been a major health problem in the past [1,2].
Typical GERD symptoms are heartburn and regurgitation. Additionally, patients may report symptoms such as epigastric pain or sleep disturbance [2]. It is of note that GERD comprises a large spectrum of clinical manifestations [2,3], including patients with typical symptoms and endoscopic diagnosis of esophagitis, but also patients without GERD symptoms, yet endoscopic diagnosis of esophagitis with varying extent of mucosal breaks, which is nowadays mainly graded according to the modified Los Angeles classification [4,5]. Non-erosive reflux disease (NERD) is characterized by the presence of troublesome reflux symptoms, abnormal pH monitoring, absence of endoscopically visible lesions [2], but with histological changes of the squamous epithelium, that is microscopic esophagitis [2,3]. NERD patients account for up to 60% of all patients with reflux symptoms. The mechanisms involved in the pathogenesis of NERD are complex and multifactorial [6]. Early diagnosis of GERD is crucial because chronic reflux esophagitis is a key risk factor for the development of Barrett´s esophagus, which is a precursor lesion for esophageal adenocarcinoma [7,8].
According to recently published practice guidelines of the American College of Gastroenterology a presumptive diagnosis of GERD can be made on the basis of typical symptoms [9,10]. Improvement of reflux symptoms on empiric medical therapy with a proton pump inhibitor (PPI) ideally confirms this symptom-based diagnosis (so-called PPI test) [9,10]. It is of note that upper gastrointestinal endoscopy is only recommended in the presence of permanent alarm symptoms and screening of patients at high risk for complications. Hence, endoscopy with biopsy from the distal esophagus and/or the gastroesophageal junction (GEJ) is currently not part of routine patient evaluation [9,10].
In this review, we will focus on the histological diagnosis of GERD, referring to different morphological features, which are altered due to acute and/or chronic acid exposure. Specifically, we will refer to GERD-induced changes of the squamous epithelium, which are mainly related to the diagnosis of acute and/or active GERD, but also to the chronic consequences of GERD at the GEJ, which are mainly characterized by metaplatic mucosal replacement.
The Histopathology of GERD – General Principles
The esophagus is lined by nonkeratinizing, stratified squamous epithelium, and the stomach by columnar epithelium, respectively [11,12]. The border between the stomach and the esophagus, that is the GEJ, contains the lower esophageal sphincter, which is characterized as a variable zone of 2-4 cm in length and a pressure of approximately 10-26 mmHg, which is well above both intragastric and intraesophageal pressures [11,12].
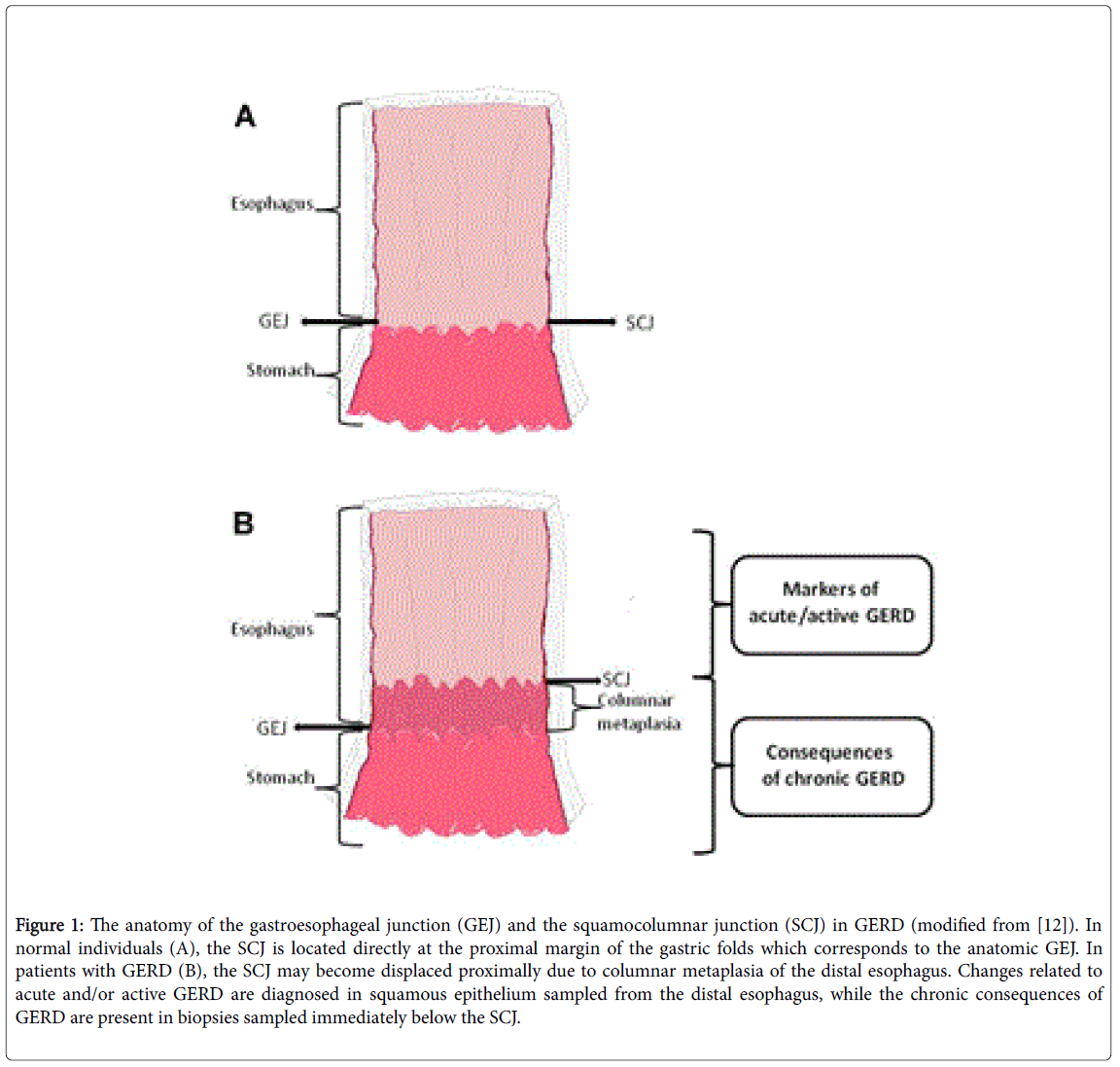
Upon endoscopy, the GEJ is the point, where the tubular esophagus meets the gastric mucosal folds [12,13], also known as the “Z-line” [11]. In “normal” individuals, the GEJ is identical with the squamocolumnar junction (SCJ), the histological transition point between esophageal squamous and gastric columnar epithelium. In patients with GERD, the SCJ moves proximally as a result of metaplasic change (Figure 1). Thus, the histological SCJ is located above the anatomical GEJ [12].
Figure 1: The anatomy of the gastroesophageal junction (GEJ) and the squamocolumnar junction (SCJ) in GERD (modified from [12]). In normal individuals (A), the SCJ is located directly at the proximal margin of the gastric folds which corresponds to the anatomic GEJ. In patients with GERD (B), the SCJ may become displaced proximally due to columnar metaplasia of the distal esophagus. Changes related to acute and/or active GERD are diagnosed in squamous epithelium sampled from the distal esophagus, while the chronic consequences of GERD are present in biopsies sampled immediately below the SCJ.
GERD is the major cause of inflammation and mucosal breaks of the squamous epithelium in the distal esophagus. As a consequence, the reparative capacity of the native squamous epithelium may be unable to tolerate the acidic and/or proteolytic nature of persistent reflux, and it adapts to the damaging stimuli by converting into metaplastic columnar epithelium. This is due to the abnormal differentiation of pluripotent esophageal stem cells into columnar epithelium of gastric or intestinal type, which is more resistant to injury from acidic gastric content [14-17].
The histological diagnosis of GERD is generally believed to be a tool of limited value [9,18,19]. The sensitivity and specificity of histological GERD diagnosis are generally believed to be low [18]. Several recent studies have, however, demonstrated that histology, if systematically applied, may render important diagnostic clues. This holds particularly true for individuals with NERD, of whom approximately two thirds have histological evidence of esophageal injury [20,21].
Various histological features of the squamous epithelium related to GERD have been identified, which may be explained as response to damage [22]. These mainly include proliferative changes of the squamous epithelium, such as basal cell layer hyperplasia, papillary elongation, and intercellular space dilation, but also intraepithelial inflammatory cell infiltration by eosinophils, neutrophils, and mononuclear cells [21,23]. The recognition of these parameters has recently been standardized in the Esohisto project, proving good interobserver agreement of histological diagnosis (Table 1) [3,24].
| Proliferative changes of the squamous epithelium | Criterion | Definition and method of assessment | Severity score |
|---|---|---|---|
| Basal cell layer Hyperplasia | Basal cell layer thickness in μm as a proportion (%) of total epithelial thickness (10×) | 0 (<15%) 1 (15–30%) 2 (>30%) |
|
| Papillary Elongation | Papillary length in μm as a proportion (%) of total epithelial thickness (10×) | 0 (<50%) 1 (50–75%) 2 (>75%) |
|
| Dilated intercellular spaces | Identify as irregular round dilations or diffuse widening of intercellular space (40×) | 0 (absent) 1 (<1 lymphocyte) 2 (≥1 lymphocyte) |
|
| Inflammatory infiltrate | Intraepithelial Eosinophils | Count in the most affected high-power field (4×0) | 0 (absent) 1 (1–2 cells) 2 (>2 cells) |
| Intraepithelial Neutrophils | Count in the most affected high-power field (40×) | 0 (absent) 1 (1–2 cells) 2 (>2 cells) |
|
| Intraepithelial mononuclear cells | Count in the most affected high-power field (40×) | 0 (0–9 cells) 1 (10–30 cells) 2 (>30 cells) |
Table 1: Histologic criteria for the recognition and assessment of microscopic lesions related to gastroesophageal reflux disease (GERD) – the Esohisto project criteria [3].
The identification (and subsequent validation) of additional parameters may further help to re-define the role of histology in the routine work-up of patients with suspected GERD. These may include changes related to GERD that are encountered outside the squamous epithelium. Multilayered epithelium, defined as hybrid epithelium with characteristics of both squamous and columnar epithelium, and cardiac mucosa, the origin of which is currently under debate, are the most promising candidates [25-29].
The Diagnosis of Active GERD
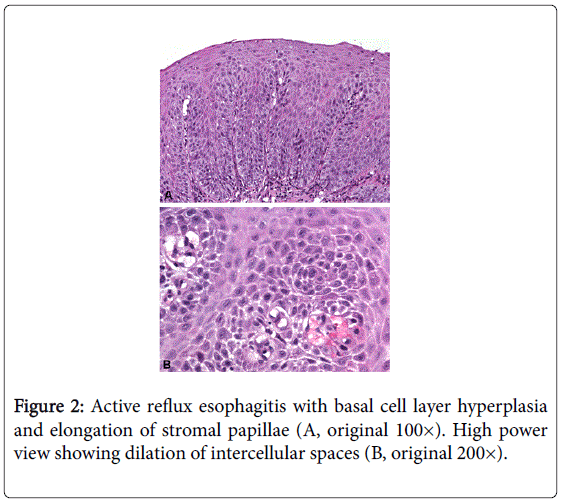
The histological diagnosis of GERD is based mainly upon reactive changes of the squamous epithelium as a response to luminal acid exposure, qualifying for a diagnosis of active and/or acute GERD. Basal cell layer hyperplasia and elongation of stromal papillae are the immediate consequences of epithelial regeneration (“hyperregeneration”) [21-23].
The thickness of the basal cell layer is measured in comparison to the total epithelial thickness [3,23,24]. In the normal esophagus, basal cells comprise up to 2-6 cell layers (<15% of total epithelial thickness) [3,24,30]. For assessing papillary elongation the most extended stromal papilla approaching the epithelial surface should be evaluated [3,24]. In healthy individuals, the papillary length is less than 50% of the total epithelial thickness [24,31]. Both parameters, that is, basal cell layer hyperplasia and papillary elongation, show good results in the histologic diagnosis of GERD (Figure 2) [32].
The dilation of intercellular spaces within the squamous epithelium has been identified as another histological feature, which is related to persistent luminal acid exposure [33]. The detachment of interepithelial cell junctions results in irregular round or diffuse spaces between the epithelial cells, which can easily be detected upon light microscopy [3,24]. In particular, patients with NERD show scores of dilated intercellular spaces three times higher than healthy controls [21,34,35]. The dilation of intercellular spaces has proven good interobserver agreement [36].
Acidic reflux causes, however, also an increase in inflammatory cells, both within the squamous epithelium and the underlying stroma. It is believed that the dilation of intercellular spaces enables acid to reach the epithelial basal membrane, thereby attracting the inflammatory response, but also adding to GERD-related nociception. Inflammatory cells that occur in GERD include neutrophils [37], eosinophils [38,39] and mononuclear cells [40,41]. It is of note that the inflammatory cell reaction is an unspecific histological feature, as microscopic esophageal inflammation may likewise occur independent of reflux disease [42].
The histological features of GERD affecting the squamous epithelium have recently been evaluated in the Esohisto project, a multinational initiative for the standardized recognition of microscopic lesions in patients with GERD [3]. In that project, the authors standardized the criteria for diagnosis, introducing a severity score for each parameter ranging from 0 to 2. The histological diagnosis of GERD based upon the Esohisto criteria proved good interobserver agreement, ranging from 64% (phase I, after development of criteria) to 97% (phase II, after refinement of criteria and consensus discussion) [3,24].
In a subsequent paper, the same group of authors introduced a combined severity score to assess the severity of individual lesions [3,24,31]. This score is restricted to basal cell layer hyperplasia, papillary elongation, dilation of intercellular spaces, and the presence of intraepithelial eosinophils, as these four features are considered to be the most informative elementary lesions (Table 2) [31]. The combined severity score is calculated by summing up lesion scores divided by the number of lesion types assessed. Scores 0-0.25 are regarded as normal, scores 0.5-0.75 qualify for a diagnosis of “mild” esophagitis, and scores ≥ 1 for a diagnosis of “severe” esophagitis, respectively [24,31].
| Criterion | Combined Severity Score | |
|---|---|---|
| Basal cell layer hyperplasia | Sum of lesion severity scores divided by the number of lesions assessed | Normal mucosa Severity score of0-0.25 |
| Papillary elongation | Mild esophagitis Severity score of0.5-0.75 | |
| Dilation of intercellularSpaces | Severe esophagitis | |
| Intraepithelial eosinophils | Severity score of≥1.00 | |
Table 2: Combined severity score for the microscopic diagnosis of refluxesophagitis [24,31].
Levels of interobserver agreement were tested for all features. Agreement was 64% for dilation of intercellular spaces, and was in the range 73-74% for basal cell layer hyperplasia, papillary elongation, and intraepithelial mononuclear cells, and 83-97% for intraepithelial eosinophils, intraepithelial neutrophils, active erosions, and healed erosions [3,24,31].
In the histoGERD trial, we validated the histological criteria as defined in the Esohisto project, demonstrating that histology is significantly associated with patients’ symptoms and also the endoscopic diagnosis of esophagitis [43]. It is of particular note, that histological changes may already be present in symptomatic individuals with normal endoscopy, indicating higher sensitivity of histological diagnosis [43]. These data suggest that histology, if systematically applied, contributes important diagnostic clues to the diagnosis of GERD. This may be particularly relevant for patients with NERD [44].
The Diagnosis of Chronic GERD
The columnar epithelium at the GEJ can be classified in three distinct epithelial types originally described by Paull et al. [45] in 1976 with further modifications made by Chandrasoma et al. [25].
Pure gastric oxyntic mucosa (OM) is formed of glands composed entirely of parietal and chief cells without mucous cells.
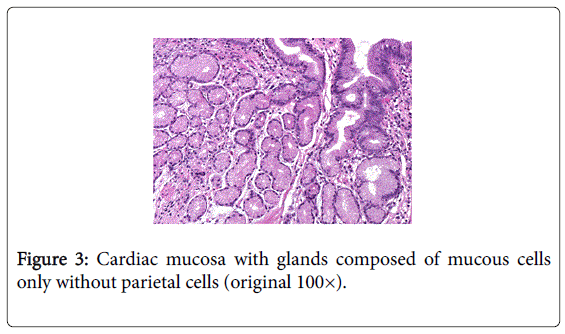
Pure cardiac mucosa (CM) is formed of glands composed of mucous cells only without parietal cells (Figure 3).
Oxytocardiac mucosa (OCM) contains glands with a mixture of mucous cells, parietal cells, and chief cells.
The three epithelial types may encounter in a zonal distribution pattern with CM accounting for the most proximal part of the columnar epithelium and OM for the most distal part, while OCM is found interposed between the other two types [25,45].
Traditionally, CM has been considered as the most proximal part of the stomach, that is “cardia ventriculi”. On the physiological level, it constitutes a natural buffer zone between the stomach and the esophagus that aims to resist reflux of acidic gastric content. A malignant growth in this region is referred to as carcinoma of the cardia and regarded as a gastric neoplasm [12,46].
Origin and significance of CM are controversial, as well as its precise location and extent. For some authors CM is congenital, thus representing a normal structure that is present from birth [47-52], possibly with expansion due to postnatal exposures [48,51]. Others refer to CM as a lesion that is acquired during postnatal life [25,53-57] and a specific and sensitive marker of GERD.
Already in 1997, Öberg et al. [55] related CM to GERD. In their study CM and carditis were associated with deterioration of lower esophageal sphincter characteristics and increased esophageal acid exposure. Chandrasoma et al. [25] investigated 71 patients with reflux disease and observed CM and/or OCM in all of them. Patients with a CM/OCM length >2 cm had a markedly higher acid exposure than patients with a CM/OCM length <2 cm. This finding suggested that the presence of CM and OCM at the GEJ are predictive of abnormal acid exposure, and that increasing CM/OCM length correlates with the amount of acid exposure. These data allowed the authors to conclude that the presence of CM/OCM can, as a sensitive histologic marker, predict the severity of GERD [25].
Several autopsy studies conducted on fetuses, children and adolescents reported CM and OCM in every individual [48-50], thereby arguing that CM is a normal finding, present at birth, hence congenital. In the histoGERD trial CM was present in two out of three individuals undergoing gastroscopy for unselected reasons [58]. It is of note that the presence of CM was related to patients´ symptoms and the endoscopic diagnosis of esophagitis. On the histological level, CM was significantly associated with the histologic features of the squamous epithelium that are related to reflux disease (compare above). These findings, in particular the association between CM and microscopic esophagitis, indicate that CM is at least in part a metaplastic lesion related to chronic GERD. In support of this hypothesis, Glickman et al. [51] reported an association between the length of CM and the diagnosis of active esophagitis also in pediatric patients. Taken together, our data and the data from others suggest that injury and repair related to GERD contribute to the development and/or expansion of CM [58].
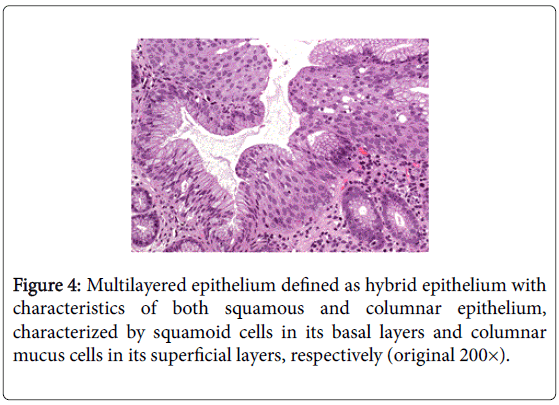
The so-called “multilayered epithelium” (MLE) is a distinct type of epithelium with morphological and immunocytochemical characteristics of both squamous and columnar epithelium (Figure 4). This epithelium has been suggested to be the precursor of Barrett's esophagus [27,28,59]. In 1993, Shields et al. [59] were the first to report the presence of a distinctive cell type at the GEJ with features intermediate between those of squamous and columnar epithelium. The surface characteristics of this cell type were strikingly similar to those of cells found in the transformation zone of the uterine cervix, an area in which squamous epithelium physiologically replaces columnar epithelium.
The presence of MLE was related to GERD-induced inflammation [60,61] and goblet cell metaplasia in patients with endoscopic evidence of esophageal columnar epithelium, that is, Barrett’s esophagus [28,59,62,63]. Glickman et al. [61] analyzed 27 patients with Barrett’s esophagus, 12 patients with GERD, and 18 controls. The authors observed MLE in 33% of patients with Barrett’s esophagus and GERD, respectively, but not in controls. In the histoGERD trial we confirmed these observations: MLE was identified in about every tenth individual undergoing upper GI endoscopy. The histologic diagnosis of MLE was associated with increasing age, indicating that MLE is an acquired lesion. In addition, MLE was associated with high BMI and presence of hiatal hernia, both well recognized risk factors for GERD [64]. Finally, the presence of MLE was significantly associated with the endoscopic diagnosis of esophagitis. Of note, MLE was identified in 28 of 450 (6.2%) individuals with normal GEJ (Los Angeles category N) and 34 of 303 (11.2%) individuals with minimal changes, suggesting a higher sensitivity of histologic diagnosis [64].
But MLE is not only interesting as a new and sensitive marker of GERD, it is also interesting from a biological point of view, as the origin of Barrett’s metaplasia is still unclear [64]. In the histoGERD trial we were able to show that the presence of MLE is significantly associated with the presence of CM (and OCM). Furthermore, MLE was significantly associated with the endoscopic diagnosis of Barrett’s esophagus.
According to Odze [12], columnar metaplasia of the distal esophagus represents a squamous to columnar metaplastic change that may develop from an esophageal stem cell through an intermediate phase characterized by MLE. Intestinal metaplasia, that is, the development of Barrett’s esophagus occurs as a second columnar to columnar metaplastic change that may develop from a stem cell located within the deep foveolar compartment of the (metaplastic) columnar epithelium, most probably under the influence of strong and persistent acid reflux [16,17]. In summary, MLE may be the precursor of columnar metaplasia, thereby providing the soil for the possible subsequent development of intestinal metaplasia, that is Barrett’s esophagus, but it does not seem to be the immediate precursor of intestinal metaplasia [64].
Conclusion
Though histology is not recommended in the current guidelines for the diagnosis and management of GERD, histologic analysis of biopsies sampled from the GEJ appears to be a valuable tool, particular in patients with NERD. The adherence to standardized and clinically validated criteria may improve diagnostic accuracy.
Specifically, histology may be used to diagnose active GERD, characterized by proliferative changes of the squamous epithelium and by inflammatory infiltrate. These criteria have recently been defined in the Esohisto project, proving low interobserber variation. The histological diagnosis of GERD should be based upon these criteria. The routine inclusion of scoring values is, however, not recommended for the pathology report.
Inflammation due to chronic GERD causes metaplastic changes. This may lead from MLE to CM, and ultimately to intestinal metaplasia and Barrett’s esophagus.
References
- Ronkainen J, Agréus L (2013) Epidemiology of reflux symptoms and GORD.Best Pract Res Clin Gastroenterol 27: 325-337.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group (2006) The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus.Am J Gastroenterol 101: 1900-1920.
- Fiocca R, Mastracci L, Riddell R, Takubo K, Vieth M, et al. (2010) Development of consensus guidelines for the histologic recognition of microscopic esophagitis in patients with gastroesophageal reflux disease: the Esohisto project. Hum Pathol 41: 223-231.
- Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, et al. (1999) Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification.Gut 45: 172-180.
- Hongo M (2006) Minimal changes in reflux esophagitis: red ones and white ones.J Gastroenterol 41: 95-99.
- Modlin IM, Hunt RH, Malfertheiner P, Moayyedi P, Quigley EM, et al. (2009) Diagnosis and management of non-erosive reflux disease--the Vevey NERD Consensus Group.Digestion 80: 74-88.
- Lagergren J, Lagergren P (2013) Recent developments in esophageal adenocarcinoma.CA Cancer J Clin 63: 232-248.
- Sharma P (2009) Clinical practice. Barrett's esophagus.N Engl J Med 361: 2548-2556.
- Katz PO, Gerson LB, Vela MF (2013) Guidelines for the diagnosis and management of gastroesophageal reflux disease.Am J Gastroenterol 108: 308-328.
- Krugmann J, Neumann H, Vieth M, Armstrong D (2013) What is the role of endoscopy and oesophageal biopsies in the management of GERD?Best Pract Res Clin Gastroenterol 27: 373-385.
- DeNardi FG, Riddell RH (1991) The normal esophagus.Am J Surg Pathol 15: 296-309.
- Odze RD (2005) Unraveling the mystery of the gastroesophageal junction: a pathologist's perspective.Am J Gastroenterol 100: 1853-1867.
- Sharma P, Morales TG, Sampliner RE (1998) Short segment Barrett's esophagus--the need for standardization of the definition and of endoscopic criteria.Am J Gastroenterol 93: 1033-1036.
- Lagergren J, Bergström R, Lindgren A, Nyrén O (1999) Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma.N Engl J Med 340: 825-831.
- Spechler SJ, Fitzgerald RC, Prasad GA, Wang KK (2010) History, molecular mechanisms, and endoscopic treatment of Barrett's esophagus.Gastroenterology 138: 854-869.
- McDonald SA, Lavery D, Wright NA, Jansen M (2015) Barrett oesophagus: lessons on its origins from the lesion itself.Nat Rev Gastroenterol Hepatol 12: 50-60.
- McDonald SA, Graham T, Lavery D, Wright NA, Jansen M (2015) The barrett´s gland in phenotype space. CMGH Cellular and Molecular Gastroenterology and Hepatology 1: 41-54.
- Schindlbeck NE, Wiebecke B, Klauser AG, Voderholzer WA, Müller-Lissner SA (1996) Diagnostic value of histology in non-erosive gastro-oesophageal reflux disease.Gut 39: 151-154.
- Takubo K, Honma N, Aryal G, Sawabe M, Arai T, et al. (2005) Is there a set of histologic changes that are invariably reflux associated?Arch Pathol Lab Med 129: 159-163.
- Zentilin P, Savarino V, Mastracci L, Spaggiari P, Dulbecco P, et al. (2005) Reassessment of the diagnostic value of histology in patients with GERD, using multiple biopsy sites and an appropriate control group.Am J Gastroenterol 100: 2299-2306.
- Dent J (2007) Microscopic esophageal mucosal injury in nonerosive reflux disease.Clin Gastroenterol Hepatol 5: 4-16.
- Livstone EM, Sheahan DG, Behar J (1977) Studies of esophageal epithelial cell proliferation in patients with reflux esophagitis.Gastroenterology 73: 1315-1319.
- Ismail-Beigi F, Horton PF, Pope CE 2nd (1970) Histological consequences of gastroesophageal reflux in man.Gastroenterology 58: 163-174.
- Yerian L, Fiocca R, Mastracci L, Riddell R, Vieth M, et al. (2011) Refinement and reproducibility of histologic criteria for the assessment of microscopic lesions in patients with gastroesophageal reflux disease: the Esohisto Project.Dig Dis Sci 56: 2656-2665.
- Chandrasoma PT, Lokuhetty DM, Demeester TR, Bremmer CG, Peters JH, et al. (2000) Definition of histopathologic changes in gastroesophageal reflux disease.Am J Surg Pathol 24: 344-351.
- Chandrasoma P (2003) Pathological basis of gastroesophageal reflux disease.World J Surg 27: 986-993.
- Boch JA, Shields HM, Antonioli DA, Zwas F, Sawhney RA, et al. (1997) Distribution of cytokeratin markers in Barrett's specialized columnar epithelium.Gastroenterology 112: 760-765.
- Glickman JN, Chen YY, Wang HH, Antonioli DA, Odze RD (2001) Phenotypic characteristics of a distinctive multilayered epithelium suggests that it is a precursor in the development of Barrett's esophagus.Am J Surg Pathol 25: 569-578.
- Shields HM, Rosenberg SJ, Zwas FR, Ransil BJ, Lembo AJ, et al. (2001) Prospective evaluation of multilayered epithelium in Barrett's esophagus.Am J Gastroenterol 96: 3268-3273.
- Behar J, Sheahan D (1975) Histologic abnormalities in reflux esophagitis.Arch Pathol 99: 387-391.
- Mastracci L, Spaggiari P, Grillo F, Zentilin P, Dulbecco P, et al. (2009) Microscopic esophagitis in gastro-esophageal reflux disease: individual lesions, biopsy sampling, and clinical correlations.Virchows Arch 454: 31-39.
- Vieth M, Peitz U, Labenz J, Kulig M, Nauclér E, et al. (2004) What parameters are relevant for the histological diagnosis of gastroesophageal reflux disease without Barrett's mucosa?Dig Dis 22: 196-201.
- Tobey NA, Carson JL, Alkiek RA, Orlando RC (1996) Dilated intercellular spaces: a morphological feature of acid reflux--damaged human esophageal epithelium. Gastroenterology 111: 1200-1205.
- Calabrese C, Fabbri A, Bortolotti M, Cenacchi G, Areni A, et al. (2003) Dilated intercellular spaces as a marker of oesophageal damage: comparative results in gastro-oesophageal reflux disease with or without bile reflux.Aliment Pharmacol Ther 18: 525-532.
- Caviglia R, Ribolsi M, Maggiano N, Gabbrielli AM, Emerenziani S, et al. (2005) Dilated intercellular spaces of esophageal epithelium in nonerosive reflux disease patients with physiological esophageal acid exposure.Am J Gastroenterol 100: 543-548.
- Vieth M, Fiocca R, Haringsma J, Delarive J, Wiesel PH, et al. (2004) Radial distribution of dilated intercellular spaces of the esophageal squamous epithelium in patients with reflux disease exhibiting discrete endoscopic lesions. Dig Dis 22: 208-212.
- Ballem CM, Fletcher HW, Mckenna RD (1960) The diagnosis of esophagitis.Am J Dig Dis 5: 88-93.
- Winter HS, Madara JL, Stafford RJ, Grand RJ, Quinlan JE, et al. (1982) Intraepithelial eosinophils: a new diagnostic criterion for reflux esophagitis.Gastroenterology 83: 818-823.
- Brown LF, Goldman H, Antonioli DA (1984) Intraepithelial eosinophils in endoscopic biopsies of adults with reflux esophagitis.Am J Surg Pathol 8: 899-905.
- Geboes K, Ectors N, Vantrappen G (1991) Inflammatory disorders of the esophagus.Hepatogastroenterology 38 Suppl 1: 26-32.
- Wang HH, Mangano MM, Antonioli DA (1994) Evaluation of T-lymphocytes in esophageal mucosal biopsies.Mod Pathol 7: 55-58.
- Vieth M, Haringsma J, Delarive J, Wiesel PH, Tam W, et al. (2001) Red streaks in the oesophagus in patients with reflux disease: is there a histomorphological correlate?Scand J Gastroenterol 36: 1123-1127.
- Schneider NI, Plieschnegger W, Geppert M, Wigginghaus B, Hoess GM, et al. (2014) Validation study of the Esohisto consensus guidelines for the recognition of microscopic esophagitis (histoGERD Trial).Hum Pathol 45: 994-1002.
- Schneider NI, Plieschnegger W, Vieth M, Langner C (2014) Esophageal biopsies in the management of GERD: complementary tool for many but not for all-reply.Hum Pathol 45: 2513-2514.
- Paull A, Trier JS, Dalton MD, Camp RC, Loeb P, et al. (1976) The histologic spectrum of Barrett's esophagus.N Engl J Med 295: 476-480.
- Hayward J (1961) The lower end of the oesophagus.Thorax 16: 36-41.
- Park YS, Park HJ, Kang GH, Kim CJ, Chi JG (2003) Histology of gastroesophageal junction in fetal and pediatric autopsy.Arch Pathol Lab Med 127: 451-455.
- Derdoy JJ, Bergwerk A, Cohen H, Kline M, Monforte HL, et al. (2003) The gastric cardia: to be or not to be?Am J Surg Pathol 27: 499-504.
- De Hertogh G, Van Eyken P, Ectors N, Tack J, Geboes K (2003) On the existence and location of cardiac mucosa: an autopsy study in embryos, fetuses, and infants.Gut 52: 791-796.
- Kilgore SP, Ormsby AH, Gramlich TL, Rice TW, Richter JE, et al. (2000) The gastric cardia: fact or fiction?Am J Gastroenterol 95: 921-924.
- Glickman JN, Fox V, Antonioli DA, Wang HH, Odze RD (2002) Morphology of the cardia and significance of carditis in pediatric patients.Am J Surg Pathol 26: 1032-1039.
- Zhou H, Greco MA, Daum F, Kahn E (2001) Origin of cardiac mucosa: ontogenic consideration.Pediatr Dev Pathol 4: 358-363.
- Chandrasoma PT, Der R, Ma Y, Dalton P, Taira M (2000) Histology of the gastroesophageal junction: an autopsy study.Am J Surg Pathol 24: 402-409.
- Sarbia M, Donner A, Gabbert HE (2002) Histopathology of the gastroesophageal junction: a study on 36 operation specimens.Am J Surg Pathol 26: 1207-1212.
- Oberg S, Peters JH, DeMeester TR, Chandrasoma P, Hagen JA, et al. (1997) Inflammation and specialized intestinal metaplasia of cardiac mucosa is a manifestation of gastroesophageal reflux disease.Ann Surg 226: 522-530.
- Lord RV, Wickramasinghe K, Johansson JJ, Demeester SR, Brabender J, et al. (2004) Cardiac mucosa in the remnant esophagus after esophagectomy is an acquired epithelium with Barrett's-like features.Surgery 136: 633-640.
- Lenglinger J, Ringhofer C, Eisler M, Sedivy R, Wrba F, et al. (2007) Histopathology of columnar-lined esophagus in patients with gastroesophageal reflux disease. Wien Klin Wochenschr 119: 405-411.
- Langner C, Schneider NI, Plieschnegger W, Schmack B, Bordel H, et al. (2014) Cardiac mucosa at the gastro-oesophageal junction: indicator of gastro-oesophageal reflux disease? Data from a prospective central European multicentre study on histological and endoscopic diagnosis of oesophagitis (histoGERD trial). Histopathology 65: 81-89.
- Shields HM, Zwas F, Antonioli DA, Doos WG, Kim S, et al. (1993) Detection by scanning electron microscopy of a distinctive esophageal surface cell at the junction of squamous and Barrett's epithelium.Dig Dis Sci 38: 97-108.
- Wieczorek TJ, Wang HH, Antonioli DA, Glickman JN, Odze RD (2003) Pathologic features of reflux and Helicobacter pylori-associated carditis: a comparative study.Am J Surg Pathol 27: 960-968.
- Glickman JN, Spechler SJ, Souza RF, Lunsford T, Lee E, et al. (2009) Multilayered epithelium in mucosal biopsy specimens from the gastroesophageal junction region is a histologic marker of gastroesophageal reflux disease. Am J SurgPathol 33: 818-825.
- Upton MP, Nishioka NS, Ransil BJ, Rosenberg SJ, Puricelli WP, et al. (2006) Multilayered epithelium may be found in patients with Barrett's epithelium and dysplasia or adenocarcinoma.Dig Dis Sci 51: 1783-1790.
- Srivastava A, Odze RD, Lauwers GY, Redston M, Antonioli DA, et al. (2007) Morphologic features are useful in distinguishing Barrett esophagus from carditis with intestinal metaplasia.Am J Surg Pathol 31: 1733-1741.
- Langner C, Wolf EM, Plieschnegger W, Geppert M, Wigginghaus B, et al. (2014) Multilayered epithelium at the gastroesophageal junction is a marker of gastroesophageal reflux disease: data from a prospective Central European multicenter study (histoGERD trial). Virchows Arch 464: 409-417.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 25651
- [From(publication date):
December-2015 - Jul 06, 2025] - Breakdown by view type
- HTML page views : 24279
- PDF downloads : 1372