The Safety and Efficacy of Midface Rejuvenation in Asians with Hyaluronic Acid Dermal Fillers, High Intensity Focused Ultrasound and Thread lift
Received: 17-Jul-2015 / Accepted Date: 08-Sep-2017 / Published Date: 15-Sep-2017
Abstract
Introduction: Midface rejuvenation is the cornerstone of a beautiful face. It is integral in the aesthetic appeal of an Asian face.
Aim: To critically appraise 3 popular non-invasive procedures regarding midface rejuvenation for Asian beautification from an evidence based approach.
Objective: To evaluate the evidence for 3 increasingly popular aesthetic procedures in Asia for non-surgical midface rejuvenation, using best evidence topics methodology. • Thread lifts • Hyaluronic acid fillers • High intensity focused ultrasound (HIFU) Hence the research question is: What is the safety and efficacy of Thread lifts vs. Hyaluronic acid fillers vs. HIFU in midface rejuvenation in Asian adults?
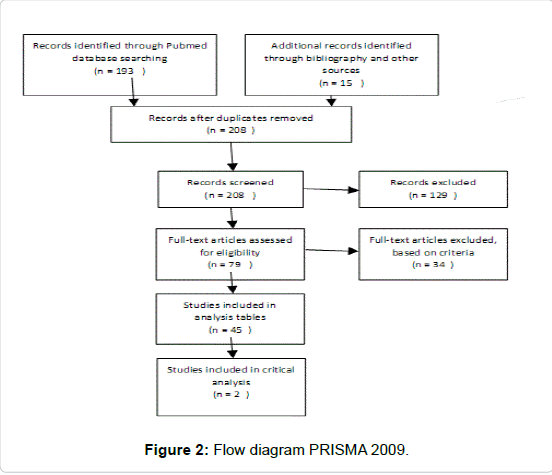
Method: A comprehensive search of the current literature was used using PubMed, Cochrane and Google Scholar. Non-English, animal studies and in-vitro studies were excluded. 45 relevant studies of variable quality were included. Data from these 41 studies were abstracted into table for the purpose of answering the focused three-part question according to Best BETs methodology.
Results: There are numerous high level evidence papers supporting the safety, efficacy and cost effectiveness of the use of hyaluronic acid fillers in midface rejuvenation. There is good evidence for the role of HIFU while Thread lifts lacks high powered data regarding their efficacy and safety.
Conclusion: Hyaluronic acid fillers are the treatment of choice for safe, efficient and cost-effective midface rejuvenation. When non-invasive treatments are preferred, HIFU is a relatively good alternative.
Keywords: Hyaluronic acid; Rejuvenation; Dermal fillers; Canfield imaging systems; Aesthetic improvement
Abbreviation
AEs: Adverse Effects; ASPS: American Society of Plastic Surgeons; CAHA: Calcium Hydroxylapatite; CE: Conformite Europene; FDA: Food and Drug Administration; FFA: Facial Fold Assessment; GAIS: Global Aesthetic Improvement Scale; GOR: Grade of recommendation; HA: Hyaluronic Acid; HADS: Hospital Anxiety Depression Scale; LAFM: Look and Feel of the Mid-face; LOE: Level of Evidence; LRS: Lemperle Rating Scale; MMVS: Medicis Midface Volume Scale; MFVDS: Midface Volume Deficiency Scale; MRI: Magnetic Resonance Imaging; NLF: Naso Labial Folds; PLLA: Poly-L-Lactic Acid; PMMA: Polymethylmethacrylate; QOL: Patient Quality of Life; SFQ: Subject Follow-up Questionnaire; WAS: Wrinkle Assessment Scale; WSRS: Wrinkle Severity Rating Scale
Introduction
As per the Cochrane collaboration, a systematic review evaluates and compiles the findings of multiple clinical trials and provides best evidence to address the chosen research question. Conclusion can then be made and future recommendations suggested. Below are the steps in a systematic review as per Cochrane:
• Identify your research question.
• Search for studies.
• Define inclusion and exclusion criteria.
• Extract studies that fulfill the above criteria.
• Perform data analysis of selected studies.
• Evaluate the degree of bias of the above.
• Present findings and assess the quality and level of evidence.
Methodology
Search
The search strategy was planned to identify all relevant articles by using Mesh terms combined with key text terms [1-9]. Different constructs of search terms were created by the use of truncation of the following terms: The search was divided into 3 stems, Thread lift, hyaluronic acid fillers and high intensity focused ultrasound. Filters were not activated fordates to widen the search result (Table 1-21) [10-25].
Key search result
Thread lift:
• Thread lift or barbed threads or silhouette sutures or PDO threads and facial rejuvenation-70 articles on PubMed.
• Thread lift or barbed threads or silhouette sutures or PDO threads, facial rejuvenation and safety or efficacy-13 articles on PubMed.
• Thread lift or barbed threads or silhouette sutures and midface lift-228 articles on Google Scholar.
• Thread lift or barbed threads or silhouette sutures, midface lift and Asian-31 results on Google Scholar.
• Combining these results, 12 studies were of sufficient quality for further analysis.
Fillers:
• Facial rejuvenation, hyaluronic acid filler and midface-23 articles on PubMed.
• Hyaluronic acid dermal fillers and facial rejuvenation-106 articles on PubMed.
• Hyaluronic acid dermal fillers, facial rejuvenation and safety or efficacy-33 results on PubMed.
• Hyaluronic acid dermal fillers, facial rejuvenation, midface and adult Asians-150 on Google Scholar.
• Pooling these results, 25 studies were of sufficient quality for further analysis.
High Intensity Focused Ultrasound (HIFU):
• High Intensity Focused Ultrasound (HIFU) or ulthera and face rejuvenation- 2 articles on PubMed.
• Ulthera or HIFU, face rejuvenation and midface-13 articles on PubMed.
• Ulthera or HIFU and facial rejuvenation-17 articles on PubMed.
• Ulthera or HIFU and face rejuvenation, midface and safety or efficacy-4 results on PubMed.
• Ulthera or HIFU, face rejuvenation and safety or efficacy-4 results on PubMed.
• HIFU or ulthera and face rejuvenation-153 articles on Google Scholar.
• HIFU or ulthera, face rejuvenation and Asian-39 results on Google Scholar.
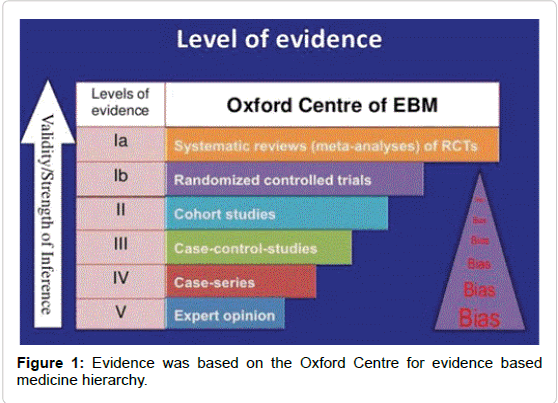
Combining these results, 8 studies were of sufficient quality for further analysis. About 45 studies of relevance and sufficient quality were further identified. Data from these 45 studies were abstracted into table for the purpose of answering the focused three-part question according to Best BETs methodology [26-32]. As the aim of this paper was to critically review existing literature on the safety and efficacy of 3 key treatments for midface rejuvenation in Asians, 2 best evidence and most relevant studies were identified for further critical analysis. Finally, with reference to cost, evidence for the longevity of the treatments was sought, and pricing from the respective clinic websites, email and phone enquiries (Figure 1 and Table 1).
| Cosmetic minimally invasive procedures | 2016 | % Change from 2015 |
|---|---|---|
| Botulinum toxin | 7 million | Up 4 |
| Soft tissue fillers | 2.6 million | Up 2 |
| Chemical Peels | 1.36 million | Up 4 |
| Laser hair removal | 1.1 million | Down 1 |
| Microdermabrasion | 7,75,000 | Down 3 |
Table 1: Top 5 cosmetic minimally invasive procedures ASPS (American Society of Plastic Surgery).
Inclusion criteria:
• Adults>19 years old
• Thread lift or High intensity Focused Ultrasound or Hyaluronic Acid fillers
• Midface or facial rejuvenation
• Highest quality papers with best evidence
Exclusion criteria:
• Non- english
• Animal studies
• In vitro studies
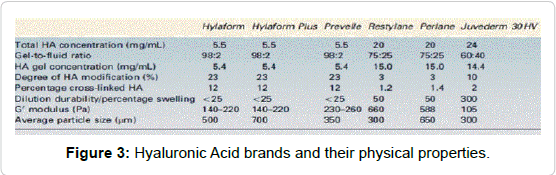
• Articles with no measurable endpoint (Figures 2,3 and Tables 2-9).
| Property | Clinical indication |
|---|---|
| Elastic modulus, G* | Related to the stiffness and firmness of the gel |
| Particle Size | Related to the smoothness, correction and filling. Affects the calibre of needle and extrusion force. Larger particles require larger bore needles |
| H.A concentration | This increases longevity and stability |
| Degree of crosslinking | This increases longevity and stability |
| Affinity for water | Related to swelling effects |
Table 2: Physical properties of HA- fillers [10-15].
| Immediate complication | Early complication | Late complication |
|---|---|---|
| Hypersensitivity | Redness, swelling and bruising Blindness, Skin necrosis, ulcer. Under and over-correction Nodules, lumps, bumps. Tyndall effect Infection | Granuloma, Edema, Migration Infection, Biofilm, Hypertrophic scarring |
Table 3: Type of filler complications.
| Immediate complication | Management |
|---|---|
| Hypersensitivity, Anaphylaxis, Urticaria | ABCs, adrenaline, antihistamine and steroid Topical steroids and oral antihistamines |
| Early complication | Management |
| Erythema, edema | Cold compress, pressure, steroid cream, Vitamin K |
| Blindness, Skin necrosis, ulceration | Stop injection, massage, use warm compress, Nitroglycerin paste, and inject Hyaluronidase. Topical Wound care and consider stem cell injection for skin necrosis |
| Under and over correction | Address patient's expectations first. Then consider the use of more fillers or hyaluronidase |
| Nodules, lumps, bumps | Massage, antibiotics, Steroid injection. If no improvement, consider biofilm, MRSA, atypical TB. Do a culture, start 2 antibiotics, 5FU,hyaluronidase and consider excision |
| Tyndall effect | Avoid superficial placement of fillers. Consider hyaluronidase |
| Infection and biofilms | Employ an aseptic technique. Cover patients with oral antibiotics post injection |
| Reactivation of herpes | For herpes, prophylactic antivirals to be given prior to injection |
| Late complication | Management |
| Granuloma | Massage, intralesional steroid injection, hyaluronidase. Excision 5 FU. Rule out biofilms in non-responsive cases |
| Hypertrophic scar | Scar revision, pulse dye laser Intralesional steroid |
| Skin defect | Grafting |
Table 4: Filler complications and their management.
| Methods | Rationale |
|---|---|
| Use blunt cannulas | This reduces risk of injecting filler into a vessel |
| Inject the filler together with adrenaline | This reduces the blood vessel diameter |
| Use smaller syringes, preferably not more than 1 cc | This reduces injection pressure, as injecting a syringe with 2cc of filler for example requires more force |
| Always aspirate before you inject | Aspiration can help determine if you are in a vessel. However, if the filler is very viscous, or the needle use very fine, this would be unsuccessful |
| Inject in small amounts slowly. Never inject into an area where there has been tissue trauma (accident, operation etc.). Know your anatomy well | As the anatomy would have been altered |
| Ocular specific complication | |
| If the patient complains of pain or vision loss, please stop injection. Bring the patient to an ophthalmologist immediately. | In intravascular complications, the key to immediately |
| Retrobulbar injection of hyaluronidase | Different hyaluronic acid fillers, due to its components, respond differentially to hyaluronidase. This will dissolve the Hylaluronic acid in the intravascular and surrounding tissue |
| Use of Diamox, ocular massage, intravenous mannitol | To reduce intraocular pressure |
Table 5: Prevention of intravascular complications.
| Treatment pearls |
|---|
| Vigorous massage |
| Warm compress |
| Use of hyaluronidase if filler is HA filler |
| Topical nitroglycerin paste |
| Oral prednisolone 40mg daily for 5 days |
| Hyperbaric oxygen |
| Use of stem cell regenerative therapy (Platelet rich plasma) |
| Follow patient up closely |
Table 6: Treatment of intravascular complications with fillers.
| Absorbable | Non-absorbable |
|---|---|
| Polydiaxanone | Polypropylene |
| Poly-L-lactic acid (PLLA) | Silicone |
| Polyester |
Table 7: Threadlift materias.
| Complications | Treatment | ||
|---|---|---|---|
| Minor | Major | Minor | Major |
| Pain | Infection | Ice massage | Antibiotics |
| Swelling | Parasthesia | Arnica massage | Extract and snip off |
| Numbness | Thread extrusion | Ice and compress | Further treatments as needed |
| Neurapraxia | Asymmetry | Improves with time | Further treatments as needed |
| Hematoma | Poor correction | Subcision and injection of filler can help | Antibiotics |
| Dimple | Parotid gland injury | with persistent dimpling | Antibiotics |
Table 8: Threadlift complication and management.
| Brand name | Date of approval | FDA approved Indications |
|---|---|---|
| Belotero Balance | 11-14-2011 | Injection into facial tissue to smooth wrinkles and folds, especially around the nose and mouth |
| Elevess | 12-20-2006 | Moderate/ severe facial wrinkles |
| Juvederm | 06-02-2006 | Moderate/ severe facial wrinkles |
| Juvederm Voluma | 10-22-2013 | Cheek augmentation for age related volume loss in mid/face (age>21) |
| Restylane | 12-12-2003 | Moderate/ severe facial wrinkles |
| Restylane Lyft | 07-01-2015 | Moderate/ severe facial wrinkles Age related volume loss (age>21) |
| Restylane Silk | 06-12-2014 | Lip augmentation and perioral wrinkles (age>21) |
Table 9: Selected Hyaluronic acid facial fillers and their FDA approved indication.
Analysis Tables
The followings are the analysis obtained, described in the tables (Tables 10-21).
| Author & published date | Evidence Level | Sample Size | Intervention | Efficacy Outcome Measures | Results | Analysis |
|---|---|---|---|---|---|---|
| Wu et al. 2016 | 2 | 88 (Asian population) | Nasolabial folds | WSRS and GAIS | Statistically significant patient satisfaction and doctor reporting | Strength: This was a multicenter, double-blinded, randomized, controlled, non-inferiority clinical trial. Study design was clear and appropriate. Study group was clearly defined, recruited from those with WSRS score of 3 or 4. Follow-up period of 13- 15 months was appropriate to assess longevity and safety of the fillers. Randomisation process was described and fair, using a software SAS 9.2. Control group was used and appropriate. Study met ethical standards with IRB approval and written informed consent.Statistical analysis used was appropriate, and results are significant. Limitation: Assessment had a subjective component, relying on doctor and patient evaluation, although objective photographs were used. The sample size was small and not representative of the population, hence a population based study may be needed. There is assessment bias as no third-party evaluators used. Follow-up data was incomplete and not accounted for |
| Pak et al. 2015 | 2 | 67 (Asian population) | Nasolabial folds | WSRS and GAIS | Statistically significant | Strength: Study design was good, in this randomised, multi-center, double-masked, matched pairs, active controlled trial. Study group was clearly defined. Inclusion and exclusion criteria was clear.\ Randomisation process was described and fair. Control group was used and appropriate. Study met ethical standards. Assessment made was valid and reliable, using validated scales. Statistical analysis used was appropriate, and results are significant. Limitation: Study lacked histological results from skin biopsy as a further objective measure of efficacy and safety. |
| Carruthers et al. 2005 | 2 | 150 | Nasolabial folds | WSRS and GAIS | Statistically significant patient satisfaction and doctor reporting | Strength: Good study design, large scale randomised double blinded comparative study. Ethical approval was obtained. Assessment was made with validated scales. Statistical analysis used was appropriate and significant. Limitation: Authors are affiliated to Medicis and this may introduce a bias. |
| Cohen et al. 2013 | 2 | 4605 Global ethnicity | Face and hands | Doctor and patient evaluation. | Statistically significant patient satisfaction and doctor reporting. | Strength: Systematic review of clinical trials for H.A fillers. Limitation: This study was funded by Medicis, and a\ few of the authors have conflict of interest, which may introduce selection and observer bias. Heterogenity of study designs, evaluation methods and results reporting limit comparison between studies. Good quality evidence for anatomical areas other than NLFs is still lacking. There was lack of data for young and elderly subjects, and those from various ethnic backgrounds. There may be under-reporting of serious side effects, with only 8 events in 8 patients out of 4605 patients, introducing publication bias. |
| Few et al. 2015 | 4 | 235 | Analysis of patient outcomes from Jones et al pivotal study 2013. | GAIS and LAFM. | Statistically significant patient satisfaction findings. Localised reaction resolved in 2 weeks. | Strength: A well designed, 2 years, single blinded, randomised controlled, multi centred study. Limitation: Clinical significance is a qualitative entity, whereas the assessment outcome was largely subjective, based on patient reporting and not all were validated. Patient were not blinded to treatment, hence introducing observer bias. Blunt cannula usage was not investigated in this study. There was no control group, comparing Juverderm Voluma with another filler. Patient numbers vary between assessments. The mean injected volume was 6.68ml for the entire face. This is uncommon in clinical practice, hence the results while valid, are not reproducible. The authors have conflict of interest, being closely associated with Allergan. This study was funded by Allergan. |
| Jones et al. 2013 | 2 | 235 | Midface filling | 6 point MFVDS. | Statistically significant patient satisfaction findings. 85.6% improved by 1 point or more at 6 months. Common location reactions, tenderness, swelling lasted not more than 2 weeks. No long term AE’s reported | Strength: A 2 year, single blinded, randomised controlled, multi centred study. MFVDS is a validated scale. Limitation: Study compared treatment group to no treatment group (control). Study was sponsored by Allergan. The authors are closely associated with Allergan. There was no control group, comparing Juverderm Voluma with another filler. Patient numbers vary between assessments. The mean injected volume was 6.68ml for the entire face. This is uncommon in clinical practice, hence the results while valid, are not reproducible. Patient were not blinded to treatment, hence introducing observer bias. |
| Nast et al. 2011 | 2 | 60 | Nasolabial folds | WSRS and GAIS were outcome measures. | Statistically significant patient satisfaction and doctor reporting | Strength: Multicentre, randomised, blinded, split face study, comparing 2 HA fillers. Limitation: Materials and funding of study was provided by Teoxane. Follow-up period was short with 7 months. |
| S Liew et al. 2016 | 3 | 29 | Nose | GAIS | Statistically significant patient satisfaction and doctor reporting | Strength: The follow-up of 12 months is good for assessment of safety and efficacy. There was a specific injection sequence combined with a structured goal-setting exercise and strict eligibility criteria. An independent assessor was used in the form of a central evaluating physician. Results were statistically significant, performed by an independent company, Datalytics Pty Ltd. Limitation: This study was limited to nose augmentation, hence results may not be reproducible for midface. Sample size was small at 29. |
| Buntrock et al. 2013 | 3 | 20 | Nasolabial folds | Efficacy outcomes were based on WSRS, subject questionnaire and biophysical in vivo methods. | Statistically significant patient satisfaction and doctor reporting. Similar localised reactions within 1st week of injection for both groups. | Strength: This was a split face, randomised, double blinded study. Study group was clearly defined. Inclusion and exclusion criteria was clear. Control group was used, NASHA filler in contralateral NLF. Study met ethical standards with the principles of Good Clinical practice and the Declaration of Helsinki. Assessment made was valid and reliable, using objective 3D in vivo skin measurement (PRIMOS) and standardised photography. Statistical analysis used was appropriate, And results are significant. Limitation: Sample size of 20 is small. Not representative of population group. Randomisation process was not described. |
| Prager et al. 2012 | 3 | 40 | Nasolabial folds | Merz 5 point scale and 3D scan PRIMOS system. | Statistically significant patient satisfaction and doctor reporting | Strength: Comparison of 3 different fillers in a prospective, split face, randomised, 2 arm study over 12 months. Study design was clear. Study group clearly defined. Inclusion and exclusion criteria was clear. Control group was used, NASHA filler or Juvederm UltraPlus in contralateral NLF. Study met ethical standards with approval received before October 2008. Assessment made was valid and reliable, using objective 3D in vivo skin measurement (PRIMOS) and standardised photography. Statistical analysis used was appropriate, and results are significant. Limitation: This study was funded by Merz and 2 authors have close association with the company. |
| Narins et al. 2003 | 1 | 138 | Nasolabial folds | WSRS and GAIS | Statistically significant | Strength: Study design was appropriate, being a pivotal, randomised, double blind, split face, multicentre study. Study group clearly defined. Inclusion and exclusion criteria was clear. Control group was used, collagen filler in contralateral NLF. Randomisation process was via a computer generating code. Study met ethical standards. Assessment made was valid and reliable, using objective 3D in vivo skin measurement (PRIMOS) and standardised photography. Statistical analysis used was the McNemar’s test which was appropriate, and results are significant. Limitation: Study funded by Q Med. The injection depth and injection volume were selected at the discretion of the individual investigator. The injection depth and injection volume were selected at the discretion of the individual investigator. This leads to nonuniformity in treatment. |
| Narins et al. 2010 | 3 | 95 | Nasolabial folds | WSRS and GAIS | Statistically significant patient satisfaction and doctor reporting in reduction of NLF severity. 24.2% reported bruising. | Strength: This was an open label extension of Narins et al 2003. Limitation: This study was sponsored by Merz, and the authors received financial incentive from the company. |
| Narins et al. 2011 | 3 | 52 | Nasolabial folds | WSRS and GAIS | Continued improvement in NLF up to 36 months after retreatment. Side effects reported were not treatment related. | Strength: Long follow-up period, as a result of the extension, this study followed patients for 36 months. Limitation: Study size of 52 subjects was small. This study was sponsored by Medicis.. Camera was provided by Canfield Imaging Systems. |
| Callan et al. 2013 | 4 | 103 | Midface filling | Validated MFVDS and GAIS | Statistically significant patient satisfaction, doctor reporting and 3D scan and photographs. | Strength: This study addressed the midface. Study design was appropriate, being a prospective cohort study with 24-month follow-up. Study group was clearly defined, between 30 to 60 years of age. Inclusion and exclusion criteria was clear. Control group was used, collagen filler in contralateral NLF. Randomisation process was via a computer generating code. Study was approved by central institutional review board, the International Conference on Harmonization, guidelines for Good Clinical Practice. Assessment made was valid and reliable, using objective 3D in vivo skin measurement (PRIMOS) and standardised photography. Statistical analysis used was the McNemar’s test which was appropriate, and results are significant. Limitation: Study was sponsored by Allergan and authors received financial incentive for conducting this study. The mode of administration (needle, cannula), site of administration (submalar, lateral, and/or medial), principal administration technique (bolus, fanning, and/or cross-hatching), in (retrograde and/or antegrade), and depth of injection or subcutaneous) were at the discretion of the physician investigator, leading to nonstandardization of treatment |
| Dover et al. 2009 | 1 | 248 | Nasolabial folds | WSRS | >50% had at least 1 grade improvement in WSRS at 24 weeks. Swelling and tenderness were the commonest complaints. 6 patients reported masses or nodules; but may not have understood difference between normal palpable products from mass formation. | Strength: Multi-centre, blinded, prospective randomised comparative study. Study design was good, being a multicentre, blinded, prospective randomised comparative study. Study group was clearly defined, being subjects seeking soft tissue augmentation for correction of bilateral nasolabial folds. Inclusion and exclusion criteria were clear. Control group was used, collagen filler in contralateral NLF. Randomisation process was via a central randomisation service. The Quorum Institutional Review Board approved the study protocol and informed consent. All subjects provided written informed consent. Assessment made was valid and reliable, using a validated scale, WSRS. Statistical analysis used was appropriate, and results are significant. Limitation: The treating investigator determined the method, depth, and volume of each injection. This may lead to outcome bias |
Table 10: Analysis of data [16-25].
| Author & date | Evidene Level | Study Size | Intervention | Safety measure | Result | Analysis |
|---|---|---|---|---|---|---|
| Joo et al. | 1 | 58 (Asian population) | Nasolabial fold | Patient and doctor evaluation | Statistically significant patient satisfaction and doctor reporting | Strength: A good study design, randomized, multicentre, double-blind, trial. Study group was clearly defined. Inclusion and exclusion criteria were clear. Study group was representative of the wider population group. Randomisation was described and fair, in the form of sealed envelopes. Control group with Perlane on the contralateral fold was used. Study met ethical standards, IRB of 2 centers in Seoul and based on Declaration of Helskinki. Assessment made was valid and reliable, based on validated scales, GAIS and WSRS. Safety profile was elicited through patient reporting. Statistical analysis was appropriate, and results are significant. Limitation: This study was funded by Medytox, the manufacturer of Neuramis. Neuramis was injected with 27-gauge ultra-thin-wall needle, and Perlane-L was injected with a 29-gauge needle. This difference can introduce outcome bias |
| Dover et al. | 1 | 248 | Nasolabial fold | Collected from subject diary entries | Statistically significant patient satisfaction and doctor reporting. | Strength and Limitation: Please refer to above table for elaboration |
| Baumann et al. | 1 | 439 | Nasolabial fold | Patient and doctor evaluation | Statistically significant patient satisfaction and doctor reporting | Strength: Study design double-masked, randomized, split face study. Study group clearly defined. Inclusion and exclusion criteria were clear. Study group fairly representative of the wider population group. Randomisation fair. Control group was used and appropriate The study was approved by the relevant institutional review boards, all subjects signed informed consent. Assessment made was valid and reliable using the WAS and patient diary. Statistical analysis used was appropriate and results are significant. HA filler demonstrated longer correction and greater patient preference. Limitation: Research funded by Allergan. Authors have close association with the company |
| Costa et al. | 2 | 25 | Right lumbar intradermally | Histological analysis of skin samples | Statistically significant histological findings | Strength: Objective assessment method used. Clear inclusion and exclusion criteria. Ethics approval was obtained. Statistical analysis was clear and result significant. First study to evaluate longevity in humans of 1 key filler from each category. Limitation: Site was lumbar area so may not be directly representative of facial skin. Small sample size of 25. Selection of 1 representative filler from each category which may introduce selection and outcome bias |
| Taylor et al. | 2 | 150 | Nasolabial fold | Patient and doctor assessment WSRS | Statistically significant patient satisfaction and doctor reporting. No keloid seen and 3 patients had post inflammatory hyper-pigmentation | Strength: Study design was multicentre, comparative,,prospective, randomised, split face, patient blinded study. Included Asian ethnicity. Study group clearly defined to ascertain safety and efficacy in patients with skin color. Inclusion and exclusion criteria was clear.Study group was representative of the wider population group. Control group was used, Restylan SubQ in contralateral NLF. The Quorum Institutional Review Board and the FDA Center for Devices and Radiological Health approved the protocol for the study. Patients provided informed consent as outlined in the Declaration of Helsink (Finland) and pursuant to institutional policies. Assessment made was valid and reliable. Statistical analysis was properly described, and results are significant. Limitation: This study was funded by Medicis. Ramdomisation process was not described in detail.Patients and investigators differed in the reporting of ‘‘mass formation’’ most likely because of a misunderstanding of the definition of the term, hence inroducing observer bias |
| Goodman et al. | 2 | 80 | Nasolabial fold | Physician and subject assessment. Nasolabial Fold Photonumeric Rating Scale | Statistically significant patient satisfaction and doctor reporting | Strength: Good study design. Inclusion and exclusion criteria was clear.Randomisation process was explained. Study obtained ethical approval. Assessment was based on validated scale. Limitation: Study was funded by Allergan, hence may introduce outcome bias. Authors have affiliation with Allergan. Physician investigator was not blinded in the study |
| Ascher et al. | 2 | 60 | Nasolabial fold | Patient and doctor evaluation | Statistically significant patient satisfaction and doctor reporting | Strength: Study design: This was a multicentre, split face, randomised, blinded study which compared 2 HA fillers. Study group clearly defined to ascertain safety and efficacy in patients with skin colour. Inclusion and exclusion criteria were clear. Study group was representative of the wider population group. Control group was used, Perlane in contralateral NLF. This study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practices, and local regulatory requirements and was approved by ethics committees. All patients provided their written informed consent prior to entering the study. Assessment made was valid and reliable. Side effects were documented by the subjects in diaries, and adverse events by the investigator. Statistical analysis was properly described, and results are significant. Limitation: The injection technique and volume were at the discretion of the injector, The short follow-up period of 6 months |
| Friedman et al. | 3 | 144000 | Nasolabial fold | Patient and doctor evaluation | Statistically significant patient satisfaction and doctor reporting | Strength: Retrospective review of large cohort of patients from Europe, America, Australasia and Asia. Limitation: Conducted by QMed and limited to NASHA filler. 1 author is member of the Q – Med advisory board. There may be under-reporting of adverse events and over estimation of number of patients treated as data was based on number of syringes sold. These would lead to under estimation of the incidence of adverse events |
| Rzany et al. | 4 | 77 | Full-face rejuvenation with Emerval range of fillers | Patient and doctor evaluation | Statistically significant patient satisfaction and doctor reporting | Strength: Study design was complex with the use of 5 different fillers. The study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practices, and local regulatory requirements and was approved by ethics committees. All participants provided their written informed consent prior to entering the study. Side effects were documented by the subjects in diaries, and adverse events by the investigator. Limitation: This was an open trial label. This was a full-face rejuvenation with the use of 6.7ml of filler on average. This is not practical in clinical setting. There was no control group. Study group is not representative of the whole population group. Only 6month follow-up. And only for Emervel range of fillers. Galderma funded the study and author is closely |
| V Bertucci et al. | 4 | 40 | Midface (for moderate to severe deficit) | Patient and doctor evaluation | Statistically significant patient satisfaction and doctor reporting | Strength: The study received institutional review board approval. All patients provided written informed consent. Outcome measure was the validated and objective MMVS and the subjective GAIS, and this study show that both responses correlate well. Limitation: Study design was open label. Study population was homogeneous, and may not reflect wider population group. There was no control group. 24week follow-up, and limited to Perlane filler. Mean total volume was 5.3+/-2.5ml, which is not common in clinical setting. This study was funded by Medicis. Dr. Bertucci has served as a consultant, speaker and investigator for Medicis, Merz, and Allergan. |
| JM Bae et al. | 4 | 320 | Malar, Chin, Glabellar | Patient and doctor evaluation | Statistically significant patient satisfaction and doctor reporting | Strength: Focused on Asian females, with attention on the malar eminence. Limitation: This was a retrospective study. Study limited to use of Juvederm voluma. Only the subjective GAIS was used as assessment outcome. The total volume of 4 to 6ml is not practical in a typical clinical setting. |
Table 11: Analysis of data.
| Author and date | Evidene Level | Study Size | Intervention | Efficacy outcome measure | Safety measure | Result | Study reference | Analysis |
|---|---|---|---|---|---|---|---|---|
| Oni et al. | 2 | 103 | Cheek, jawline an submental skin | 3D Photographic assessment and AutoCad software measurement. | Patient and doctor evaluation | Statistically significant patient satisfaction and doctor reporting | 93 | Strength: Study was on cheek, jawline and neck. Large study size. Study group was clearly defined Inclusion and exclusion criteria was clear. Study group was representative of the wider population group. Control group was used and appropriate Study met ethical standards. Assessment made was valid and reliable using both qualitative and quantitative measures. Statistical analysis used was appropriate, and results are significant. Limitation: Nonrandomised, no comparative manner. Study was funded by Ulthera. Patients with skin laxity and high BMI were unlikely to benefit from treatment. F/U period was short. Treatment density was 40% of recommended guidelines. Good to explore more about pain management. |
| Alam et al. | 2 | 36 | Full face and neck | Rating scales and photographic analysis were used. | Patient and doctor evaluation | Statistically significant patient satisfaction and doctor reporting | 23 | Study design was a ratter blinded prospective cohort study. Ethics approval was obtained prior to start. Limitations: Lower face tightening difficult to quantify due to lack of fixed landmarks. Modest treatment parameters as study were evaluating safety and efficacy, so efficacy was not optimised. Higher energy densities, more passes, different depths can be explored. Small sample size of 36 subjects. |
| Sasaki et al. | 3 | 35 | Full face and neck | GAIS and specific mirroring software | Patient and doctor evaluation | Statistically significant patient satisfaction and doctor reporting | 94 | Limitations: Patients present with a range of skin qualities tissue laxity and facial fat distribution. Patient selection factors were not identified. Little is known about which clinical factors are reliable predictors of good outcome. Those with favourable clinical prognosticators had negligible results. Labelled and off-labelled sites need further studies determining optimal parameters |
| Suh et al. | 3 | 22 (Asisn Population) | Full face and neck | Histological evidence and photographic documentation | Patient and doctor evaluation | Statistically significant patient satisfaction and doctor reporting | 95 | Strength: Combined data of clinical and histological evidence supporting safety and efficacy of HIFU in Asians. Limitation: No standard photographic methodology and objective parameters to demonstrate mid and lower face tightening. Small sample size is of 22 |
Table 12: Analysis of data.
| Filler Brand | Price in SGD/ syringe |
|---|---|
| Perlane | 850 |
| Juvederm Voluma | 1000 |
| Teosyal Deep | 750 |
| Neuramis Volume | 600 |
Table 13: Cost of commonly used H.A Fillers in Singapore.
| HIFU Brand | Price in SGD |
|---|---|
| Ulthera (city clinic) | 3000 |
| Ulthera ( sub-urban clinic) | 2500 |
| Doublo | 1800 |
| Ultraformer | 1800 |
Table 14: Cost of HIFU treatment for midface rejuvenation in Singapore.
| Threadlift Brand | Price in SGD |
|---|---|
| Silhouette Soft | 3000 |
| Korean PDO | 1800 |
| Korean PLLA | 2200 |
| Korean PCL | 2400 |
Table 15: Cost of Threadlift treatments for midface rejuvenation in Singapore.
| Level of evidence | Hyaluronic acid | HIFU | Thread-lift |
|---|---|---|---|
| 1 | 4 | 0 | 0 |
| 2 | 9 | 2 | 0 |
| 3 | 6 | 2 | 0 |
| 4 | 5 | 4 | 12 |
| 5 | 0 | 0 | 0 |
| Total | 25 | 8 | 12 |
Table 16: Summary table: Treatment modalities and level of evidence of papers relevant to this study.
| Category of HA Filler | Brand | Brand |
|---|---|---|
| Biphasic | Perfectha | Perlane |
| Monophasic monodensified | Juvederm | Teosyal |
| Monophasic polydensified | Neuramis Volume | Belotero |
Table 17: H.A Filler by category.
| Category of Skin tightening | Brand | Cost/month |
|---|---|---|
| HIFU | Ultherapy | $125 |
| HIFU | Doublo | $150 |
| HIFU | Ultraformer | $150 |
| RF | Thermage | $166 |
| RF | Exilis | $166 |
Table 18: HIFU brand and monthly cost [59-65].
| Thread material | Brand | Cost/month |
|---|---|---|
| PLLA | Silhouette soft | $250 |
| PDO | Korean Ultra V lift | $200 |
| PLLA / PCL | Korean Art lift | Lack of data on longevity so difficult to estimate cost |
Table 19: Thread brand and monthly cost.
| Question | Findings |
|---|---|
| Does the title reflect the content? | Yes, it does |
| Are the authors credible? | The authors are from Department of Dermatology, St Paul Hospital, College of Medicine, Catholic University of Korea, and the Department of Dermatology, College of Medicine, Chung-Ang University. These are highly credible institutions in Korea |
| Does the abstract summarise the key components of the paper? | Yes it does |
| Is the rationale for undertaking the research clearly outlined? | Yes it is |
| Is the literature review comprehensive and up-to-date? | It is fairly up to date, but with 20 references, not comprehensive |
| Is the aim of the research clearly stated? | Yes the aim is to determine the efficacy and safety of a new filler, Neuramis deep compared to Perlane for nasolabial fold treatment |
| Are all ethical issues identified and addressed? | This randomized, double-blind, intra-individual controlled clinical trial was approved by the institutional review boards of two centers in Seoul, Republic of Korea (St. Paul’s Hospital and Chung- Ang University Hospital), and was conducted based on the guidelines for standard clinical practice and the Declaration of Helsinki. All patients gave written consent to participate in the study |
| Is the methodology identified and justified? | Yes. This was a randomized, multicenter, double-blinded, intra-individual, clinical trial was approved by the Ministry of Food and Drug Safety in the Republic of Korea. The registration number of MFDS is PC13DDMT0036. However the justification that a special 27G ultra-thin wall needle, which inner diameter was larger than conventional needle, was used for Neramis filler but a regular 29G needle for Perlane filler may not be acceptable |
Table 20: Generic critique of study by Joo et al. 2016 A randomised clinical trial to evaluate the efficacy and safety of lidocaine containing monophasic hyaluronic acid filler for nasolabial folds [65-68].
| Question | Findings |
|---|---|
| Is the study design clearly identified, and is the rationale for choice of design evident? | This clinical trial was designed to investigate the clinical efficacy and safety of Neuramis for the correction of moderate or severe nasolabial folds. The philosophy for this research is that there is no recent study that compares 2 lidocaine containing fillers for safety and efficacy. The rationale for a randomized, multi-center, double-blind, intra-individual trial was to offer the highest level of evidence |
| Is there an experimental hypothesis clearly stated? Are the key variables clearly defined? | The interpretation of the confidence interval was based on the null hypothesis, stating that the expected difference in clinical efficacy between the treatment groups was lower than the no- inferiority margin of −0.5. A lower limit of estimated confidence interval exceeding −0.5 would indicate that Neuramis was not inferior to Perlane-L |
| Is the population identified? | Yes. Please see below |
| Is the sample adequately described and reflective of the population? | 60 patients aged between 30 to 70 years were randomized. Inclusion criteria is as such: (1) men and women aged 30 to 70 years who agreed not to have other treatments to correct nasolabial folds during the study period and had a desire for cosmetic correction; and (2) at initial diagnosis, patients had to have bilaterally symmetrical naso- labial folds on both sides rated as 3 (moderate) or 4 (severe) based on the Wrinkle Severity Rating Scale |
| Is the method of data collection valid and reliable? | Yes. A similar volume of both products was injected (up to 1 ml) at baseline for both products until optimal correction was achieved. To assess efficacy, WSRS and GAIS scores were documented. To assess clinical safety, visual analogue pain scale, adverse events, vital sign measurements, and clinical laboratory information was recorded |
| Is the method of data analysis valid and reliable? | Yes. The researchers are experts in their field and have good documentation on the data analysis. For the primary efficacy endpoint parameter, a lower limit of the 97.5 percent one-sided confidence interval was calculated for the mean treatment difference between the two groups (Neuramis and Perlane-L). In the secondary efficacy evaluation, all statistical tests were con- ducted at a significance level of 0.05 in both sides unless specified otherwise. Continuous variables were analyzed using the paired t test or Wilcoxon signed rank test. Categorical variables were used for secondary efficacy evaluation presented in a shift table and analyzed using the McNemar test |
| Are the results presented in a way that is appropriate and clear? | Yes |
| Is the discussion comprehensive? | To a certain extent yes. This was a non-inferiority study. It stated that Neuramis achieved higher WSRS scores compared to Perlane, elaborated on the difference between monophasic and biphasic fillers in terms of rheology, crosslinking, correlating to different clinical efficacy |
| Are the results generalizable? | The sample size is 58, and with level 1 evidence, yes, it is generalizable |
| Are the results transferable? | Yes. The filler brands were indicated, needle size documented, and technique explained |
| Is the conclusion comprehensive? | Yes. Neuramis showed non- inferior clinical efficacy 6 months after injection, based on both the investigators’ evaluations and participants’ self-assessments. The injection pain and safety profiles of Neuramis were comparable to those of Perlane-L |
Table 21: Critique of a quantitative study by Joo et al.
A randomized clinical trial to evaluate the efficacy and safety of lidocaine containing monophasic hyaluronic acid filler for nasolabial folds (Joo et al.)
This study is ranked as level 1 in the hierarchy of evidence-based medicine. Using the selected critique framework to critically review this study, it has been found that this study was well conducted with detailed documentation of observations. However, there are limitations to this study [33-38]. Different needles were used for the respective fillers, a special 27G ultra-thin wall needle, whose inner diameter was larger than conventional needle, was used for Neramis filler but a regular 29G needle for Perlane filler [39-58]. The Neuramis Deep Lidocaine and Restylane Perlane-L used in this clinical trial were generously supported by Medytox, Inc., Seoul, Republic of Korea, the makers of Neuramis. The data collection, method of data analysis, analysis of results and conclusion were well documented. As such the results are credible, generalizable and transferable. Hence there is strong evidence to support safety and efficacy of lidocaine containing hyaluronic acid fillers [59-63].
Future trend for dermal fillers
Hyaluronic acid fillers will not be strictly limited to volume restoration and the development of fillers with greater longevity. Research will be centred on other clinical indications, for example anti-ageing and skin rejuvenation. Skinboosters comprises of a hyaluronic acid filler. It can be delivered through a microneedling injector machine into the skin, enhancing skin hydration and healing. The microneedles aids trans-epidermal penetration and deposition of the product into the dermis [64]. Kim et al. conducted a study on 150 patients who had 1,000 injections of 1 cc Skinboosters-HA via an injector gun. Results show that the skin texture, hydration and thickness significantly improved after this procedure. The dermis of the face and hand were thickened about 4% after dermal injection. The injection depth was confirmed by biopsy. Injections into the dermis changed both skin texture and thickness. It was hence postulated that the microneedling and deposition of hyaluronic acid can stimulate fibroblasts to increase collagen stimulation, hence improving skin thickness and texture [64,65]. Yet another product for skin rejuvenation is Rejuran Healer, a poly-neucleotide filler. Evidence to date is mainly derived from case studies as the product is relatively new, but the results have been promising for both safety and efficacy. About 2 Korean studies conducted, 1 of which was a case series while another, a Phase III randomised clinical trial demonstrated no adverse events and improvement in pores, wrinkles and skin tone [66,67].
Intense focused ultrasound tightening in Asian skin: Clinical and pathologic results: Clinical. Dermatologic Surg (Suh et al.)
This study is ranked as level 3 in the hierarchy of evidence-based medicine. The research used skin biopsies to evaluate pathological results of intense focused ultrasound tightening in Asian skin. Using the selected critique framework to critically review this study, it has been found that this study has fair documentation. Photographic device and objective parameters to demonstrate mid- to lower facial tightening. Another limitation was the small sample size at 22, of which only 11 patients had skin biopsy performed, and short follow-up of 2 months. This study is the first report to combine clinical and histologic data supporting the safety and efficacy of intense ultrasound therapy to the facial tissue of Asian patients. The sample size is small at 22, but the data collection, method of data analysis, analysis of results and conclusion were well presented. As such the results are credible, generalizable and transferable. Hence, there exists good evidence to support the safety and efficacy of intense focused ultrasound tightening in Asian skin. But the strength of this evidence is influenced by the limitations to the study.
Future trend for high intensity focused ultrasound treatment
Despite the fairly extensive individual safety record, there is little data regarding ultrasound combination therapy, hence the future for ultrasound lies in identifying the protocol and methodology for such combination treatments. At the microscopic level, histological evaluation has demonstrated increased neocollagenesis [68]. Already, there are a number of trials conducted on combination therapy with high intensity focused ultrasound treatment for skin rejuvenation. A study by Friedmann et al. indicates that IPL, MFUS, and PLLA may be safely performed in a single treatment session to target multiple tissue planes concurrently without increased adverse events [69]. An expert consensus hence supports a combination approach using multiple modalities in specific sequence for the safe and effective treatment of the aging face [70].
Thread lift procedure
PDO Thread lift was originally developed in Korea to contour the classical Asian rounded features into a westernised V -shaped face, perceived to be more attractive. However, less clear are the data on the extent of the optimum result and the longevity of treatment effect. Thread lifting studies has not been conclusively standardized to ensure superior clinical results. The current demand appears to be driven by market forces rather than scientific studies. Hence, although results are promising, the current studies are of poor quality and demonstrate low level of evidence, with wide variation in methodology, technique and generally small sample size. Hence, in my opinion, more robust RCTs need to be conducted, with standardization of technique, choice of material and longer follow-up period of more than 6 months in order to better evaluate its safety and efficacy. However, I do appreciate that most Thread lift companies are small, and may not have the finances of large aesthetic product companies, for example Allergan, Galderma, Merz, so such trials may be challenging. Furthermore, a split face study for Thread lift is difficult as there is no intervention that can serve as an established standard of control presently.
Summary
Nonsurgical facial rejuvenation remains the cherry picking of aesthetic medicine and has sparked a decade long, if not life-long search for innovative techniques. Ageing centres on the midface, hence my paper focused on midface rejuvenation. The reduction in treatment times, and shorter post-recovery period, combined with the removal of the need for surgical drains has enhanced the popularity of non-surgical facial rejuvenation for both patients and doctors. The evolution of fillers applied in the field of aesthetic procedure is in its second decade, high intensity focused ultrasound, the first decade and thread lifting techniques now into its third decade. As we discussed in this chapter, the future lies in the evolution of combination therapy to enhance skin rejuvenation. Currently, many of these combination studies are anecdotal. Further research is needed to identify the types of combination, the sequence, timing and number of sessions required for optimal results. Longer follow-up time and larger sample size clinical studies would help answer these questions. Hopefully in future, more robust RCTs could be conducted focusing on the combination of these non-surgical treatments to yield high quality surgical results, with the minimal downtime of non-surgical procedures.
Future Recommendation
One way is for more established treatment centres to work closely with single treatment centres to pool data of Asian patients who have undergone Thread lift, High intensity focused ultrasound treatment and HA filler injections for midface rejuvenation. The type and dosage of the HA filler injections, energy intensity and treatment protocol for HIFU used and the type, number and arrangement of threads inserted can be collected in a central database system. With such detailed information available in a central database, analysis and systematic reviews can be carried out, so as to provide evidence-based practice in evaluating the safety and efficacy of midface rejuvenation in Asians with hyaluronic acid dermal fillers, high intensity focused ultrasound and Thread lift.
References
- Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS medicine 6: e1000097.
- The Oxford center for evidence-based medicine-Levels of evidence (2009).
- Plastic surgery statistics-Cosmetic and reconstructive procedure trends: American Association of Plastic Surgeons.
- Monheit GD, Coleman KM (2006) Hyaluronic acid fillers. Dermatol Ther 19: 141-150.
- Kablik J, Monheit GD, Yu L, Chang G, Gershkovich J (2009) Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg 35: 302-312.
- Sherman R (2006) Avoiding dermal filler complications. Clin Dermatol 27: S23-S32.
- De Boulle K, Heydenrych I (2015) Patient factors influencing dermal filler complications: Prevention, assessment and treatment. Clin Cosmet Investig Dermatol 8: 205-210.
- Bailey SH, Cohen JL, Kenkel JM (2011) Etiology, prevention and treatment of dermal filler complications. ‎Aesthet Surg J 31: 110-121.
- Cavallini M, Gazzola R, Metalla M, Vaienti L (2013) The role of hyaluronidase in the treatment of complications from hyaluronic acid dermal fillers. Aesthet Surg J 33: 1167-1174.
- Funt D, Pavicic T (2013) Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol 6: 295-298.
- Ballin AC, Brandt FS, Cazzaniga A (2015) Dermal fillers: An update. Am J Clin Dermatol 16: 271-283.
- Kim JH, Ahn DK, Jeong HS, Suh IS (2014) Treatment algorithm of complications after filler injection: Based on wound healing process. J Korean Med Sci 29: S176-S182.
- Zoe DD (2009) Injectible anti ageing techniques. Cosmet Dermatol 42: 342-376.
- McCleve DE, Goldstein JC (1995) Blindness secondary to injections in the nose, mouthand face: Cause and prevention. Ear Nose Throat J 74: 182-188.
- Matarasso A, Paul MD (2013) Barbed sutures in aesthetic plastic surgery: Evolution of thought and process. Aesthet Surg J 33: 17S-31S.
- Wu WT (2014) Commentary on facial rejuvenation with fine barbed threads: The simple MIZ-lift. Aesthet Plast Surg 38: 75-77.
- Yeo SH, Lee YB, Han DG (2017) Early complications from absorbable anchoring suture following thread-lift for facial rejuvenation. Arch Aesthetic Plast Surg 23: 11-16.
- Wu Y, Sun N, Xu Y, Liu H, Zhong S, et al. (2016) Clinical comparison between two hyaluronic acid-derived fillers in the treatment of nasolabial folds in Chinese subjects: BioHyalux vs. Restylane. Arch Dermatol Res 308: 145-151.
- Pak C, Park J, Hong J, Jeong J, Bang S, et al. (2015) A phase III, randomized, multi-center, double-masked, matched-pairs, active-controlled trial to compare the efficacy and safety between neuramis deep and restylane in the correction of nasolabial folds. Arch Plast Surg 42: 721-728.
- Carruthers A, Carey W, Lorenzi C, Remington K, Schachter D, et al. (2005) Randomized, doubleâ€blind comparison of the efficacy of two hyaluronic acid derivatives, restylane perlane and hylaform, in the treatment of nasolabial folds. Dermatol Surg 31: 1591-1598.
- Cohen JL, Dayan SH, Brandt FS, Nelson DB, Axford Gatley RA, et al. (2013) systematic review of clinical trials of smallâ€and largeâ€gelâ€particle hyaluronic acid injectable fillers for aesthetic soft tissue augmentation. Dermatol Surg 39: 205-231.
- Few J, Cox SE, Paradkar-Mitragotri D, Murphy DK (2015) A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: Patient-reported outcomes at 2 years. Aesthet Surg J 35: 589-599.
- Jones D, Murphy DK (2013) Volumizing hyaluronic acid filler for midface volume deficit: 2â€Year results from a pivotal singleâ€blind randomized controlled study. Dermatol Surg 39: 1602-1612.
- Nast A, Reytan N, Hartmann V, Pathirana D, Bachmann F, et al. (2011) Efficacy and durability of two hyaluronic acid–based fillers in the correction of nasolabial folds: Results of a prospective, randomized, doubleâ€blind, actively controlled clinical pilot study. Dermatol Surg 37: 768-775.
- Liew S, Scamp T, de Maio M, Halstead M, Johnston N, et al. (2016) Efficacy and safety of a hyaluronic acid filler to correct aesthetically detracting or deficient features of the Asian nose: A prospective, open-label, long-term study. Aesthet Surg J 36: 760-772.
- Buntrock H, Reuther T, Prager W, Kerscher M (2013) Efficacy, safetyand patient satisfaction of a monophasic cohesive polydensified matrix versus a biphasic nonanimal stabilized hyaluronic acid filler after single injection in nasolabial folds. Dermatol Surg 39: 1097-1105.
- Prager W, Wissmueller E, Havermann I, Bee EK, Howell DJ, et al. (2012) A prospective, splitâ€face, randomized, comparative study of safety and 12â€month longevity of three formulations of hyaluronic acid dermal filler for treatment of nasolabial folds. Dermatol Surg 38: 1143-1150.
- Narins RS, Brandt F, Leyden J, Lorenc ZP, Rubin M, et al. (2003) A randomized, doubleâ€blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg 29: 588-595.
- Narins RS, Coleman WP, Donofrio LM, Jones DH, Maas C, et al. (2010) Improvement in nasolabial folds with a hyaluronic acid filler using a cohesive polydensified matrix technology: Results from an 18â€month openâ€label extension trial. Dermatol Surg 36: 1800-1808.
- Narins RS, Brandt FS, Dayan SH, Hornfeldt CS (2011) Persistence of nasolabial fold correction with a hyaluronic acid dermal filler with retreatment: Results of an 18â€month extension study. Dermatol Surg 37: 644-650.
- Callan P, Goodman GJ, Carlisle I, Liew S, Muzikants P, et al. (2013) Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: A 24 month study. Clin Cosmet Investig Dermatol 6: 81-89.
- Dover JS, Rubin MG, Bhatia AC (2009) Review of the efficacy, durability and safety data of two nonanimal stabilized hyaluronic acid fillers from a prospective, randomized, comparative, multicenter study. Dermatol Surg 35: 322-331.
- Joo HJ, Woo YJ, Kim JE, Kim BJ, Kang H (2016) A randomized clinical trial to evaluate the efficacy and safety of Lidocaine-containing monophasic hyaluronic acid filler for nasolabial folds. Plast Reconstr Surg 137: 799-808.
- Baumann LS, Shamban AT, Lupo MP, Monheit GD, Thomas JA, et al. (2007) Comparison of smoothâ€gel hyaluronic acid dermal fillers with crossâ€linked bovine collagen: A multicenter, doubleâ€masked, randomized, withinâ€subject study. Dermatol Surg 33: S128-S135.
- DaCosta A, Biccigo DG, De Souza ET, Mercadante LM, Oliveira PR, et al. (2017) Durability of three different types of hyaluronic acid fillers in skin: Are there differences among biphasic, monophasic monodensifiedand monophasic polydensified products? Aesthet Surg J 37: 573-581.
- Taylor SC, Burgess CM, Callender VD (2010) Efficacy of variableâ€particle hyaluronic acid dermal fillers in patients with skin of color: A randomized, evaluatorâ€blinded comparative trial. Dermatol Surg 36: 741-749.
- Goodman GJ, Bekhor P, Rich M, Rosen RH, Halstead MB, et al. (2011) A comparison of the efficacy, safetyand longevity of two different hyaluronic acid dermal fillers in the treatment of severe nasolabial folds: A multicenter, prospective, randomized, controlled, single-blind, within-subject study. Clin Cosmet Investig Dermatol 4: 197-205.
- Ascher B, Bayerl C, Brun P, Kestemont P, Rzany B, et al. (2011) Efficacy and safety of a new hyaluronic acid dermal filler in the treatment of severe nasolabial lines: 6â€month interim results of a randomized, evaluatorâ€blinded, intraâ€individual comparison study. J Cosmet Dermatol 10: 94-98.
- Friedman PM, Mafong EA, Kauvar AN, Geronemus RG (2002) Safety data of injectable non-animal stabilized hyaluronic acid gel for soft tissue augmentation. Dermatol Surg 28: 491-494.
- Rzany B, Cartier H, Kestemont P, Trevidic P, Sattler G, et al. (2012) Fullâ€face rejuvenation using a range of hyaluronic acid fillers: Efficacy, safetyand patient satisfaction over 6 months. Dermatol Surg 38: 1153-1161.
- Bertucci V, Lin X, Gatley RA, Theisen MJ, Swift A (2013) Safety and effectiveness of large gel particle hyaluronic acid with lidocaine for correction of midface volume loss. Dermatol Surg 39: 1621-1629.
- Bae JM, Lee DW (2013) Threeâ€dimensional remodeling of young Asian women's faces using 20â€mg/mL smooth, highly cohesive, viscous hyaluronic acid fillers: A retrospective study of 320 patients. Dermatol Surg 39: 1370-1375.
- Oni G, Hoxworth R, Teotia S, Brown S, Kenkel JM (2014) Evaluation of a microfocused ultrasound system for improving skin laxity and tightening in the lower face. Aesthet Surg J 34: 1099-1110.
- Sasaki GH, Tevez A (2012) Clinical efficacy and safety of focused-image ultrasonography: A 2-year experience. Aesthet Surg J 32: 601-612.
- Suh DH, Shin MK, Lee SJ, Rho JH, Lee MH, et al. (2011) Intense focused ultrasound tightening in Asian skin: Clinical and pathologic results. Dermatol Surg 37: 1595-1602.
- Gliklich RE, White WM, Slayton MH, Barthe PG, Makin IR (2007) Clinical pilot study of intense ultrasound therapy to deep dermal facial skin and subcutaneous tissues. Arch Facial Plast Surg 9: 88-95.
- Laubach HJ, Makin IR, Barthe PG, Slayton MH, Manstein D (2008) Intense focused ultrasound: Evaluation of a new treatment modality for precise micro coagulation within the skin. Dermatol Surg 34: 727-734.
- Fabi SG, Massaki A, Eimpunth S, Pogoda J, Goldman MP (2013) Evaluation of microfocused ultrasound with visualization for lifting, tighteningand wrinkle reduction of the décolletage. J Am Acad Dermatol 69: 965-971.
- Chan NP, Shek SY, Yu CS, Ho SG, Yeung CK, et al. (2011) Safety study of transcutaneous focused ultrasound for nonâ€invasive skin tightening in Asians. Lasers Surg Med 43: 366-375.
- Han SE, Go JY, Pyon JK, Oh KS (2016) A prospective evaluation of outcomes for midface rejuvenation with mesh suspension thread: “Reeborn liftâ€. J Cosmet Dermatol 15: 254-259.
- Lycka B, Bazan C, Poletti E, Treen B (2004) The emerging technique of the antiptosis subdermal suspension thread. Dermatol Surg 30: 41-44.
- Savoia A, Accardo C, Vannini F, Di Pasquale B, Baldi A (2014) Outcomes in thread lift for facial rejuvenation: A study performed with happy lift revitalizing. Dermatol Ther 4: 103-114.
- Wu WT (2004) Barbed sutures in facial rejuvenation. Aesthet Surg J 24: 582-587.
- Isse NG, Fodor PB (2005) Elevating the midface with barbed polypropylene sutures. Aesthet Surg J 25: 301-303.
- de Benito J, Pizzamiglio R, Theodorou D, Arvas L (2011) Facial rejuvenation and improvement of malar projection using sutures with absorbable cones: Surgical technique and case series. ‎Aesthet Plast Surg 35: 248-253.
- Sulamanidze M, Sulamanidze G, Vozdvizhensky I, Sulamanidze C (2011) Avoiding complications with aptos sutures. Aesthet Surg J 31: 863-873.
- Rachel JD, Lack EB, Larson B (2010) Incidence of complications and early recurrence in 29 patients after facial rejuvenation with barbed suture lifting. Dermatol Surg 36: 348-354.
- Garvey PB, Ricciardelli EJ, Gampper T (2009) Outcomes in threadlift for facial rejuvenation. Ann Plast Surg 62: 482-485.
- Kaminer MS, Bogart M, Choi C, Wee SA (2008) Longâ€term efficacy of anchored barbed sutures in the face and neck. Dermatol Surg 34: 1041-1047.
- Villa MT, White LE, Alam M, Yoo SS, Walton RL (2008) Barbed sutures: A review of the literature. ‎Plast Reconstr Surg 121: 102e-108e.
- Zhang H, Huang S, Yang X, Zhai G (2014) Current research on hyaluronic acid-drug bioconjugates. Eur J Med Chem 86: 310-317.
- Hitchcock TM, Dobke MK (2014) Review of the safety profile for microfocused ultrasound with visualization. J Cosmet Dermatol 13: 329-335.
- Caldwell K, Henshaw L, Taylor G (2005) Developing a framework for critiquing health research. J Health, Social and Environmental Issues 6: 45-54.
- Kim J (2014) Effects of injection depth and volume of stabilized hyaluronic acid in human dermis on skin texture, hydrationand thickness. Arch Aesthetic Plast Surg 20: 97-103.
- Landau M, Fagien S (2015) Science of hyaluronic acid beyond filling: Fibroblasts and their response to the extracellular matrix. Plast Reconstr Surg 136: 188S-195S.
- Park KY, Seok J, Rho NK, Kim BJ, Kim MN (2016) Longâ€chain polynucleotide filler for skin rejuvenation: Efficacy and complications in five patients. Dermatol Ther 29: 37-40.
- Pak CS, Lee J, Lee H, Jeong J, Kim EH, et al. (2014) A phase III, randomized, double-blind, matched-pairs, active-controlled clinical trial and preclinical animal study to compare the durability, efficacy and safety between polynucleotide filler and hyaluronic acid filler in the correction of Crow's feet. A J Korean Med Sci 29: S201-S209.
- Casabona G, Michalany N (2014) Microfocused ultrasound with visualization and fillers for increased neocollagenesis: Clinical and histological evaluation. Dermatol Surg 40: S194-S198.
- Friedmann DP, Fabi SG, Goldman MP (2014) Combination of intense pulsed light, sculptra and ultherapy for treatment of the aging face. J Cosmet Dermatol 13: 109-118.
- Carruthers J, Burgess C, Day D, Fabi SG, Goldie K, et al. (2016) Consensus recommendations for combined aesthetic interventions in the face using botulinum toxin, fillersand energy-based devices. Dermatol Surg 42: 586-597.
Citation: Chang D (2017) The Safety and Efficacy of Midface Rejuvenation in Asians with Hyaluronic Acid Dermal Fillers, High Intensity Focused Ultrasound and Thread lift. Cosmetol & Oro Facial Surg 2: 125.
Copyright: © 2017 Chang D. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 16597
- [From(publication date): 0-2017 - Apr 20, 2025]
- Breakdown by view type
- HTML page views: 15571
- PDF downloads: 1026