The Role of Whole Genome Gene Expression Studies in Deciphering Cortical Grey Matter Pathology in Multiple Sclerosis
Received: 11-Jan-2021 / Accepted Date: 25-Jan-2021 / Published Date: 01-Feb-2021 DOI: 10.4172/jceni.1000125
Abstract
The chronic cortical pathology of Multiple Sclerosis (MS) composes of demyelination and neurodegeneration and has been studied since the early pathoanatomical descriptions of MS. Due to technical difficulties in detecting the extent of cortical damage accumulating during the disease process; it has only been recognized as a major aspect of MS pathology 20 years ago. Whole genome gene expression studies from cortical human brain tissue have been an invaluable tool to gain novel insights into the molecular pathology of the so-called normal-appearing cortical grey matter and demyelinated grey matter lesions and have aided in discovering new pathomechanisms. These studies however are notoriously difficult to perform and interpret, due to the heterogeneity of the disease itself and the complex architecture of the human brain cortex.
Keywords: Cortical grey matter; Molecular pathology; RNA expression; Genome; Gene expression
Introduction
Multiple Sclerosis (MS) is the most common chronic inflammatory demyelinating disease of the central nervous system showing both neurodegenerative and immunological aspects. The main pathological hallmarks of MS are foci with complete loss of myelin in the grey and white matter termed lesions. The cause of MS remains elusive but the development of MS includes a complex genetic trait and several environmental risk factors, which act in concert and contribute to the main pathomechanisms including inflammation, de- and remyelination, axonal and neuronal loss, astroglia activation, and metabolic changes [1]. MS has been traditionally viewed as an autoimmune disease mediated primarily by an aberrant T-cell and B-cell reaction directed against the central nervous system myelin and oligodendrocytes [2]. Whether the initial event triggering MS is an aberrant immune-system damaging the central nervous system (outside-in hypothesis) or a primary degenerative mechanism, secondarily triggering an immune- response (inside-out hypothesis), remains unclear as of today, and while there is evidence for both concepts, the data provided are not yet conclusive [3-5].
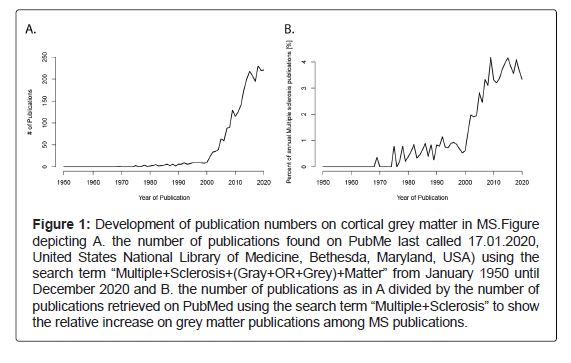
Changes within the white matter have been the primary focus of MS research since the first neuropathological description and they were long considered to be the major pathological hallmark [6,7], whereas changes within the grey matter were recognized early on [8-12] but mostly neglected due to technical difficulties to visualize the extent of cortical demyelination. Only in the past two decades have changes within the grey matter and especially the cerebral cortical grey matter become more and more recognized (Figure 1). Cortical demyelination may be present from the earliest clinical disease stages [13] and increases strongly when patients reach the progressive stage [14].
Figure 1: Development of publication numbers on cortical grey matter in MS.Figure depicting A. the number of publications found on PubMe last called 17.01.2020, United States National Library of Medicine, Bethesda, Maryland, USA) using the search term “Multiple+Sclerosis+(Gray+OR+Grey)+Matter” from January 1950 until December 2020 and B. the number of publications as in A divided by the number of publications retrieved on PubMed using the search term “Multiple+Sclerosis” to show the relative increase on grey matter publications among MS publications.
Cortical Grey Matter Lesion Pathology
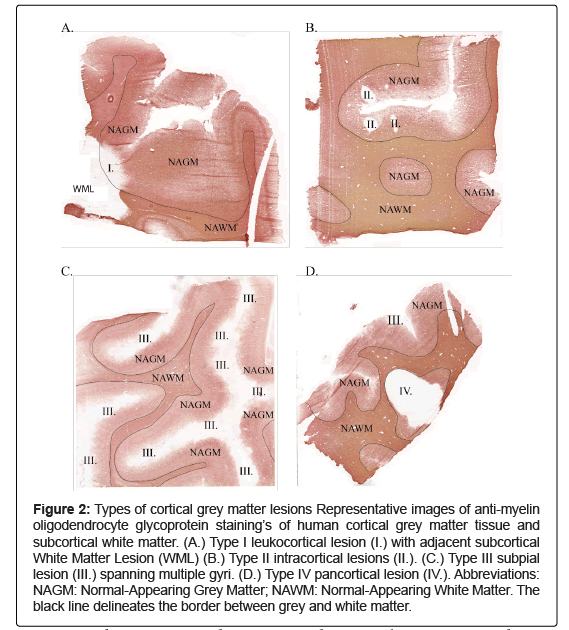
A histological classification system for cortical grey matter lesions was proposed by Peterson, et al. and updated in 2003 and has since been widely accepted and used [15,16]. This classification system distinguishes four lesion types: Type I lesions (Figure 2A), also termed leukocortical lesions, are in direct contact with the white matter border but do not reach the meninges. They are often confluent with white matter lesions. Type II or intracortical lesions contact neither the white matter border nor the meninges and are often perivascular (Figure 2B). Type III or subpial lesions are in direct contact with the meninges, often extend unto cortical layer 3 or 4, but do not reach the border to the subcortical white matter (Figure 2C). Subpial cortical lesions are the most common type of cortical lesions in MS and are the only lesion type not detected in any other disease with demyelination of the cortex such as tuberculous or luetic meningoencephalitis, progressive multifocal leukoencephalopathy, Rasmussen’s encephalitis, or paraneoplastic encephalitis [17,18]. This specificity of subpial lesions suggests that demyelination in MS is not simply a bystander effect of inflammation but part of a mechanism unique to this disease. Subpial lesions have a band or ribbon like appearance and may stretch over several adjacent gyri, in one specific case 69% of the forebrain cortical area was demyelinated [14]. Subpial cortical lesions have further been shown to associate with meningeal inflammation while parenchymal T- and B-cell infiltrates are rare [19,20]. Last, type IV or pancortical lesions extend from the meninges through all cortical layers and contact the white matter border (Figure 2D). These lesions might signify a specific pattern, or they may simply be a fusion of type I, II or III lesion, having grown to span all cortical layers.
Figure 2: Types of cortical grey matter lesions Representative images of anti-myelin oligodendrocyte glycoprotein staining’s of human cortical grey matter tissue and subcortical white matter. (A.) Type I leukocortical lesion (I.) with adjacent subcortical White Matter Lesion (WML) (B.) Type II intracortical lesions (II.). (C.) Type III subpial lesion (III.) spanning multiple gyri. (D.) Type IV pancortical lesion (IV.). Abbreviations: NAGM: Normal-Appearing Grey Matter; NAWM: Normal-Appearing White Matter. The black line delineates the border between grey and white matter.
Cortical grey matter lesions may be staged into active, chronic active or chronic inactive lesions. To differentiate between these stages a histological marker for the Major Histocompatibility Complex (MHC) class II is used: active lesions are characterized by a distinct MHC class II positive cell border and lesion core, and hypercellularity. Chronic active lesions are also characterized by a MHC class II cell border, but their core shows levels of MHC class II positive cells at the lesion core comparable to normal-appearing cortex. Chronic inactive lesions do not show any distinct MHC class II positive lesion border and have a core comparable to normal-appearing grey matter [15]. For staging the leukocortical lesions, the white matter part of the lesion has been used as a surrogate [15].
Apart from the demyelinated lesions, changes have also been described within the so-called normal-appearing white and normal- appearing grey matter. These changes are more pronounced during the progressive phases of the disease and include perivascular inflammatory infiltrates, edema, diffuse microglia activation and diffuse axonal injury, and astrocytic gliosis [14]. These changes are in part attributed to focal lesions, leading to Wallerian (anterograde) and retrograde degeneration of the neurons. Further, changes within the normal- appearing white and grey matter develop independent of focal lesions and partially associate with meningeal inflammation of the spinal cord and the cortex [21,22].
The Role of Whole Genome Gene Expression Studies in Cortical Grey Matter Lesion Pathology
Whole genome gene expression studies are an invaluable tool of descriptive research and may lead to both novel insights and novel questions, without being strictly hypothesis driven. To date only few gene expression studies have been performed with material from normal-appearing cortical grey matter and cortical grey matter lesions in MS. One study was performed by Dutta, et al. [23,24] and compared the normal-appearing motor cortex of six MS cases to motor cortex of six controls without neurological disease. They found a downregulation of mitochondrial genes involved in the respiratory chain, specific for neurons, and a lower expression of genes involved in the pre and postsynaptic components of GABAergic signaling. These results suggest a disturbance in the energy household of neurons, possibly contributing to neurodegeneration [23]. They further described an upregulation of the ciliary neurotrophic factor CNTF and functionally related genes promoting neuroprotection and anti-apoptotic pathways, suggestive of adaption mechanisms of the neurons [24].
In another study brain tissue samples from six MS cases and eight control cases without neurological disease were compared [25]. In contrast to the earlier study, cortical grey matter as well as cortical grey matter lesions were included. They observed a higher expression of immunoglobulin related genes in MS grey matter deriving from plasma cells located in the meninges.
Another study was designed and performed to distinguish MS specific gene expression changes from more general pathological changes [26]. This was achieved by comparing the gene expression profiles of three MS cases to three cases with tuberculous meningitis, three cases with Alzheimer’s disease and three control cases without neurological diseases. By this multi-comparison, they detected changes in genes related to T-cell mediated inflammation, microglia activation, oxidative injury, DNA damage and repair, and remyelination.
A study from our lab compared the gene expression profiles of 8 control cases and normal-appearing grey matter of 13 MS cases [27]. We identified a lower expression of astrocyte specific genes involved in the astrocyte-neuron lactate shuttle, which is important for neuronal homeostasis, and genes involved in the glutamate-glutamine cycle, which is also a support mechanism of astrocytes for the neurons. We further identified a higher expression of interleukin 1 beta within the normal-appearing grey matter, suggestive of inflammasome activation.
In a recent study from our lab, we analyzed a total of 106 normal- appearing grey matter samples from MS cases and cortical grey matter tissue samples from control cases [28]. This study revealed a distinct, bimodal expression pattern of HLA-DRB1, with each case having either a high or a low gene expression throughout all tissue samples used. This finding was especially interesting, as tissue showing any sign of inflammation in the histological analysis had been excluded from the study, using strict definitions of normal-appearing tissue. HLA-genotyping revealed that all carriers of the MS associated HLA- DRB1*15:01 allele also showed a high HLA-DRB1 and HLA-DRB5 gene expression, with a few carriers of other alleles also showing a high HLA- DRB1 gene expression. Strikingly, this was true in both MS and control cases. The HLA-DRB1*15:01 allele has been reported to increase the risk for developing MS about threefold, making it the strongest genetic risk factor for developing MS [29]. Further, the high HLA-DRB1 gene expression associated with a high HLA-DRA gene expression and with a higher HLA-DRB1 protein expression and with increased cortical grey matter lesion size. Our study thus hints at a link between the strongest genetic risk factor for developing MS, a higher gene and protein expression of HLA-DRB1, and a major pathological hallmark of MS: demyelinated cortical grey matter lesions. Our study, though preliminary in this respect, may hint at a unique vulnerability caused by increased HLA-DR expression within the brain, possibly facilitating the onset and/or progression of the disease. Such vulnerability could combine aspects of an inside-out and an outside-in hypothesis.
Discussion and Conclusion
In summary, gene expression studies performed on cortical grey matter of MS cases have so far revealed gene expression profiles suggestive of a stressed energy household of the neurons, aggravated by a failing support of the astrocytes and the myelin sheaths and have hinted at an increased vulnerability of the cortical tissue related to the strongest genetic risk factor.
Using human brain tissue from autopsies for gene expression studies to further increase our understanding of human brain diseases is an invaluable tool, though far away from the onset of disease, allowing us insights into chronic disease and adaptation mechanisms. The limitations of this approach however need to be carefully explored and confounding factors such as the presence of other neurological diseases, age-related changes, and post-mortem related degradation need to be considered. Further, MS is a heterogeneous disease, clinically and histologically, and gene expression studies are usually only performed on a number of cases too small to reflect this heterogeneity. It is for example unclear whether the different cortical lesion types have a common origin or should rather be studied as separate entities. Another concern arises from the architecture of the cortex: to compare different lesions with one another or to compare a lesion to normal-appearing grey matter, it is necessary to acquire tissue samples which are as comparable as possible, respecting the cellular and molecular differences between different areas of the cortex. Thus, a simple comparison of too few cases will likely not reveal all aspects of the molecular pathology, and instead, we should aim for larger studies in the future.
Today, whole genome gene expression studies have become but a part of the vast field of emerging omics analysis. We see the role of these techniques in general and especially in the difficult case of cortical grey matter diseases in hypothesis generation, initiating novel insights, but always requiring mechanistically oriented research to follow-up on the propositions.
Conflict of Interests
The authors declare no conflicts of interest.
Author Contributions
Concept (L.S.E.), draft (L.S.E., S.J.), manuscript revisions (L.S.E., S.J., P.H., N.S.-W.).
Funding
This study was supported by the Roche Translational Medicine Hub, by the Swiss National Science Foundation (31003A_159528/1), by the Swiss Multiple Sclerosis Society, and by the French MS Society (ARSEP) all to N.S.-W and by the Swiss National Science Foundation (323530_171139) to L.S.E.
Ethical Approval
Ethical approvals for the human tissues used in were given by the UK Multicentre Research Ethics Committee, MREC/02/2/39. For protocol and details see our recent publication [28].
References
- Olsson T, Barcellos LF, Alfredsson L (2017) Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nature reviews Neurology 13: 25-36
- Kawachi I, Lassmann H (2017) Neurodegeneration in multiple sclerosis and neuromyelitis optica. J. Neurol. Neurosurg. Psychiatry 88: 137-145.
- Stys PK, Zamponi GW, Van Minnen J, Geurts JJ (2012) Will the real multiple sclerosis please stand up?. Nat Rev Neurosci 13: 507-514.
- Zeis T, Enz L, Schaeren-Wiemers N (2016) The immunomodulatory oligodendrocyte. Brain research 1641: 139-48.
- Hemmer B, Kerschensteiner M, Korn T Â (2015) Role of the innate and adaptive immune responses in the course of multiple sclerosis. The Lancet Neurology 14: 406-419.
- Charcot M (1868) Histologie de la sclerose en plaque. Gaz Hosp 41: 554-556.
- Rindfleisch E (1863) Histologisches Detail zu der grauen Degeneration von Gehirn und Rückenmark.(Zugleich ein Beitrag zu der Lehre von der Entstehung und Verwandlung der Zelle.). Virchows Archiv 26: 474-483.
- Bourneville DM, Guérard L (1869) De la sclérose en plaques disséminées: nouvelle étude sur quelques points de la sclérose en plaques disséminées par Bourneville Adrien Delahaye.
- Frommann C (1878) Untersuchungen über die Gewebsveränderungen bei der Multiplen Sklerose des Gehirns und des Rückenmarks. Gustav Fischer.
- Sander DM (1898) Hirnrindenbefunde bei multipler Sklerose. European Neurology 4: 427-436.
- Dinkler (1904) Zur Kasuistik der multiplen Herdsklerose des Gehirns und Rückenmarks. Deutsche Zeitschrift für Nervenheilkunde 26: 233-247.
- Schob F (1907) Ein Beitrag zur pathologischen Anatomie der multiplen Sklerose. European Neurology 22: 62-87.
- Lucchinetti CF, Popescu BF, Bunyan RF, Moll NM, Roemer SF, et al. (2011) Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 365: 2188-2197.
- Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, et al. (2005) . Brain 128: 2705-2712.
- Peterson JW, Bo L, Mork S, Chang A, Trapp BD (2001) Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann neurol 50: 389-400.
- Bø L, Vedeler CA, Nyland HI, Trapp BD, Mørk SJ (2003) Subpial Demyelination in the Cerebral Cortex of Multiple Sclerosis Patients. J Neuropathol Exp Neurol 62: 723-732.
- Moll NM, Rietsch AM, Ransohoff AJ, Cossoy MB, Huang D, et al. (2008) Cortical demyelination in PML and MS: Similarities and differences. Neurology 70: 336-343.
- Junker A, Wozniak J, Voigt D, Scheidt U, Antel J, et al. (2020) Extensive subpial cortical demyelination is specific to multiple sclerosis. Brain Pathol 30: 641-652.
- Haider L, Zrzavy T, Hametner S, Hoftberger R, Bagnato F, et al. (2016) The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain 139: 807-815.
- Androdias G, Reynolds R, Chanal M, Ritleng C, Confavreux C (2010) Meningeal T cells associate with diffuse axonal loss in multiple sclerosis spinal cords. Ann Neurol 68: 465-4Â 76.
- Dutta R, McDonough J, Yin X, Peterson J, Chang A, et al. (2006) Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann neurol 59: 478-489.
- Dutta R, McDonough J, Chang A, Swamy L, Siu A, et al. (2007) Activation of the ciliary neurotrophic factor (CNTF) signalling pathway in cortical neurons of multiple sclerosis patients. Brain 130: 2566-2576.
- Torkildsen O, Stansberg C, Angelskar SM, Kooi EJ, Geurts JJ, et al. (2010) Â Upregulation of immunoglobulin-related genes in cortical sections from multiple sclerosis patients. Brain pathol 20: 720-729.
- Fischer MT, Wimmer I, Hoftberger R, Gerlach S, Haider L, et al. (2013) Disease-specific molecular events in cortical multiple sclerosis lesions. Brain 136: 1799-1815.
- Zeis T, Allaman I, Gentner M, Schroder K, Tschopp J, et al. (2015) Metabolic gene expression changes in astrocytes in Multiple Sclerosis cerebral cortex are indicative of immune-mediated signaling Brain Behav Immun 48: 313-325.
- Enz LS, Zeis T, Schmid D, Geier F, van der Meer F et al. (2020) Increased HLA-DR expression and cortical demyelination in MS links with HLA-DR15. Neurol Neuroimmunol Neuroinflamm 7: e656.
- International Multiple Sclerosis Genetics Consortium, Wellcome Trust Case Control Consortium, Sawcer S, Hellenthal G, Pirinen M, Spencer CCA et al. (2011) Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476: 214-219.
Citation: Enz LS, Jaggi S, Huemer P, Schaeren-Wiemers N (2021) The Role of Whole Genome Gene Expression Studies in Deciphering Cortical Grey Matter Pathology in Multiple sclerosis. J Clin Exp Neuroimmunol. 6: 125. DOI: 10.4172/jceni.1000125
Copyright: © 2021 Enz LS et al. This is an open-access article distributed under the terms of the creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.