Research Article Open Access
The Role of Matricaria recutita L. and Asparagus officinalis L. against the Neurotoxicity of Diazinon in Rats
Fahmy G. Elsaid1,2*, Ali A. Shati1 and Mohammad A. Sarhan1
1Biology Department, Science College, King Khalid University, Saudi Arabia
2Zoology Department, Faculty of Science, Mansoura University, Egypt
- *Corresponding Author:
- Fahmy G. Elsaid
Zoology Department,Faculty of Science
Mansoura University, Egypt
Tel: +966536906036 E-mail: fahmygad@mans.edu
Received September 13, 2014; Accepted October 20, 2014; Published October 28, 2014
Citation: Elsaid FG, Shati AA, Sarhan MA (2014) The Role of Matricaria recutita L. and Asparagus officinalis L. against the Neurotoxicity of Diazinon in Rats. J Neuroinfect Dis 6:164. doi: 10.4172/2314-7326.1000164
Copyright: © 2014 Elsaid FG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
Diazinon (DZN) is an organophosphorus insecticide that widely used in agriculture. It has a variety of harmful effects on human. Asparagus and chamomile have antioxidant properties and used as antidotes of DZN in this study. Thirty five adult male Sprague Dawely rats were divided into: control group; DZN group: subdivided into two subgroups receiving ¼ LD50 and ½ LD50 dose of DZN for 30 days; DZN and asparagus extract group: subdivided into two subgroups receiving ¼ LD50 and ½ LD50 dose of DZN respectively and treated with asparagus extract (300 mg/kg b. wt.); DZN group and chamomile extract: subdivided into two subgroups receiving DZN respectively, and treated with chamomile extract (300 mg/kg b. wt.). The results herein showed that the biochemical changes associated with the exposure to the DZN are dose dependent. The tumorigenicity of DZN was represented by the significant increase of arginase and the alpha-L- fucosidase in the sera of all DZN groups. In addition, the molecular changes were investigated by the changes in Cu/ Zn-dependent superoxide dismutase, glutathione-S-transferase and glutathione peroxidase enzymes that deflected with administration of rats with DZN. Oral administration of watery extracts of asparagus or chamomile able to restore the total antioxidant capacity as recorded by the increase of superoxide dismutase, glutathione content and their relative enzymes in the investigated tissues. Due to their antioxidants, it is recommended asparagus and chamomile as anti-neurotoxic agents.
Keywords
Diazinon; Antioxidants; Oxidative stress; Neurotoxicity;Gene expression; Asparagus; Chamomile
Introduction
Diazinon (DZN) is an organophosphate insecticide used mainly in agriculture and in sheep dips, and is designed as an irreversible acetylcholine esterase inhibitor [1,2]. It is classified as moderately hazardous class-II organophosphate insecticide [3]. Many systems could be affected by organophosphate intoxication are the immune system, urinary system, reproductive system, pancreas and haematological and biochemical changes [4-8]. In addition, showed that neonatal DZN exposure, at doses below the threshold for cholinesterase inhibition has been lasting effects on emotional responses, with preferential effects on males [9,10]. The microsomal enzymes in the liver oxidize DZN are generating more potent acetylcholinesterase inhibitors, such as diazoxon, hydroxydiazoxon and hydroxydiazinon [11]. Diazinon affects mitochondrial membrane transportation in rat liver [12]. Moreover, it interrupts cytochrome P450 system in human liver [13]. Oxidative stress can also be induced by pesticides, either by the overproduction of free radicals or by alteration in antioxidant defence mechanisms, including detoxification and scavenging enzymes [14]. Oxidative stress has been reported to play an important role in the toxicity of various pesticides, including organochlorines, carbamates and pyrethrods [15,16]. The higher oxidative stress in pesticide sprayers is evidenced by increased concentration of plasma and red blood cell thiobarbituric acid reactive substances (TBARS), changes in antioxidant status, and altered activities of cellular enzymes [17]. Treatment of rats with DZN significantly enhances renal lipid peroxidation, which is accompanied by a decrease in the activities of renal antioxidant enzymes (e.g. catalase (CAT), glutathione peroxidise, glutathione reductase (GSH-R), glucose- 6-phosphate dehydrogenase, glutathione-s-transferase (GST) and depletion in the level of glutathione reduced (GSH). In blood, normal erythrocyte function depends on the intactness of cell membrane, which is the target for many toxic factors, including pesticides. Erythrocyte GSH together with glutathione peroxidase (GSH-Px), GSH-R, GST, gamma-glutamyl transferase (γ-GT), superoxide dismutase (SOD) and CAT efficiently scavenge toxic free radicals and are partly responsible for protecting against lipid peroxidation due to acute/chronic pesticide exposure [18]. There is a relationship between pesticide exposure and the decrease of antioxidant enzymes [19].
Oxidative stress and genotoxic effects of DZN were documented through the changes in total antioxidant capacity (TAC), reduced GSH and oxidative DNA damage [20]. Exposure to low-level of pesticides is known to produce a variety of biochemical and molecular changes, some of which may be responsible for the adverse biological effects reported in human and experimental studies. Many studies have reported that DZN can induce molecular changes and alter gene expression. Jamshidi et al. have found that administration of DZN to rats at doses of 60 mg/ kg significantly decreased expression of glutamate dehydrogenase, the key enzyme of Langerhans islet for the secretion of insulin, gene 18- hour post-administration [21]. Other studies showed that DZN had affected the expression of neurotrophic factors that coordinate neuronal cell differentiation and brain assembly [22]. In addition, Timofeeva et al. found that persisting effect of developmental DZN cholinergic and serotonergic neurotransmitter systems and gene expression as well as behavioural function [9].
Matricaria recutita L. (family Asteraceae, commonly known as German chamomile) is one of the most widely used and welldocumented medicinal plants in the world [23]. Chamomile is also extensively consumed as a tea or tonic. Chamomile is used both internally and externally to treat an extensive list of conditions. It is used externally for wounds, ulcers, eczema, gout, skin irritations, neuralgia, sciatica, rheumatic pain, and haemorrhoids and internally to treat anxiety, hysteria, nightmares, insomnia and other sleep problems, convulsions and even delirium tremens [24,25]. The main chemical constituents of the German chamomile are terpenoids like α-bisobolol, chamazulene, sesquiterpenes and flavonoids like apigenin, luteolin and quercetin. Green asparagus (Asparagus officinalis L.) is a healthy and nutritious vegetable, containing antioxidants, such as rutin, ascorbic acid, tocopherol, ferulic acid and GSH. The major flavonoid antioxidant in asparagus has been reported to be rutin, with the content of 286.5 ± 6.0 mg/kg fresh weight [26,27]. Among 23 commonly consumed vegetables, antioxidant activity of asparagus, based on dry weight, has been ranked as the greatest [28]. Rodriguez et al. and Sun et al. emphasized the antioxidant activity of asparagus as it contains flavonoids and phenolic components [29,30].
The study aimed to investigate the role of aqueous extracts of asparagus and chamomile in ameliorating the biochemical and molecular changes accompanied with the administration of diazinon.
Materials and Methods
Rats
Thirty five adult male Sprague Dawley rats, weighing 180-200 g, were obtained from the Experimental Animal Unit, College of Science, King Khalid University, Saudi Arabia and were used this study. All rats received food and water ad libitum and were kept in a room with the temperature regulated to 22 ± 1°C. The experiment was approved by the Animal Ethical Committee, College of Science, King Khalid Universit
Methods
Asparagus and chamomile extracts
Crude extracts were prepared from asparagus (Matricaria recutita L., family Asteraceae) and chamomile (Matricaria recutita L., family Asteraceae). 5 g of each herb was separately, grinding in a blender and was then incubated in 100 ml of boiling distilled water for 30 min. Once at room temperature, the extracts were filtered once by four layers of cheesecloth and once by a single layer of Whatmann 1 filter paper. Finally, each extract was centrifuged at 6000 g for 10 min, decanted, fractioned into 10 mL aliquots and freeze-stored at -20° C. Just prior to each experiment, an aliquot of the extract was thawed. The route of administration of both extracts was orally administered using a stomach tube.
Experimental design
Animal grouping: The protocol was designed to investigate the physiological and molecular changes associated with the exposure of experimental animals to the pesticides such as diazinon (O,O-diethyl- O-[2-isopropyl-6-methyl-4-pyrimidine-yl]phosphorothionate) for 30 days. The LD50 and the regime schedule were selected according to the previous study [31,32]. After determination of the LD50 dose (60 mg/kg b. wt.) we used the quarter (15 mg/kg. b. wt.) and half (30 mg/kg. b. wt.) of this dose and orally administered to the animals using gavage for 30 days. The treated animals were divided into: Control group: rats were received corn oil alone as a vehicle for DZN; DZN group: subdivided into two subgroups receiving ¼ LD50 and 1/2 LD50 dose of DZN; DZN and asparagus extract group: subdivided into two subgroups receiving ¼ LD50 and 1/2 LD50 dose of DZN respectively and orally administered with watery extract of asparagus at 300 mg/kg of b. wt.; DZN group and chamomile extract: subdivided into two subgroups receiving ¼ LD50 and 1/2 LD50 dose of DZN respectively, and orally administered with watery extract of chamomile at 300 mg/kg of b. wt.
Blood sampling: At the end of the experimental period, blood samples were collected by decapitation and centrifuged at 2500 rpm for 15 min, and then sera were kept in clean eppendorf tubes at -20°C until later analysis.
Preparation of tissue homogenate: Different studies have been focused on the brain homogenate, but herein, we aimed to investigate the effect of DZN on the main compartments of the brain. So, at the end of 30 days, a piece of cerebrum, cerebellum and spinal cord were taken freshly from each animal on ice according to the rat brain atlas. Homogenates of the tissues were prepared in 1.0 ml of phosphate buffer per100 mg of tissues by using an electrical homogenizer. The samples were spun at 3000 rpm at 4°C, and the supernatant was used for the biochemical analysis.
For RNA extraction, samples from rat cerebrum, cerebellum and spinal cord were removed, rinsed with ice cold saline solution, weighed directly frozen by dropping into liquid nitrogen and stored at -80°C for further determinations.
Biochemical analysis
Determination of malondialdehyde
To analyse lipid peroxidation in brain regions samples 2-Thiobarbituric acid-Reactive Substances (TBARS) were measured according to Ohkawa et al. method at 532 nm. The concentration of MDA is calculated using an extinction coefficient of MDA-TBA complex, which is 1.56×105 M-1cm-1 and the results was expressed as n mol MDA /g tissue [32].
Determination of reduced Glutathione (GSH) concentration
Total glutathione content was measured according to the method described by Beutler et al. using glutathione reduced colorimetric method [33].
Determination of superoxide dismutase (SOD) activity
SOD activity was determined in the sample according to the method of Nishikimi et al. [34]. Absorbance was read at 505 nm using a spectrophotometer. A standard curve was plotted for each standard against the percentage of inhibition, which was used to determine the superoxide dismutase activity (U/ml).
Determination of the glutathione peroxidase (GSH-Px) assay
Glutathione peroxidase activity in the sample was measured according to the Paglia and Valentine’s method [35]. The activity of GSH-Px was measured at 340 nm by measuring the decrease of NADPH absorbance using an extension coefficient of 6.22 mM-1cm-1.
Determination of glutathione reductase (GSH-R) activity
GSH-R activity was measured as described by Goldberg and Spooner [36]. The principle of the method based on that GSH-R catalyses the reduction of oxidized glutathione (GSSG) in the presence of NADPH, which is oxidized NADPH+. The decrease in absorbance is measured at 340 nm.
Determination of glutathione-S-transferase (GST) activity
The method of Habig et al. [37] was used. It depends on measuring the conjugation of reaction 1-chloro-2,4-dinitrobenzene (CDNB) with reduced glutathione. The conjugation is accompanied by an increase in absorbance at 340 nm. The rate of the increase is directly proportional to the GST activity in the sample.
Determination of total antioxidant capacity (TAC)
TAC was measured as described by Koracevic et al. [38]. The principle of the method based on the determination of the antioxidative capacity is performed by the reaction of antioxidants in the sample with a definite amount of exogenously provide H2O2. The antioxidants in the sample eliminate a certain amount of the provided hydrogen peroxide. The residual is H2O2 determined colorimetrically by an enzymatic reaction which involves the conversion 3,5, dichloro-2-hydroxyl benzene sulphonate to a colored product.
Determination of serum arginase activity
Serum arginase estimation was based upon the colorimetric determination of urea by condensation with diacetyl monoxime in an acid medium in the presence of ferric chloride and carbazide according to the method of Marsh et al. [39].
Determination of serum α-l-fucosidase activity
The assay used for α-L-fucosidase, was based on the enzymatic cleavage of the synthetic substrate p-nitro phenyl α -L-fucopyranoside to p-nitrophenyl and l-fucose L. The yellow color of p-nitrophenyl in an alkaline medium was measured quantitatively at 405 nm [40].
Gene Expression
RNA extraction
To determine mRNA expression levels of GST, GSH-Px and SOD (Cu/Zn-dependent superoxide dismutase) total RNA was isolated from cerebrum, cerebellum and spinal cord using RNeasy Mini kit, (Qiagen) according to the manufacturers’ protocol. Following the photometrical determination of RNA concentration and purity at 260 nm and 280 nm. RNA quality was checked by testing the integrity of the 18S- and 28Sribosomal RNA bands and by controlling the absence of genomic DNA in 1.0% agarose gels.
Complementary DNA (cDNA)
According to the manufacturers’ protocol of Omniscript RT Kit- Qiagen, USA; total RNA (2 μg) was transcribed into cDNA in a 20 μL final volume of reaction buffer [2 μL 10x Buffer; 2 μL dNTP mix (5 mM each); 1 μL RNase inhibitor (10 U/μL); 2 μL oligo dT primers (100 μM); 1μL Omniscript RT and the corresponding amount of RNA (100mg)] by incubation for 1h at 37°C. The reaction was stopped by incubation at 99°C for 5 min. cDNA’s were stored at -20°C until usage.
Amplification of transcripts by Polymerase Chain Reaction
According to the manufacturer’s protocol of GoTaq® DNA polymerase Promega, USA. For rat GST, GSH-Px and Cu/Zn-SOD, PCR was performed with 100 ng of the synthesized cDNA and specific primers for a given gene of interest (Table 1). In a typical PCR, a fresh master mix was prepared by mixing (per sample) 2.5 μl 10x PCR buffer, 1 μl MgCl2 (25 mM), 2μl dNTPs mix (2.5mM of each dNTP), 0.5μl Taq DNA polymerase (final concentration 2.5 U/ml), and 17μl water. To the resulting 23 μl per sample, 1μl of a pre-made mix of forward and reverse primers (5 μM each; final concentration 200 nM) and 1μl cDNA template was added, for a final reaction volume of 25 μl. The thermal cycler was started with an initial denaturation at 94°C for 3 min, denaturation at 94°C for 30 sec, annealing at 58°C for 30 sec and extension at 72°C for 60 sec. These steps were repeated for 30 cycles. Last extension was carried out at 72°C for 5 min and then finally reaction was kept at 4°C. After the reaction was completed, PCR tubes were stored at -20°C until further use. All PCR amplifications were performed on a Primus 25 advanced thermocycler (Germany). For each PCR reaction, 5 μL was electrophoresed in a 1% agarose gel in Tris-borate- EDTA buffer. The DNA was visualized and photographed using the BioRad Gel documentation system.
| Primer Name | Sequence | Product Size |
|---|---|---|
| GST-F | 5’CCTCACCCTTTACCAATCTA3’ | 350bp |
| GST-R | 5’TTCGTCCACTACTGTTTACC3’ | |
| Cu-Zn SOD – F | 5’TCTAAGAAACATGGCGGTCC3’ | 311bp |
| Cu-Zn SOD – R | 5’CAGTTAGCAGGCCAGCAGAT3’ | |
| GSH-Px- F | 5’CTCTCCGCGGTGGCACAGT3’ | 290bp |
| GSH-Px- R | 5’CCACCACCGGGTCGGACATAC3’ |
Table 1: List of primers used in this study.
Statistical Analysis
The biochemical data were expressed as mean ± SD and statistical and correlation analyses were performed using the oneway ANOVA followed by a post-hoc least significant difference (LSD) test. P-values<0.05 were considered as statistically significant. Statistical analyses were performed with the statistical package for the social sciences for Windows (SPSS, version 16.0, Chicago, IL, USA).
Results
Biochemical results
Table 2 shows the activity of superoxide dismutase (SOD), total antioxidant capacity and lipid peroxidation (malondialdehyde level) in all groups of rats. Rats that administered with DZN showed a significant increase in the activity of SOD in cerebrum, cerebellum and spinal cord tissues when compared to the rats in the control group. However, rats administered with DZN along with asparagus or chamomile (300 mg/ kg b. wt.) showed a significant improvement in the activity of SOD in the cerebrum, cerebellum and spinal cord tissues when compared to the ¼ and 1/2 LD50 doses of DZN groups. The change in SOD activity in all investigated tissues was more pronounced in 1/2 LD50 groups than ¼ LD50 one. Reversely, the total antioxidant capacity in the cerebrum, cerebellum and spinal cord was depleted significantly in the ¼ LD50 groups as compared to the control one, but the depletion was more noticed in the 1/2 LD50 in all tissues. Oral administration of watery extracts of asparagus or chamomile able to restore the total antioxidant capacity in the investigated tissue in all treated groups when compared to their matched ones. Lipid peroxidation (measured as MDA level) was a significant increase in DZN non treated groups when compared to the control group. The increment was more distinct in the 1/2LD50. This increase of MDA leads to augmentation of oxidative stress in all tissues, the administration of aqueous tested extracts alleviated this increase especially in ¼LD5.
| Parameters | Tissues | Control | DZN 1/4 LD50 | DZN 1/4 LD50 + Chamomile | DZN 1/4 LD50 + Asparagus | DZN 1/2 LD50 | DZN 1/2 LD50 + Chamomile | DZN 1/2 LD50 + Asparagus |
|---|---|---|---|---|---|---|---|---|
| SOD (U/mg tissue) | Cerebrum | 7.7 ± 2.8 | 44.6 ± 2.8*** | 36.8 ± 2***, Ã?£Ã?£Ã?£ | 36.4 ± 0.5***, Ã?£Ã?£Ã?£ | 54.9 ± 1.7***,c | 43.8 ± 2.5***,ᴵᴵᴵ,c | 34.8 ± 3.1***,ᴵᴵᴵ |
| Cerebellum | 18.2 ± 2 | 39.5 ± 0.4*** | 33.9 ± 1.7***, Ã?£Ã?£Ã?£ | 27.5 ± 0.4***, Ã?£Ã?£Ã?£ | 55 ± 3.6***,c | 50.3 ± 3.3***,ᴵᴵᴵ,c | 40.6 ± 0.9***,ᴵᴵ,c | |
| Spinal cord | 16.6 ± 3.3 | 35.4 ± 3.6*** | 37.4 ± 2.1*** | 27.6 ± 2.3***, Ã?£Ã?£Ã?£ | 42.7 ± 2.8***,c | 35.6 ± 0.9***,ᴵᴵᴵ | 34.7 ± 0.6***,ᴵᴵᴵ,c | |
| TAC (U/ g tissue) | Cerebrum | 327.3 ± 23.2 | 254 ± 17.2*** | 282.4 ± 10.5** | 304.6 ± 21.9Ã?£Ã?£ | 234.7 ± 13.5*** | 274.2 ± 45.6***,á´µ | 284.7 ± 11.5**,ᴵᴵ |
| Cerebellum | 354.4 ± 12.1 | 260.5 ± 4.8*** | 301.1 ± 26.3***, Ã?£Ã?£Ã?£ | 301.9 ± 12.1***, Ã?£Ã?£Ã?£ | 245.1 ± 11.2*** | 263.3 ± 6.3***,á´µ,c | 273.1 ± 8.6***,ᴵᴵ,b | |
| Spinal cord | 397.8 ± 19.9 | 272.7 ± 24.1*** | 281.3 ± 10.1*** | 295.1 ± 10.6*** | 278.6 ± 16.9*** | 345.3 ± 37.9***,ᴵᴵᴵ,c | 393.4 ± 16.6ᴵᴵᴵ,c | |
| MDA (n mol/g tissue) | Cerebrum | 99.1 ± 3.7 | 207.8 ± 5.5*** | 170.8 ± 23.9***, Ã?£Ã?£Ã?£ | 172.1 ± 16.8***, Ã?£Ã?£Ã?£ | 162.5 ± 24.6***,c | 117.3 ± 7.0ᴵᴵᴵ,c | 111.3 ± 15.2ᴵᴵᴵ,c |
| Cerebellum | 111.9 ± 7.9 | 219.7 ± 20.6*** | 176.6 ± 4.3***, Ã?£Ã?£Ã?£ | 166.6 ± 13***, Ã?£Ã?£Ã?£ | 203.5 ± 29.9*** | 128.3 ± 18.2ᴵᴵᴵ,c | 119 ± 6.3ᴵᴵᴵ,c | |
| Spinal cord | 102.3 ± 4.3 | 168.9 ± 8.9*** | 119.6 ± 4.9**, Ã?£Ã?£Ã?£ | 107.9 ± 7.3 Ã?£Ã?£Ã?£ | 186.7 ± 14.4***,bc | 116.1 ± 6.1*,ᴵᴵᴵ | 121 ± 14.6**,ᴵᴵᴵ,a |
Values were expressed as means ± SD of five animals in each group.
*P<0.05 (significant), **P<0.01 (high significant) ***P<0.001 (very high significant), when all groups compared with control group.
�£�£P<0.01 (high significant) �£�£�£P<0.001 (very high significant), when all LD25+ chamomile and LD25+asparagus groups compared with LD25group.
ᴵP<0.05 (significant), ᴵᴵP<0.01 (high significant) ᴵᴵᴵP<0.001 (very high significant), when all LD50+ chamomile and LD50+asparagus groups compared with LD50group.
a P<0.05 (significant), b P<0.01 (high significant) c P<0.001 (very high significant), when 1/4LD50 and treated 1/4LD50 with chamomile and asparagus groups compared with their matched 1/2LD50 and treated 1/2LD50 with chamomile and asparagus groups.
Table 2: Superoxide dismutase (SOD), total antioxidants capacity (TAC) and lipid peroxidation in different animal groups.
As indicated in Table 3 the enzymatic antioxidants such as GST, GSH-Px and GSH-R and non-enzymatic antioxidant such as GSH were adversely changed in all investigated tissues in all groups when compared with the control. The cerebrum, cerebellum and spinal cord tissues were more sensitive to DZN as represented by the high significant decrease in GST, GSH-Px and GSH-R activity in ¼ and 1/2 LD50 groups. Administration of aqueous extracts of asparagus and chamomile is effective mediators as they alleviate the inhibition of these antioxidant enzymes when compared to their pesticide administered groups. The decrement of the above enzymes may be due to the significant decrease in GSH of cerebrum, cerebellum and spinal cord of the ¼ and 1/2 LD50 rats’ groups.
| Parameters | Tissues | Control | DZN 1/4 LD50 | DZN 1/4 LD50 + Chamomile | DZN 1/4 LD50 + Asparagus | DZN 1/2 LD50 | DZN 1/2 LD50 + Chamomile | DZN 1/2 LD50 + Asparagus |
|---|---|---|---|---|---|---|---|---|
| GST (U/ g tissue) | Cerebrum | 290.5 ± 9.9 | 246.9 ± 13.6*** | 260.5 ± 11.2***, Ã?£ | 281 ± 4.3***, Ã?£Ã?£Ã?£ | 210.7 ± 11.8***,c | 245.3 ± 8.8***,ᴵᴵᴵ,a | 264.6 ± 9.5***,ᴵᴵᴵ,b |
| Cerebellum | 320.3 ± 17.8 | 292.7 ± 9.5** | 301.3 ± 7.2* | 312.4 ± 16.7 Ã?£ | 273.6 ± 14.5*** | 285.7 ± 9.4*** | 303.9 ± 12.9ᴵᴵᴵ | |
| Spinal cord | 278.7 ± 26.5 | 236.8 ± 11.9** | 246.3 ± 17.4* | 229.2 ± 47.9** | 226.6 ± 13** | 237.4 ± 11.9** | 245.4 ± 12.6* | |
| GSH-Px (U/ g tissue) | Cerebrum | 178.04 ± 3.2 | 138.9 ± 13.2*** | 154.7 ± 17.5**, Ã?£ | 172.9 ± 9.0 Ã?£Ã?£Ã?£ | 122.7 ± 8.3***,a | 165.9 ± 14.1 ᴵᴵᴵ, | 170.8 ± 2.3 ᴵᴵᴵ |
| Cerebellum | 180.9 ± 9.2 | 100.6 ± 5.2*** | 156.7 ± 11.4*, Ã?£Ã?£Ã?£ | 151.4 ± 17.6**, Ã?£Ã?£Ã?£ | 87.8 ± 14.8*** | 171.2 ± 27.1ᴵᴵᴵ | 164.5 ± 12.5ᴵᴵᴵ | |
| Spinal cord | 209.2 ± 9.5 | 110.3 ± 6.0*** | 142.8 ± 8.7***, Ã?£Ã?£Ã?£ | 154.5 ± 18.0***, Ã?£Ã?£Ã?£ | 99.1 ± 5.4*** | 174.8 ± 20.2***,ᴵᴵᴵ,c | 184.9 ± 4.2**,ᴵᴵᴵ,c | |
| GSH-R (U/ g tissue) | Cerebrum | 72.4 ± 5.2 | 56.9 ± 5.4*** | 61.5 ± 6.6*** | 66.8 ± 8.5***, Ã?£ | 54.8 ± 7.5*** | 59.1 ± 7.6*** | 76.1 ± 9.6***,ᴵᴵᴵ |
| Cerebellum | 86.7 ± 7.1 | 60.1 ± 3.9*** | 69.9 ± 12.1* | 78.1 ± 12.5 Ã?£ | 57.1 ± 4.7*** | 69.3 ± 15.7* | 73.6 ± 16.4á´µ | |
| Spinal cord | 112.3 ± 8.5 | 76.9 ± 9.2*** | 76.5 ± 12.9*** | 83.6 ± 10.6*** | 68.7 ± 6.9*** | 69.9 ± 4.3*** | 84.4 ± 10.2***,á´µ | |
| GSH (U/ g tissue) | Cerebrum | 460.7 ± 30.4 | 240.8 ± 25.6*** | 389.3 ± 52.5***, Ã?£Ã?£Ã?£ | 400.3 ± 8.8**, Ã?£Ã?£Ã?£ | 252.1 ± 23.7*** | 373.6 ± 24.3***, ᴵᴵᴵ | 332.1 ± 25.9***, ᴵᴵᴵ,c |
| Cerebellum | 384.9 ± 16.1 | 231.0 ± 12.4*** | 348.5 ± 9.9***, Ã?£Ã?£Ã?£ | 301.2 ± 12.5***, Ã?£Ã?£Ã?£ | 225.6 ± 30.4*** | 262.5 ± 9.1***, ᴵᴵᴵ,c | 267.4 ± 2.9***, ᴵᴵᴵ,b | |
| Spinal cord | 390.7 ± 43.1 | 259.1 ± 13.7*** | 418.9 ± 5.0 Ã?£Ã?£Ã?£ | 383.4 ± 39.2 Ã?£Ã?£Ã?£ | 237.7 ± 28.4*** | 259.9 ± 13.1***,c | 358.9 ± 26.4ᴵᴵᴵ |
*P<0.05 (significant), **P<0.01 (high significant) ***P<0.001 (very high significant), when all groups compared with control group.
�£�£P<0.05 (significant), �£�£�£P<0.001 (very high significant), when all LD25+ chamomile and LD25+asparagus groups compared with LD25group.
ᴵP<0.05 (significant), ᴵᴵᴵP<0.001 (very high significant), when all LD50+ chamomile and LD50+asparagus groups compared with LD50group.
aP<0.05 (significant), bP<0.01 (high significant) cP<0.001 (very high significant), when 1/4LD50 and treated 1/4LD50 with chamomile and asparagus groups compared with their matched 1/2LD50 and treated 1/2LD50 with chamomile and asparagus groups
Table 3: Glutathione contents and their relative antioxidant enzymes different animal groups.
The data in Table 4 show a significant increase in arginase and alpha-L-fucosidase in the DZN administered groups as compared to control groups. The administration of asparagus and chamomile extracts showed an improvement in the levels of tumor markers in the treated groups when compared with the control and DZN non treated groups, especially with asparagus extract.
| Parameters | Control | DZN 1/4 LD50 | DZN 1/4 LD50 + Chamomile | DZN 1/4 LD50 + Asparagus | DZN 1/2 LD50 | DZN 1/2 LD50 + Chamomile | DZN 1/2 LD50 + Asparagus |
|---|---|---|---|---|---|---|---|
| Arginase (U/L) | 141.4 ± 9 | 238.1 ± 48.2*** | 235.2 ± 23.9*** | 196.5 ± 15.3**, Ã?£ | 325.7 ± 33.7***,c | 239.9 ± 18.7***,ᴵᴵᴵ, | 241.9 ± 19.8***,ᴵᴵᴵ,a |
| Alpha-L-Fucosidase (U/L) | 3.1 ± 0.2 | 4.5 ± 0.8** | 3.9 ± 0.9 | 4 ± 0.4 | 5.3 ± 1.3*** | 5 ± 0.8***,ᴵᴵ | 5.2 ± 0.4***,á´µ |
Values were expressed as means ± SD of five animals in each group.
*P<0.05 (significant), **P<0.01 (high significant) ***P<0.001 (very high significant), when all groups compared with control group. �£P<0.05 (significant),�£�£P<0.01 (high significant)
�£�£�£P<0.001 (very high significant), when all LD25+ chamomile and LD25+asparagus groups compared with LD25group.
ᴵP<0.05 (significant), ᴵᴵP<0.01 (high significant) ᴵᴵᴵP<0.001 (very high significant), when all LD50+ chamomile and LD50+asparagus groups compared with LD50group.
aP<0.05 (significant), bP<0.01 (high significant) cP<0.001 (very high significant), when 1/4LD50 and treated 1/4LD50 with chamomile and asparagus groups compared with their matched 1/2LD50 and treated 1/2LD50 with chamomile and asparagus groups.
Table 4: Serum tumour markers.
Molecular Results
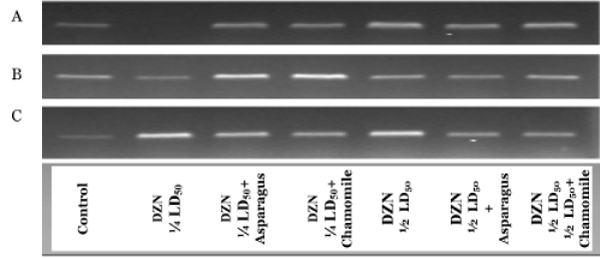
In Figure 1, complementary DNA was produced and amplified using RNA purified from the cerebrum (A), cerebellum (B) and spinal cord (C) tissues of rat groups administered with DZN and treated of these DZN groups with asparagus or chamomile. In all tissues, the differences in band density between DZN groups and other matched treated groups (orally administered with aqueous extracts of asparagus and chamomile) were observed after R T-PCR r eactions f or Cu-Zn SOD, but were not observed for cerebrum administered with ¼ LD50 of DZN. The expression of Cu-Zn SOD gene in the cerebellum in ¼ LD50+asparagus and the 1/2 LD50+chamomile group was differed but nearly the same pattern of expression of this gene in spinal cord tissues in different rat groups when matched altogether. These results indicated the activities of the key antioxidant enzyme (Cu-Zn SOD) were associated with the expressions of their respective gene.
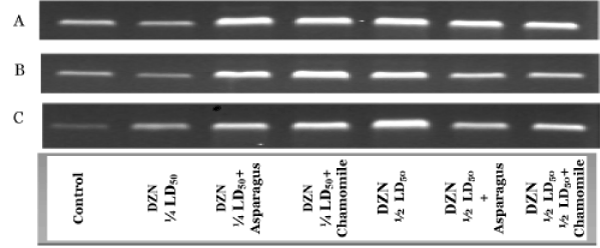
Figure 2 showed the RT-PCR product of GSH-Px gene in the cerebrum (A), cerebellum (B) and spinal cord (C) tissues in different animal groups. The differences in band density between DZN groups and other their matched treated groups (orally administered with aqueous extracts of asparagus and chamomile) were markedly observed after RT-PCR reactions for GSH-Px in all nervous tissues (Figure 2). The mRNA expressions of GSH-Px in all organs were significantly appeared in 1/2LD50 groups than in ¼ LD50 ones, and the co-administration with asparagus or chamomile compared with control (Figure 2A-C). These results specified the activities of the GSH-Px were associated with the expressions of their respective gene.
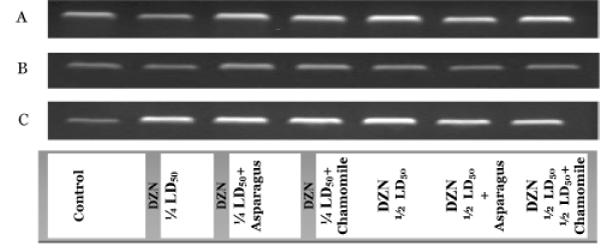
RT-PCR products of GST gene in the cerebrum (A), cerebellum (B) and spinal cord (C) tissues in different animal groups are shown in Figure 3. In cerebrum, cerebellum and spinal cord tissues, the differences in band density between DZN groups and other their matched treated groups (orally administered with aqueous extracts of asparagus and chamomile) were observed after RT-PCR reactions for GST. The mRNA expressions of GST in the cerebellum weren’t significantly appeared in all groups when compared with control (Figure 3B and C). These results indicated the activities of the important xenobiotic antioxidant enzyme (GST) were associated with the expressions of their respective gene.
Discussion
Diazinon is not only used in control of vegetables and fruits, but also it is used in ectoparasiticide formulations for sheep and cattle. Diazinon not only has toxic effects orally, but it also has toxic effects by a dermal way [41]. Diazinon is readily absorbed from the gastrointestinal tract and is rapidly metabolized within a few hours [42]. It is well known that DZN has neurotoxic effects by inhibiting and blocking cholinesterase activity in plasma, erythrocytes and brain [43,44]. The present study indicated that DZN administration induced oxidative stress, as verified by compromised antioxidant defences and increased lipid peroxidation in the different brain regions. MDA is a major oxidation product of peroxidized polyunsaturated fatty acids and increased MDA content is an important indicator of lipid peroxidation (LPO) [45]. Our results are in agreement with the studies of El-Shenawy et al. [31] who demonstrated a significant increase in LPO level of liver tissue by ¼ and 1/2 LD50 of DZN treatment. Organophosphorus compounds caused an increase of level LPO [46]. Moreover pesticide intoxication produced oxidative stress by the generation of free radicals and induced tissue LPO in mammals and other organisms [46,47]. Many insecticides are hydrophobic molecules, which bind extensively to biological membranes, especially to the phospholipids bilayers [48]. Ogutcu et al. [49] showed that DZN caused an increase of MDA level, the end product of lipid peroxidation. The increase of MDA level is an indicator of free radical formation of DZN in heart tissue of rats. So the neurotoxicity was documented here by the increase in LPO especially with the high dose of DZN. Antioxidants can reduce the damage of ROS to the human body. Therefore, intake of vegetables could significantly decrease the death rate of Cardio- and cerebrovascular diseases, immune dysfunction and cancer [50]. Upon of these, oral administration of aqueous extracts of asparagus and chamomile (300 mg/ kg b. wt.) able to alleviate the LPO in the investigated tissues due to their antioxidant activity.
Cellular enzymatic and non-enzymatic antioxidants such as SOD, GST, GSH-Px, GSH-R and GSH normally encounter the oxidative stress. The results of this study showed an increased in MDA concentration in cerebrum, cerebellum and spinal cord tissues, which may be related to the increased in ROS. SOD is the key enzyme that dismutates superoxide onto oxygen and hydrogen peroxide, such as SOD protects the biological systems from oxidative stress. The current study showed a significant increase in the activity of SOD in groups of rats administered with DZN. This increase could be attributed to an enhanced production of ROS during metabolism of DZN in the investigated tissues to moderate this exhibition. In phase 2 metabolism phenols, phenol dihydrodiols, quinones, and dihydrodiols are conjugated to form sulfates or glucuronides. Spontaneous formation of conjugates is possible as well. The resulting sulfates, glucuronides or protein adducts are detoxifying products of hazardous diazinon metabolites (DZNOxon). DNA adducts, however, can lead to mutation and the initiation of cancer cells if they are not repaired [51]. Since glutathione is required to maintain the normal reduced state of cells and to counteract all the deleterious effects of oxidative stress. Thus, GSH is involved in many cellular processes, including the detoxification of endogenous and xenobiotic compounds. The key enzyme that detoxifies the synthesized DZN-oxon is GST (phase II enzyme) that binds epoxides to glutathione and forms epoxide-GSH conjugates [52]. The decrease in the activity of GSH-Px in the investigated tissues in DZN groups could be due to the decrease of the reduced form of GSH. The decrease in the reduced form of GSH in cerebrum, cerebellum and spinal cord tissues after the exposure to DZN may be due to the inhibition of GSH-R activity. This reflected by a significant decrease in the total antioxidant capacity in the examined tissues. The administration of a watery extract of asparagus, and chamomile has the ability to recover the imbalance state in the redox state associated with DZN administration. The improvement in all changes was more prominent in all treated low doses of DZN groups when compared with high dose groups. The improvement action of watery extracts of asparagus and chamomile may be due to their antioxidant property [24,30].
It has been reported that the organophosphate pesticide dichlorvos induces blood–brain barrier dysfunction in mice but not in rats [53]. Diazinon, firstly, is detoxified in the liver and affects the mitochondria. The detoxification process of DZN converts them to a variety of reactive metabolites. Diazinon absorbed in the body is metabolized to DZNoxon through oxidative desulfuration by CYPs such as CYP1A2 and CYP3A2, and then DZN-oxon is hydrolyzed by A- and B-esterase such as paraoxonase and carboxyl esterase into diethylphosphate and 2-isopropyl-4-methyl-6-hydroxypyrimidine [52]. Moreover, paraoxonase and carboxyl esterase can mediate detoxification of DZN by hydrolysis, resulting in the production of diethylthiophosphate [54], which is mainly excreted in urine and bile [41]. Beside the main toxic action of DZN to inhibit acetylcholineesterase activity, it is established that some organophosphate inhibits different ATPases. Diazinon induced oxidative stress demonstrated by the decreased total antioxidant capacity and enhanced lipid peroxidation [55]. The decrease in total antioxidant capacity of all tissues in the DZN groups can lead reactive metabolites of DZN cruelty to attack the biomolecules such as DNA, protein and lipids. These DNA adducts can then, in case no reparation mechanisms start, cause tumorigenesis. This may explain the significant increase in arginase and alpha-l-fucosidase activity in DZN groups (Table 4). Oral administration with asparagus or chamomile has antitumor action as they decrease the level of tumor markers in DZN ¼ LD50 and 1/2 LD50 asparagus and chamomile when compared to their matched non-treated groups.
Diazinon induced neurotoxicity in cortical culture, which was independent from its effect on cholinesterase inhibition activity. This effect was inhibited by the caspase inhibitor. This suggests that DZN induce apoptotic neuronal death [56]. Oxidative stress is responsible for an increase in the accumulation of the ROS in cells, which may subsequently lead to an increase in the expression of genes encoding antioxidant enzymes. Variances in band density of amplicones of Cu- Zn SOD, GSH-Px and GST between rats in the control group and DZN groups, co-administration with asparagus or chamomile suggest that these enzymes may be differentially expressed in cells exposed to oxidative stress when compared with control ones. The DNA adducts may be the main reason of mutation of the investigated genes and then alter their expression. Moving to the end, the molecular changes in the antioxidant enzymes was reflected by the deflection of their activity in the nervous tissues.
Conclusions
Diazinon has a high toxic effect on the nervous system, especially in the cerebrum, cerebellum and spinal cord in rats. The neurotoxic effect of diazinon comes from their ability to initiate the oxidative stress by increasing the high levels of lipid peroxidation. Moreover, the inhibition of most enzymatic antioxidants and non-enzymatic antioxidants plays a vital role in the malfunction of nervous system. Diazinon has carcinogenic action as represented by the high levels of arginase and alpha-L-fucosidase in sera of rats. The administration of asparagus and chamomile extracts ameliorates the biochemical and molecular changesin Cu-Zn SOD, GSH-Px and GST associated with diazinon exposure. Asparagus and chamomile have antidote and antitumor action due to their antioxidants effect.
Acknowledgements
The authors are indebted to the work was supported by the Deanship of Scientific Research, King Khalid University [grant number, kku-sci-11/09].
References
- Bailey HC, Deanovic L, Reyes E, Kimball T, Larson K (2000) Diazinon and chlorpyrifos in urban waterways in northern California, USA. Environ. Toxicol. Chem 19: 82–87
- Davies DB, Holub BJ (1980) Toxicological evaluation of dietary diazinon in the rat. Arch Environ ContamToxicol 9: 637-650.
- Shah MD, Iqbal M (2010) Diazinon-induced oxidative stress and renal dysfunction in rats. Food ChemToxicol 48: 3345-3353.
- Neishabouri EZ, Hassan ZM, Azizi E, Ostad SN (2004) Evaluation of immunotoxicity induced by diazinon in C57bl/6 mice. Toxicology 196: 173-179.
- Rodrigo L, Hernández AF, López-Caballero JJ, Gil F, Pla A (2001) Immunohistochemical evidence for the expression and induction of paraoxonase in rat liver, kidney, lung and brain tissue. Implications for its physiological role. ChemBiol Interact 137: 123-137.
- Joshi SC, Mathur R, Gajraj A, Sharma T (2003) Influence of methyl parathion on reproductive parameters in male rats. Environ ToxicolPharmacol 14: 91-98.
- Hagar HH, Azza H, Fahmy (2002) A biochemical, histochemical, and ultrastructural evaluation of the effect of dimethoate intoxication on rat pancreas. ToxicolLett 133: 161-170.
- deBlaquière GE, Waters L, Blain PG, Williams FM (2000) Electrophysiological and biochemical effects of single and multiple doses of the organophosphate diazinon in the mouse. ToxicolApplPharmacol 166: 81-91.
- Timofeeva OA, Roegge CS, Theodore A, Slotkin TA and Levin ED (2008) Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon. Neurotoxicol. Terat. 30: 38-45
- Kappers WA, Edwards RJ, Murray S, Boobis AR (2001) Diazinon is activated by CYP2C19 in human liver. ToxicolApplPharmacol 177: 68-76.
- WHO (1998) Diazinon, environmental Health Criteria 198, International Program on Chemical Safety, United Nations Environmental Program, World Health Organization, Geneva, Switzerland.
- Nakagawa Y, Moore G(1999) Role of mitochondrial membrane permeability transition in p-hydroxybenzoate ester-induced cytotoxicity in rat hepatocytes. Biochemical Pharmacology 58: 811–816.
- Sams, C, Cocker, J and Lennard, MS (2003) Metabolism of chlorpyrifos and diazinon by human liver microsomes. Toxicology Letters 144: 146-153
- Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A (2004) Pesticides and oxidative stress: a review. Med SciMonit 10: RA141-147.
- Lee CH, Kamijima M, Kim H, Shibata E, Ueyama J, Suzuki T (2006) T: 8-Hydroxydeoxyguanosine levels in human leukocyte and urine according to exposure to organophosphorus pesticides and paraoxonase 1 genotype. Int Arch Occup Environ Health. 80: 217-27.
- Kale M, Rathore N, John S, Bhatnagar D (1999) Lipid peroxidative damage on pyrethroid exposure and alterations in antioxidant status in rat erythrocytes: a possible involvement of reactive oxygen species. ToxicolLett 105: 197-205.
- Prakasam A, Sethupathy S, Lalitha S (2001) Plasma and RBCs antioxidant status in occupational male pesticide sprayers. ClinChimActa 310: 107-112.
- Shah MD, Iqbal M (2010) Diazinon-induced oxidative stress and renal dysfunction in rats. Food ChemToxicol 48: 3345-3353.
- López O, Hernández AF, Rodrigo L, Gil F, Pena G, et al. (2007) Changes in antioxidant enzymes in humans with long-term exposure to pesticides. ToxicolLett 171: 146-153.
- Tsitsimpikou C, Tzatzarakis M, Fragkiadaki P, Kovatsi L, Stivaktakis P, et al. (2013) Histopathological lesions, oxidative stress and genotoxic effects in liver and kidneys following long term exposure of rabbits to diazinon and propoxur. Toxicology 307: 109-114.
- Jamshidi HR, Ghahremani MH, Ostad SN, Sharifzadeh M (2009) Effects of diazinon on the activity and gene expression of mitochondrial glutamate dehydrogenase from rat pancreatic Langerhans islets, Pest. Biochem. Physiol 93: 23-27
- Slotkin TA and Seidler FJ (2007) Comparative developmental neurotoxicity of organophosphates in vivo: transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res. Bull 72: 232-274
- Salaman I (1992) Chamomile: a medicinal plant. The Herb, Spice, and Medicinal Plant Digest 10: 1-4
- NewallCA, Anderson LA, Phillipson JD (1996) Herbal Medicines: A Guide for Health-Care Professionals. Pharmaceutical Press, London
- Martens D (1995) Chamomile: the herb and the remedy. The Journal of the Chiropractic Academy of Homeopathy 6: 15-18
- Tsushida T, Suzuki M and Kurogi M (1994) Evaluation of antioxidant activity of vegetable extracts and determination of some active compounds. J Jap Soc Food SciTechnol 41: 611-618
- Makris DP1, Rossiter JT (2001) Domestic processing of onion bulbs (Allium cepa) and asparagus spears (Asparagus officinalis): effect on flavonol content and antioxidant status. J Agric Food Chem 49: 3216-3222.
- Vinson, A. Hao, Y., Su, XH and Zubik L(1998) Phenol antioxidant quantity and quality in foods: vegetables. J Agricul Food Chem 46 : 3630-3634
- Rodríguez R, Jaramillo S, Rodríguez G, Espejo JA, Guillén R, et al. (2005) Antioxidant activity of ethanolic extracts from several asparagus cultivars. J Agric Food Chem 53: 5212-5217.
- Sun T, Powers JR and Tang J (2007) Evaluation of the antioxidant activity of asparagus, broccoli and their juices. Food Chemistry 105: 101-106
- El-Shenawy NS, El-Salmy F, Al-Eisa RA and El-Ahmary B (2010) Amelioratory effect of vitamin E on organophosphorus insecticide diazinon-induced oxidative stress in mice liver. Pesticide BiochemPhysiol 96: 101-107
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical biochemistry 95: 351-358
- beutler E, Duron O, Kelly Bm (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61: 882-888.
- Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazinemethosulfate and molecular oxygen. BiochemBiophys Res Commun 46: 849-854.
- Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70: 158-169.
- Goldberg DM, Spooner RJ (1983) In Methods of Enzymatic Analysis (Bergmeyen, H.V. Ed.) 3: 258-265
- Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J BiolChem 249: 7130-7139.
- Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V (2001) Method for the measurement of antioxidant activity in human fluids. J ClinPathol 54: 356-361.
- marshWh, Fingerhut B, Miller H (1965) Automated And Manual Direct Methods For The Determination Of Blood Urea. ClinChem 11: 624-627.
- Zietke K, Okada S and O’Brien JS (1972) Fucosidosis diagnosis by serum assay aL-fucosidase. J Lab Clinic Med 79: 1649– 1654
- Garfitt SJ, Jones K, Mason HJ, Cocker J: (2002) Exposure to the organo-phosphate diazinon: data from a human volunteer study with oral and dermal doses, Toxicol. Lett 134: 105–113
- FAO/WHO (1970) Evaluation of some pesticide residues in food, WHO Food Additive Series No. 42, World Health Organization, Geneva
- Tomokuni K, Hasegawa T (1985) Diazinon concentrations and blood cholinesterase activities in rats exposed to diazinon. ToxicolLett 25: 7-10.
- Neishabouri EZ, Hassan ZM, Azizi E, Ostad SN (2004) Evaluation of immunotoxicity induced by diazinon in C57bl/6 mice. Toxicology 196: 173-179.
- Kalender S, Ogutcu A, Uzunhisarcikli M, Açikgoz F, Durak D, et al. (2005) Diazinon-induced hepatotoxicity and protective effect of vitamin E on some biochemical indices and ultrastructural changes. Toxicology 211: 197-206.
- Hazarika A, Sarkar SN, Hajare S, Kataria M, Malik JK (2003) Influence of malathion pretreatment on the toxicity of anilofos in male rats: a biochemical interaction study. Toxicology 185: 1-8.
- Oruç EO, Uner N (2000) Combined effects of 2,4-D and azinphosmethyl on antioxidant enzymes and lipid peroxidation in liver of Oreochromisniloticus. CompBiochemPhysiol C ToxicolPharmacol 127: 291-296.
- Lee A, East J, Balgaug P (1991) Interactions of insecticides with biological membranes, Pestic.Sci 32: 317–327
- Ogutcu A, Suludere Z, Kalender Y (2008) Dichlorvos-induced hepatotoxicity in rats and the protective effects of vitamins C and E. Environ ToxicolPharmacol 26: 355-361.
- Verlangieri AJ, Kapeghian JC, el-Dean S, Bush M (1985) Fruit and vegetable consumption and cardiovascular mortality. Med Hypotheses 16: 7-15.
- AngererJ, Mannschreck C, Gündel J (1997) Biological monitoring and biochemical effect monitoring of exposure to polycyclic aromatic hydrocarbons. Int Arch Occup Environ Health 70: 365-377.
- Ueyama J, Wang D, Kondo T, Saito I, Takagi K, et al. (2007) Toxicity of diazinon and its metabolites increases in diabetic rats. See comment in PubMed Commons below ToxicolLett 170: 229-237.
- Sinha C, Shukla GS (2003) Species variation in pesticide-induced blood-brain barrier dysfunction. Hum ExpToxicol 22: 647-652.
- Sams C, Cocker J, Lennard MS (2004) Biotransformation of chlorpyrifos and diazinon by human liver microsomes and recombinant human cytochrome P450s (CYP). Xenobiotica 34: 861-873.
- Amirkabirian N, Teimouri F, Esmaily H, Mohammadirad A, Aliahmadi A, et al. (2007) Protection by pentoxifylline of diazinon-induced toxic stress in rat liver and muscle. ToxicolMech Methods 17: 215-221.
- Rush T, Liu XQ, Hjelmhaug J, Lobner D (2010) Mechanisms of chlorpyrifos and diazinon induced neurotoxicity in cortical culture. Neuroscience 166: 899-906.
- Michiels C, Raes M, Toussaint O, Remacle J (1994) Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free RadicBiol Med 17: 235-248.
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 14597
- [From(publication date):
February-2015 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 10180
- PDF downloads : 4417