Review Article Open Access
The Role of Mast Cells and Neuroglia in Neuroinfectious Diseases
| Azize Yasemin Goksu Erol* | |
| Department of Histology and Embryology, Faculty of Medicine, Akdeniz University, Antalya, Turkey | |
| Corresponding Author : | Azize Yasemin Goksu Erol Department of Histology and Embryology Faculty of Medicine, Akdeniz University Antalya, Turkey Tel: 90-242- 3101575 Fax: 90-242-3106629 E-mail: yasemin.goksu@gmail.com |
| Received August 29, 2015; Accepted October 20, 2015; Published October 20, 2015 | |
| Citation: Erol AYG (2015) The Role of Mast Cells and Neuroglia in Neuroinfectious Diseases. J Neuroinfect Dis 6:190. doi:10.4172/2314-7326.1000190 | |
| Copyright: © 2015 Yasemin A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
The central nervous system may be infected by a wide range of microorganisms. However, immune response in the central nervous system is too limited and delayed, and penetration of antimicrobials from blood-brain barrier is also limited. As a result, morbidity and mortality rates of these infectious diseases are still high despite advanced antimicrobial therapies. Many of these infections are life threatening, and an important percentage of individuals who survive may experience severe and permanent neurological deficit. In the last decades, various experimental studies have provided some insight into the molecular and cellular basis of the human neuroinfectious diseases, including the host response to pathogens, pathobiology of neuroinflammation, and mast cell–microglia crosstalk. In this content, mast cells are multifunctional cells which are known as central players in classic IgE-associated allergic disorders. Actually, they have a critical and protective role in host defense against parasites, bacteria, fungi and viruses in the context of both innate and adaptive immune responses through releasing a wide range of pro-inflammatory and immunoregulatory molecules and their characteristic surface receptors for cytokines and chemokines. In this content, mast cells have an important role in protection against infections of the nervous sytem, as well. Recent studies show that mast cells form the major link between neurons and inflammation via neuropeptides. Namely, neuropeptides may induce human mast cell degranulation and chemokine production. This review will provide an overview of the innate and adaptive immune responses, neuroinflammation, the roles of neuroglia and mast cells, and mast cell-glia crosstalk. Development of novel therapies targeting neuroinflammation and an agent which is efficacious in acute and neurogenic inflammation called N-palmitoylethanolamine will also be discussed.
| Keywords |
| CNS; Infection; Mast cell; Neuroglia; Neuroinflammation |
| Introduction |
| The central nervous system (CNS) may be infected by a wide range of microorganisms, including bacteria, viruses, fungi and parasites. Although advanced antimicrobial therapies have been developed, these infectious diseases still remain a major cause of morbidity and mortality, and prompt medical attention/intervention is needed. Many of these infections are life threatening and many of the individuals who survive experience severe and permanent neurological deficit. The main factors accounting for the devastating effects of these infections include 1- limited and delayed immune response in the CNS which is attributable to the local deficiency in complement system and immunoglobulins, 2- limited penetration of many antimicrobial agents from blood–CNS barrier, and 3- the unique characteristic of the CNS anatomy [1]. |
| Furthermore, when infectious pathogenic organisms enter the nervous system, they may cause a wide range of clinical syndroms, such as progressive multifocal leukoencephalopathy (PML), chronic meningitis, herpes simplex encephalitis, West Nile virus neurologic infections, Creutzfeldt–Jakob disease, neurosarcoidosis, AIDS–related viral infections of the nervous system, etc. The host response to pathogens differs among individuals depending on the individual’s current immune status and efficiency of immune responses. For instance, polyomavirus (JC virus), the etiological agent of PML, infects 50% or more of the adult population throughout the world, however, only a small percentage of infected individuals become complicated with PML, an extraordinarily rare complication of this infection in otherwise normal persons. Although the majority of PML cases occur in severely immunesuppressed individuals, PML has been increasingly diagnosed in patients treated with biological therapies such as monoclonal antibodies (mAbs) that modulate immune system functions [2]. |
| In recent years, researchers have focused on understanding the pathophysiology of neuronal injury associated with neuroinfectious diseases in order to develop new diagnostic markers and therapeutic targets. In this content, various experimental studies have provided some insight into the molecular and cellular basis of the human neuroinfectious diseases, including the host response to pathogens, pathobiology of neuroinflammation, the roles of glial and mast cells (MCs), and mast cell–microglia crosstalk. In the last decades, interactions between nervous system and immune system are also of great interest by many researchers. The investigation of mast cell–glia communication has provided new perspectives for the development of novel therapies targeting neuroinflammation. One of the suggestions as being the basis of novel therapies is modulating activation of non-neuronal cells that normally control neuronal sensitization [3,4]. |
| Furthermore, an important member of the immune system is the mast cell which mainly express critical effector functions in allergy and anaphylaxis. MCs have also been recognized as crucial effectors in both innate and adaptive immune responses. Studies using MC-deficient mice have shown that MCs protect the host against bacteria, fungi, protozoa, and even viruses through releasing proinflammatory and chemotactic mediators. Indeed, a variety of antimicrobial activities of MCs have been reported in the literature in the last decades [5]. |
| In response to bacterial infection, MCs increase the recruitment of neutrophils and aid in bacterial clearance. Interestingly, they are capable of phagocytosis, a vital component of the innate immune system, which results in bacterial cell death with lysis of the dead organisms. On the other hand, MCs can modulate the adaptive immune responses and are capable of antigen presentation [6]. |
| In addition, MCs are both sensors and effectors in communication among nervous, vascular, and immune systems. MCs form the major link between neurons and inflammation via neuropeptides. Neuropeptides activate human mast cell degranulation and chemokine production. In this content, the connection between MCs and MC mediators may lead to a better understanding of the neuro-immune-endocrine systems in physiology and pathology. Moreover, widening the knowledge of cytokine network will help us to understand how immune system cells influence the central nervous system [7]. |
| Innate and adaptive immunity |
| The host response to pathogens has two components, the innate and adaptive responses. The innate system includes anatomical defenses which restrict pathogen invasion, the complement system and myeloid cells that first sense and attack to pathogens. Neutrophils, monocytes, basophils, eosinophils and MCs are cells of myeloid lineage and together with natural killer cells, they are involved in innate immune responses. These cells detect and respond to pathogens within the bloodstream. On the other hand, tissue macrophages which are also of myeloid origin residing within tissues, may be the first to encounter pathogens. These cells release chemotactic signals as a determinant of pathogen activity in that tissue and promote the transition of circulating leukocytes into the area of infected tissue. In fact, innate immunity, by itself, may be insufficient to protect a host against an invading pathogen or to prevent disease from occurring. However, if innate immunity fails, the organism may be detected and attacked by the mechanisms of adaptive immunity. |
| On the other hand, the adaptive immune responses involve lymphocytes which target to destroy pathogens and their toxic molecules. It is crucial that they attack only in response to molecules that are foreign to the host and not to the molecules of the host itself. The ability to distinguish ‘foreign’ from ‘self’ in this way is a fundamental feature of the adaptive immune system. There are two classes of such responses: 1- antibody responses which are carried out by B lymphocytes, 2- cell-mediated immune responses which involve T cells. In antibody responses, B lymphocytes are activated to secrete antibodies, called immunoglobulins. The antibodies circulate in the bloodstream and permeate the other body fluids, where they bind specifically to the foreign antigen that stimulated their production. Antibody binding inactivates viruses and microbial toxins (i.e. tetanus toxin) by blocking their ability to bind to receptors on host cells. Binding of antibody also marks invading pathogens for destruction, primarily by making it easier for phagocytic cells of the innate immune system to ingest them. |
| Indeed, the innate and adaptive immune responses both function to protect the body against invading organisms, but they differ in a number of ways: (1) The innate immune system is constitutively present and reacts immediately to infection, whereas the adaptive immune response takes some time to develop. (2) The innate immune system is not specific in its response, however, the adaptive immune system is antigen-specific and reacts only with the organism that induced the response. (3) The adaptive immune system exhibits immunological memory, remembers that it has encountered an antigen, and reacts more rapidly on subsequent exposure to the same organism, whereas the innate immune system does not possess a memory [8-11]. |
| Neuroinflammation |
| Infection and injury induce a physiologic response called inflammation in order to remove detrimental stimuli and initiate tissue healing. In the central nervous system, this process is termed neuroinflammation. In acute neuroinflammation, microglia become activated and phagocytose dying cells and release pro-inflammatory cytokines, thus they prevent the dissemination of the area of injury [4,12,13]. |
| However, prolonged neuroinflammation causes deleterious effects through activating pro-inflammatory cytokines which results in increased oxidative stress, and neuronal death. The cytokines released by necrotic neurons induce further activation of microglia and astrocytes, resulting in positive feedback loops working independently in which the original pro-inflammatory molecules are no longer needed. |
| Neuroinflammation is a common mechanism influencing the severity, progression, and complications of neuroinfectious diseases, and is an important candidate target for neuroprotective therapies. For instance, sustained neuroinflammation is implicated in HIV-associated neurocognitive disorder [14,15]. Neuroinflammation is also a key feature in other nervous system pathologies such as neurodegenerative diseases [16], stroke, spinal cord injury [17], chronic pain, and neuropsychiatric disorders such as anxiety, depression and schizophrenia. Furthermore, inflammation in the periphery of the body may impact on central nervous system behaviors, such as cognitive performance. |
| On the other hand, neuroinflammation may also increase the sensitivity of the brain to stress, thus effecting stress-related neuropsychiatric disorders. And vice versa is also true: namely, stress may stimulate inflammation. Corticotropin-releasing factor (CRF), which is secreted from the hypothalamus under stress, together with neurotensin (NT), can stimulate brain MCs to release inflammatory and neurotoxic mediators that disrupt the blood-brain barrier (BBB), stimulate microglia and cause focal inflammation. As a result, brain MCs may be involved in the pathogenesis of “brain fog,” headaches, and autism spectrum disorders (ASDs), which worsen with stress [18,19]. |
| Mast cells |
| MCs were first described by Paul Ehrlich in 1878 on the basis of their staining characteristics and large cytoplasmic granules, and have long been recognized as key cells of type I immunoglobulin E (IgE)- associated hypersensitivity reactions. Although MCs share similarities with basophil granulocytes in blood, contrasting with basophils, MCs circulate in blood in an immature form as committed precursors until they migrate to a tissue site to settle and complete their differentiation in tissues [10,20]. |
| MCs are present in most tissues in the vicinity of blood vessels, particularly near surfaces exposed to the environment. Thereby, they are distributed in almost all organs and vascularized tissues. They are present in the the gastrointestinal tract, skin, and the respiratory tracts, where they are in close contact with the outside environment. MCs are also normal resident cells of the hair follicles, peritoneum, synovium, and many other organs. |
| Although they have long been implicated in the pathogenesis of allergic diseases and inflammatory disorders, and well-studied with their protective responses to parasites, their functional role has been found to be more complex than recognized. MCs play key functional roles in distinct nonimmunological activities, such as wound healing following injury, tissue homeostasis or remodeling, fibrosis, carcinogenesis, and angiogenesis. MCs are found to be critical protagonists in host defence against microorganisms [5,20-24]. |
| Indeed, MCs are effector cells of the innate immune system and are involved in various cell-mediated immune reactions and in infectious diseases as a component of the host reaction to bacteria, parasite, fungi, and even virus infections. This is accomplished through the secretion of cytokines and other soluble mediators. In response to bacterial infection, MCs were found to increase the recruitment of neutrophils and aid in bacterial clearance. Recent studies have shown that MCs can modulate the host’s innate immune response to gram negative bacteria by contributing to the process of phagocytosis through the release of pro-inflammatory mediators and presentation of bacterial antigens to T cells [25,26]. MCs have been shown to phagocytose and kill enterobacteria, and are capable of processing bacterial antigens for presentation through class I MHC molecules to T cell hybridomas after phagocytic uptake of live bacteria [27]. |
| MCs have been found to have anti-viral effects, as well. Histamine, protease enzymes and leukotrienes are important mast cell mediators for effective host responses. The cytokines and chemokines produced by MCs in response to pathogens alter the nature of the innate immune response and its effectiveness in eliminating infection. Moreover, vasoactive amines released by MCs in the central nervous system have been proposed to play a facilitating role in the development of the inflammatory response to Sindbis virus [28]. |
| MCs produce a wide range of mediators, including biogenic amines, cytokines enzymes, growth factors [nerve growth factor (NGF)], lipid metabolites, ATP, neuropeptides, nitric oxide, and heparin. There are two different types of mediator release; one is via degranulation which is a very rapid process, the other is the release of de novo formed mediators which results in a longer lasting activation [4,29,30]. |
| Mast cells in the nervous system |
| As mentioned earlier, MCs are found at host-environment interfaces and in many vascularized tissues in the body [31]. They are also resident in the brain and are localized to the diencephalon and hippocampus and surrounding leptomeninges [32-35]. MCs are found in peripheral tissues innervated by small calibre sensory nerve fibres and within the endoneurial compartment of peripheral nerves, and cerebral blood vessels. During development they enter the brain by way of penetrating blood vessels, with which they remain associated [36]. |
| Specifically, many MCs are found on the brain side of blood vessels adjacent to astrocytic and neuronal processes [35]. MCs are also found on the brain side of the blood-brain barrier (BBB) [37,38]. About 97% of MCs reside on the abluminal (brain) side of the blood vessels, thus, they are able to communicate with neurons, astrocytes, microglia, extracellular matrix, and blood vessels [3]. |
| Under baseline conditions, in the absence of stress, disease, or trauma, the numbers of MCs are considerably smaller than that of neurons, microglia, and other brain-resident cells. Despite their small numbers, activated MCs can impact neurons, BBB, microglia, and astrocytes [3]. |
| Neuroglia |
| In the host response to pathogens in central nervous system, glial cells are of great importance. Central nervous tissue contains very little extracellular material and consists of a huge number of neurones and their processes embedded in a mass of supportive cells, known as neuroglia. Neuroglia which are highly branched cells occupy the spaces between neurones and comprise all the non-neural cells of the CNS. The neuroglia have intimate functional relationships with neurones and they provide both mechanical and metabolic support. Four main types of neuroglia are recognised: astrocytes, microglia, oligodendrocytes, and ependymal cells [11,39]. |
| Microglia are small cells, derived from cells of mesenchymal origin which invade the CNS at a late stage of fetal development. In response to tissue damage, microglia transform into large amoeboid phagocytic cells and are thus considered to be the CNS representatives of the macrophage- monocyte defence system. Microglia act as sensors for disturbed brain tissue homeostasis and accumulate locally in response to the invasion of a foreign material in the brain or neuronal injury [3,12]. |
| In addition to being the support cells of the CNS, astrocytes are recognized as active players in the regulation of neural repair, synaptic function, and CNS immunity [14,39,40]. |
| Astrocytes respond to CNS trauma and infection through a complex combination of molecular and cellular events. Astrocytes react to CNS disturbances with enhanced intermediate filament expression, progressive cellular hypertrophy and proliferation [41,42]. |
| Following infection, astrocytes secrete cytokines and chemokines, such as CXC motif ligand 10 (CXCL10), Chemokine ligand 2 (CCL2), interleukin-6 (IL-6), and BAFF, which influence both innate and adaptive immune responses [43]. These responses elicit local CNS immune responses through inflammatory mediators and induce recruitment of additional effector cells of the immune system from the peripheral circulation. |
| Mast cell- glia ├?┬▒nteraction |
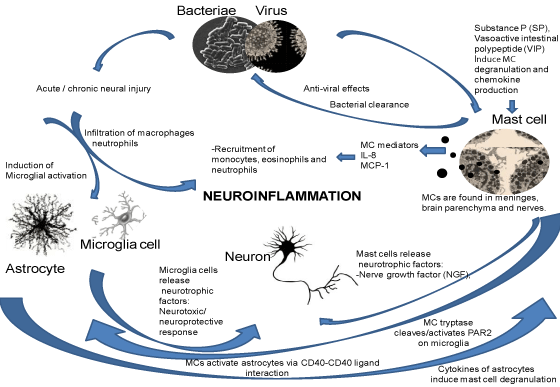
| Pro-inflammatory signals released from other non-neuronal cells of immune origin can induce glial cells. In this context, MCs are of particular relevance. Potential interactions between MCs and microglia are based on several molecular mechanisms determined by in vitro experiments [4,44]. For example, activation of P2 receptors on microglia by ATP stimulates the release of IL-33, which binds to mast cell receptors. This event triggers the release of Il-6, IL-13, and monocyte chemoattractant protein 1, which in turn affect microglial activity. Similarly, mast cell tryptase activates microglial protease activated receptor-2 (PAR2), facilitating the release of pro-inflammatory mediators TNF-a, IL-6, both of which subsequently upregulate the expression of PAR2 receptors on microglia (Figure 1). In this content, PAR-2 contributes to neuroprotection and/or neurodegeneration in the brain under pathological conditions. Therefore, PAR-2 has been suggested to be a novel therapeutic target for the treatment of brain disorders. |
| Moreover, ATP, released from damaged cells is a potent stimulus for microglia in vitro. ATP may act as an autocrine or a paracrine factor for MCs, thus ATP released from one mast cell can provide a rise in Ca2+ in the neighbouring cells [4,45-47]. |
| Besides microglia, MCs also have a dynamic relationship with astrocytes. MCs and astrocytes are co-localized in the perivasculature and thalamus [48]. In vitro studies show that astrocytes can be activated by MCs via activation of CD40-CD40 ligand interactions, and MCs are also stimulated to release histamine, leukotrienes, and cytokines [49]. Astrocytes also have histamine receptors (H1R and H2R) [50], and cytokines released from astrocytes can induce mast cell degranulation [3,43]. |
| N-palmitoylethanolamine |
| MCs and glial cells are known to possess endogenous homeostatic molecules, such as N-acylethanolamines. These molecules can be up regulated by the induction of tissue damage or stimulated by inflammatory responses. N-palmitoylethanolamine (PEA) is a member of N-acylethanolamines with a key role of maintaining cellular homeostasis when provoked by external stressors such as inflammation. PEA is produced and hydrolyzed by microglia. It downmodulates mast cell activation and, it increases in glutamate-treated neocortical neurons ex vivo and in injured cortex. |
| When applied exogenously, PEA has been found to be efficacious in mast cell-mediated experimental models of acute and neurogenic inflammation. It has shown that this fatty acid amide has also neuroprotective effects, proved by a model of spinal cord trauma and a delayed post-glutamate paradigm of excitotoxic death. It was also useful against amyloid β-peptide-induced learning and memory impairment in mice. Thus, inhibiting or modulating the enzymatic breakdown of PEA represents a complementary therapeutic approach to treat neuroinflammation. |
| Conclusion |
| Exploring mast cell-mast cell mediator connection and mast cellglia cell interactions may lead to a better understanding of the neuroimmune- endocrine system functions in both physiologic and pathologic conditions. Understanding the pathophysiology of neuronal injury and the immune response associated with neuroinfectious diseases may further direct us to new therapeutic approaches. |
| Moreover, targeting endogenous regulators of neuroinflammation may provide a strategy for treating a wide range of nervous system disorders. |
References
- Croteau D (2014) Neuroinfectious diseases. Best Practices in Neurological Care: 126-143.
- White MK, Khalili K (2011)Pathogenesis of progressive multifocal leukoencephalopathy—revisited. J Infect Dis 203:578-586.
- Dong H, Zhang X, Qian Y (2014) Mast cells and neuroinflammation. Med Sci Monit Basic Res20: 200-206.
- Skaper SD,Facci L, Giusti P (2013) Mast cells, glia and neuroinflammation: partners in crime?Immunology141: 314-327.
- Göksu AY, Özdemir Ö (2007) Growing Importance And Newly Defined Roles Of Mast Cells In Microbiology. Turkiye Klinikleri Journal of Medical Sciences 27: 577-588.
- Ribatti D, Crivellato E (2009) The Controversial Role of Mast Cells in Tumor Growth. Int Rev Cell Mol Biol 275: 89-131.
- Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP (2007) Neuropeptides activate human mast cell degranulation and chemokine production. Immunology.123: 398-410.
- Alberts B, Johnson A, Lewis J, et al. (2002) General Principles of Cell Communication, Molecular Biology of the Cell. (4thedn),Garland Science, New york, USA.
- http://www.textbookofbacteriology.net/
- Stevens A, Lowe JS, Young B (2002) Wheater's basic histopathology, a colour atlas and text. (4thedn), Edinburgh, Toronto, Canada.
- Bruce R, Kettenmann H (2012) Neuroglia. (3rdedn), Oxford University Press, USA.
- Graeber MB, Li W, Rodriguez ML (2011) Role of microglia in CNS inflammation. Febs Lett 585:3798-3805.
- David S, Kroner A (2011) Repertoire of microglial and macrophage responses after spinalcord injury. Nat Rev Neurosci 12:388-399.
- Kim Mai Lee and Andrew G MacLean (2015) New advances on glial activation in health and disease. World J Virol4: 42-55.
- McArthur JC, Steiner J, Sacktor N, Nath A (2010) Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol 67:699-714.
- Mayo L, Trauger SA, Blain M, Nadeau M, Patel B, et al. (2014) Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat Med 20:1147-1156.
- Hulsebosch CE (2008) Gliopathy ensures persistent inflammation and chronic pain after spinal cord injury. Exp Neurol 214:6-9.
- Theoharides TC, Stewart JM, Panagiotidou S, Melamed I (2015) Mast cells, brain inflammation and autism. Eur J Pharmacol S0014-2999: 398-402
- Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ (2005) Identification of mastcell progenitors in adult mice. Proc Natl Acad Sci USA 102:11408-11413.
- Goksu Erol AY, Tokyol C, Ozdemir O, Yilmazer M, Arioz TD, et al. (2011) The role of mast cells and angiogenesis in benign and malignant neoplasms of the uterus.Pathol Res Pract 207: 618-622.
- Göksu Erol AY, Uzunköy A, Özdemir Ö (2010) New roles of mast cells in postoperative wound healing and adhesion formation. Journal of surgical arts 2: 1-10.
- Goksu Erol AY, Aktepe F (2013) Angiogenesis: Insights from a Systematic Overview, Mast Cells and Angiogenesis in Tumoral and Non-Tumoral Disease. Nova Science Publishers, Hauppauge NY, USA.
- Malaviya R, Ikeda T, Ross E, Abraham SN (1996) Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 381: 77-80.
- Malaviya R, Abraham SN (2001) Mast cell modulation of immune responses to bacteria. Immunol Rev 179: 16-24.
- Malaviya R, Twesten NJ, Ross EA, Abraham SN, Pfeifer JD (1996) Mast cells process bacterial Ags through a phagocytic route for class I MHC presentation to Tcells. J Immunol 156: 1490-1496.
- Mokhtarian F, Griffin DE (1984) The role of mast cells in virus-induced inflammation in the murine central nervous system. Cell Immunol 86: 491-500.
- Leslie M. (2007) Mast cells show their might. Science 317: 614-616.
- Gilfillan AM, Austin SJ, Metcalfe DD (2011) Mast cell biology: introduction and overview. Adv Exp Med Biol 716: 2-12
- Metcalfe DD, Baram D, Mekori YA (1997) Mast cells. Physiol Rev 77: 1033-1079.
- Hough LB (1988) Cellular localization and possible functions for brain histamine: recent progress. Prog Neurobiol 30: 469-505.
- Kiernan JA (1976) A comparative survey of the mast cells of the mammalian brain. J Anat 121: 303-311.
- Silver R, Silverman AJ, Vitkovic L, Lederhendler II (1996)Mast cells in the brain: evidence and functional significance. Trends Neurosci 19: 25-31.
- Silverman AJ, Sutherland AK, Wilhelm M, Silver R(2000)Mast cells migrate from blood to brain. J Neurosci 20: 401-408.
- Lambracht-Hall M, Dimitriadou V, Theoharides TC (1990) Migration of mast cells in the developing rat brain. Dev Brain Res 56:151-159.
- Khalil M, Ronda J, Weintraub M, Jain K, Silver R, et al. (2007) Brain mast cell relationship to neurovasculature during development. Brain Res 1171: 18-29.
- Florenzano F, Bentivoglio M (2000) Degranulation, density, and distribution of mast cells in the rat thalamus: a light and electron microscopic study in basal conditions and after intracerebroventricular administration of nerve growth factor. J Comp Neurol424: 651-669.
- Farina C, Aloisi F, Meinl E (2007) Astrocytes are active players in cerebral innate immunity. Trends Immunol 28: 138-145.
- Ransom B, Behar T, Nedergaard M (2003) New roles for astrocytes (stars at last) Trends Neurosci 26: 520-522.
- Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci32: 638-647.
- Gallo V, Deneen B (2014) Glial development: the crossroads of regeneration and repair in the CNS. Neuron83:283-308.
- Dong Y, Benveniste EN (2001) Immune function of astrocytes. Glia36:180-190.
- Skaper SD, Giusti P, Facci L (2012) Microglia and mast cells: two tracks on the road to neuroinflammation. FASEB J 26: 3103-3117.
- Osipchuk Y, Cahalan M (1992) Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature 359:241-245.
- Zhang S, Zeng X, Yang H, Hu G, He S (2012) Mast cell tryptase induces microglia activation via protease-activated receptor 2 signaling. Cell. Physiol. Biochem. 29: 931-940.
- Zhang H, Yang H, He S (2010) TNF increases expression of IL-4 and PARs in mast cells. Cell Physiol Biochem 26: 327-336
- Kim DY, Jeoung D, Ro JY (2010) Signaling pathways in the activation of mast cells cocultured with astrocytes and colocalization of both cells in experimental allergic encephalomyelitis. J Immunol 185: 273-283.
- Kim DY, Hong GU, Ro JY (2011) Signal pathways in astrocytes activated by cross-talk between of astrocytes and mast cells through CD40-CD40L. J Neuroinflammation 8: 25
- Hosli L, Hosli E, Schneider U, Wiget W (1984) Evidence for the existence of histamine H1- and H2-receptors on astrocytes of cultured rat central nervous system. Neurosci Lett 48: 287-291
- Kaper SD, Facci L, Giusti P (2013) Glia and mast cells as targets for palmitoylethanolamide, an anti-inflammatory and neuroprotective lipid mediator. Mol Neurobiol 48: 340-352.
- Kaper SD, Facci L, Barbierato M, Zusso M, Bruschetta G, et al. (2015) N-Palmitoylethanolamine and Neuroinflammation: a Novel Therapeutic Strategy of Resolution. Mol Neurobiol 52: 1034-1042.
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 12024
- [From(publication date):
December-2015 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10956
- PDF downloads : 1068
