The Role of IL-17 Imbalance in Promoting the Pyroptosis in ImmuneMediated Liver Injury through STAT3-IFI16 Axis
Received: 13-Jul-2023 / Manuscript No. DPO-23-105946 / Editor assigned: 17-Jul-2023 / PreQC No. DPO-23-105946 (PQ) / Reviewed: 31-Jul-2023 / QC No. DPO-23-105946 / Revised: 07-Aug-2023 / Manuscript No. DPO-23-105946 / Accepted Date: 07-Aug-2023 / Published Date: 14-Aug-2023 DOI: 10.4172/2476-2024.8.S13.006 QI No. / DPO-23-105946
Abstract
Autoimmune Hepatitis (AIH), characterized with excessive production of proinflammatory cytokines and progressive damage of hepatocytes, is an inflammatory disease of unclear pathogenesis. AIH affects all age group and occurs mainly in women. Pyroptosis is a novel programmed cell death featured with a distinct morphology associated with cell bursting and release of proinflammatory cytokines. A deeper understanding of the pathogenesis of AIH and its related pyroptosis will contribute to the development of novel therapy for AIH patients. We aimed to investigate the role of Interleukin 17 (IL-17) in immune-mediated liver injury caused by Concanavalin A-induced AIH based on integrated study using primary mouse hepatocytes and BALB/c mouse model. The levels of cytokines were measured by ELISA, and RT-qPCR was conducted to detect the mRNA expression of STAT3 and IFI16. The protein expression of STAT3 and IFI16 was measured by western blotting. Immunohistochemical staining and transmission electron microscopy were applied to evaluate the liver histopathological changes of the mice under different treatments. The primary mouse hepatocytes modeling demonstrated that the levels of IFI16, IL-1b, IL-18, LDH and STAT3 were significantly increased in hepatocytes treated with IL-17, and further elevated followed by STAT3-Overexpressed (STAT3-OE) lentivirus treatment relative to the controls (p<0.01). Importantly, cell pyroptosis was observed in hepatocytes treated with IL-17, and severe cell damage was observed after STAT3-OE lentivirus treatment. In addition, a binding interaction between IFI16 and STAT3 was detected in IL-17 treated hepatocytes. In the BALB/c mouse modeling, glutathione transaminase activity was enhanced in ConA-induced AIH mice compared to that in control group (p<0.01). Aggravated liver damage in mice treated with STAT3-OE lentivirus was observed. However, liver damage was alleviated in mice treated with anti-IL-17 neutralizing antibody or STAT3-knockdown lentivirus. Hence, IL-17 plays an important role in activating STAT3 and up-regulating IFI16, which may promote the pyroptosis in the progression of AIH-related liver injury through STAT3-IFI16 axis. Therapeutic potential of targeting IL-17-STAT3-IFI16 pathway for AIH patients deserve to be further investigated.
Keywords: Autoimmune hepatitis; IL-17; Pyroptosis; IL-17-STAT3- IFI16 axis; Liver injury
Abbreviations
IL-17: Interleukin 17; AIH: Autoimmune Hepatitis; ConA: Concanavalin A; IFI16: Interferon Gamma-Inducible Protein 16; IL-1β: Interleukin-1β; IL-18: Interleukin-18; STAT3: Signal Transducer and Activator of Transcription 3; LDH: Lactate Dehydrogenase; TEM: Transmission Electron Microscopy; H&E: Hematoxylin and Eosin; TNF-a: Tumor Necrosis Factor-Alpha; IFN-g: Interferon- gamma; Th17: T-helper; NLRP3: NOD-Like Receptor Protein 3; RT- qPCR: Real-Time quantitative Polymerase Chain Reaction; PVDF: Polyvinylidene Difluoride; HRP: Horseradish Peroxidase; ECL: Enhanced Chemiluminescence; ELISA: Enzyme-Linked Immune Sorbent Assay.
Introduction
Autoimmune Hepatitis (AIH) occurs mainly in women and contributes to various liver diseases [1-4]. AIH is an inflammation disease due to the loss of immunological tolerance to hepatocytes, which promotes the progression of liver damage and excessive production of cytokines [5-7]. It is evaluated by serum transaminases levels, presence of autoantibodies, and evidence of interface hepatitis [8-11]. Study on the pathogenesis of AIH may help advance novel intervention for AIH patients. Proinflammatory cytokines such as Interleukin-17 (IL- 17), Interleukin-1b (IL-1b), Interleukin-18 (IL-18), Tumor Necrosis Factor-Alpha (TNF-a), and Interferon-Gamma (IFN-g) are key factors associated with AIH [12-18]. T-helper 17 (Th17) cells mainly secrete IL-17 and play an important function in the host defense against infection and autoimmunity [19]. IL-17, involving in stimulating hepatic inflammation and immune cell infiltration, acts as a regulator facilitating T-cell activation and inflammatory responses [20-21]. It accelerates the production and release of proinflammatory cytokines, and promotes the activation of Signal Transducer and Activator of Transcription 3 (STAT3) pathway [22]. Although IL-17/Th17 pathway plays an important role in regulating immune responses in AIH development, the mechanism of AIH progression still remains unclear. Several studies have reported that elevated plasma/serum levels of IL- 17 were detected in AIH patients relative to healthy controls [23-24]. A deeper understanding the role of IL-17 in AIH pathogenesis will have significant impact on developing novel therapies for AIH patients.
Pyroptosis is a programmed inflammatory cell death, resulting in release of intracellular pro-inflammatory cytokines and activation of a strong inflammatory response [24-25]. It was found that IL-17 induces pyroptosis in osteoblasts through NOD-Like Receptor Protein 3 (NLRP3) inflammasome pathway [26]. Other studies showed that STAT3 and Interferon Gamma-Inducible protein 16 (IFI16) are involved in the initiation of the pathogenesis of autoimmune diseases such as sclerosis and lupus [27-29]. IL-17 may contribute to the cell pyroptosis in AIH through promoting the binding interaction of activated STAT3 with IFI16, and the formation of inflammasome complexes. Although aberrant activation of STAT3 has been reported in autoimmune diseases [29], the mechanism of IL-17-STATS-IFI16 axis in AIH is unclear. In present work, we developed a comprehensive study on the function of IL-17 in immune-mediated hepatocyte pyroptosis in AIH using the primary hepatocytes model and BALB/c mouse model. Our findings suggest that IL-17 plays a pivotal role in the progression of pyroptosis in AIH through IL-17-STAT3-IFI16 axis by activating STAT3 and up- regulating IFI16. Therapeutic potential of targeting IL-17-STAT3-IFI16 pathway for AIH patients deserve to be further studied.
Materials and Methods
Animals
Male BALB/c mice were purchased from Shanghai SLAC Laboratory Animal Co. Ltd (License Key: SCXK 2017-0005; Certificate No: 20170005049224, Shanghai, China) and bred in our facility. Mice were housed in temperature-controlled environment (22°C-25°C) with a 12 h light-dark cycle and ad libitum access to food and water, following the standards set by the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the Affiliated Hospital of Shaoxing University (protocol ID. 20-10-06 SU).
Mice aged 7 weeks to 9 weeks old were used in this study. Littermate WT mice were used as a control for the ConA-induced AIH experiment. Mice were randomly divided into seven groups for different treatments (Table 1). There were 6 mice in each group, and each mouse was treated through tail vein injection except for the lentivirus infection injected intraperitoneally. Mice were sacrificed at indicated time and blood from orbital sinus was collected. Serum was separated by centrifuging at 3,000 rpm for 10 min at 4 and used for the detection of liver function (glutathione transaminase activity) and cytokine levels.
| Primary Mouse Hepatocytes | Mouse | ||
|---|---|---|---|
| Group | Characteristics | Group | Characteristics |
| Control | no treatment | Control (n=6) | no treatment |
| IL-17 | IL-17 | Model (n=6) | ConA |
| NC | IL-17 | IL-17 (n=6) | anti IL-17 neutralizing antibody |
| ConA | |||
| STAT3-OE | IL-17 | NC (n=6) | ConA |
| STAT3-OE lentivirus | |||
| si-NC | IL-17 | si-NC (n=6) | scrambled shRNA lentivirus |
| scrambled shRNA lentivirus | ConA | ||
| si-STAT3 | IL-17 | STAT3-OE (n=6) | STAT3-OE lentivirus |
| si-STAT3 lentivirus | ConA | ||
| - | - | si-STAT3 (n=6) | si-STAT3 lentivirus |
| ConA | |||
Table 1: Background characteristics of the study groups.
Model or NC: Mice were challenged with 15 mg/kg ConA (C2010, Sigma-Aldrich, St. Louis, MO, USA) to establish AIH.
Control: Mice were received an equal amount of 0.9% normal saline (IN9000, Solarbio Life Sciences, Beijing, China).
IL-17: Mice were administrated with anti-IL-17 neutralizing antibody (1 mg/kg) 30 min before injected with 15 mg/kg ConA.
STAT3-OE: Lentivirus with a short hairpin RNA (shRNA, Santa Cruz Biotechnology, Inc. Santa Cruz, CA, USA) up-regulated expression of STAT3 was given at 108 virus per mouse every day for 7 days before the mouse was injected with 15 mg/kg ConA.
si-STAT3: Lentivirus with a shRNA down-regulated expression of STAT3 was given at 108 virus per mouse every day for 7 days before the mouse was injected with 15 mg/kg ConA.
si-NC: Lentivirus with a negative scrambled shRNA lentivirus unregulated expression of STAT3 was given at 108 virus per mouse every day for 7 days before the mouse was injected with 15 mg/kg ConA.
Primary mouse hepatocytes
Primary mouse hepatocytes were purchased from Liver Biotechnology Co. Ltd (Cat# LV-PMH002, Shenzhen, China). Hepatocyte cells were divided into six groups for different treatments (Table 1). Each treatment was conducted in triplicate in 150 mm tissue culture dish (Cat# FB0875714, ThermoFisher Scientific, MA, USA). Cells were cultured in RPMI-1640 media (Cat# 11875093, ThermoFisher Scientific, MA, USA) supplemented with 10% fetal bovine serum (FBS), 100 mg/mL streptomycin and 100 IU/mL penicillin in a humidified incubator with 5% CO2 at 37°C.
Control: Cells were cultured as described above.
IL-17: Cells were treated with 100 ng/mL of IL-17 recombinant protein (Cat# P01621, Solarbio Life Sciences, Beijing, China) for 24 hours.
NC: Cells were treated with 100 ng/mL of IL-17 recombinant protein for 24 hours.
STAT3-OE: Cells were treated with 100 ng/mL of IL-17 recombinant protein for 24 hours, followed by treatment with lentivirus up-regulated STAT3 for 48 hours.
si-STAT3: Cells were treated with 100 ng/mL of IL-17 recombinant protein for 24 hours, followed by treatment with lentivirus down- regulated STAT3 for 48 hours.
si-NC: Cells were treated with 100 ng/mL of IL-17 recombinant protein for 24 hours, followed by treatment with lentivirus unregulated STAT3 for 48 hours.
mRNA expression assay
Total RNA was extracted with Trizol reagent (Cat# 15596018, Invitrogen, CA, USA) following the manufacturer’s instructions. A NanoDrop ND-2000 instrument (Thermo Fisher Scientific, USA) was used to evaluate the quality of the RNA samples. 2 mg of total RNA was used in reverse transcription with Prime Script RT Reagent Kit (Cat# R222-01, Vazyme Biotech, Nanjing, China) according to the manufacturer’s protocol.
Real-time quantitative PCR (RT-qPCR)
Real-Time quantitative Polymerase Chain Reaction (RT-qPCR) was conducted using IO-RAD CFX Connect Real-Time System (Applied Biosystems, CA, USA) with ChamQ SYBR Color qPCR Master Mix (Cat# Q411-02, Vazyme Biotech, Nanjing, China). The Primers were purchased from Sunny Biotech (Shanghai, China) as listed in Table 2. The mRNA expression levels of target genes were calculated by the comparative Cycle Threshold (CT) method (2-ΔΔCT) using ACTB as internal control according to the formula: 2-ΔΔCT (STAT3/or IFI16: DCt=mRNACt-ACTBCt, DDCT=DCt-average control DCt).
| Name | Primer sequence (5’-3’) | PCR product size |
|---|---|---|
| Actin (F) | AGAGGGAAATCGTGCGTGAC | 189 bp |
| Actin (R) | CCAAGAAGGAAGGCTGGAAA | |
| STAT3 (F) | GACATTCCCAAGGAGGAGGC | 89 bp |
| STAT3 (R) | TACGGGGCAGCACTACCT | |
| IFI16 (F) | GACAACCAAGAGCAATACACCA | 86 bp |
| IFI16 (R) | ATCAGTTTGCCCAATCCAGAAT |
Table 2: Primers used in this study.
Western blot
The cell culture media were removed and hepatocyte cells were lysed with radio-immunoprecipitation assay (RIPA) buffer (Cat# 89900, ThermoFisher Scientific, MA, USA) supplemented with protease inhibitor cocktail (Cat# 78440, ThermoFisher Scientific, MA, USA). The detached cells were re-suspended in 500 ml of ice-cold RIPA buffer and transferred into a microcentrifuge tube. After rotation for 20 min at 40 , lysates were centrifuged at 20,000 g for 20 min at 40 to °C °C remove cell debris. The cleared supernatants were transferred into fresh tubes and protein concentrations were determined using BCA Protein Assay kit (Cat# 23227, Thermo Fisher Scientific, Waltham, MA, USA). An aliquot of 20 mg of protein was mixed with an equivalent volume of loading buffer (pH 7.4, containing b-mercaptoethanol) and boiled for 5 min before separated using the sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE, 8%-10% polyacrylamide). After electrophoresis, proteins were transferred onto Polyvinylidene Difluoride (PVDF) membranes (Millipore, Billerica, MA, USA) and blotted against primary antibodies at 4℃ overnight. Membranes were washed three times with TPBS (0.05% Tween 20 in PBS, pH 7.4) and incubated with 5% nonfat milk to block the nonspecific binding sites. Antibodies against STAT3 (Cat# GTX104616, 1:1000) was purchase from GeneTex (CA, USA), and IFI16 (Cat# ab169788, 1:1000) and internal control GAPDH (Cat# ab181602, 1:5000) were purchase from Abcam (MA, USA). After washing three times with TPBS, the secondary antibody (Cat# ab150077, 1:5000, Abcam, MA, USA) conjugated with Horseradish Peroxidase (HRP) was incubated with the membranes at room temperature for 1 h and washed five times with 1 × TBST for 5 min each time. Protein bands were revealed by the Enhanced Chemiluminescence (ECL) imaging system (SageCreation Science, Beijing, China) with SuperSignalTM West Femto Maximum Sensitivity Substrate (Thermo Scientific, USA).
Dual-luciferase reporter assay
The binding interaction between STAT3 and IFI16 were analyzed by dual-luciferase reporter assay. The plasmid of pcDNA3.1, STAT3- pcDNA3.1, Wt-IFI16-pGL3 and Mut-IFI16-pGL3 were transfected with ExFect 2000 reagent (Cat# T202-03, Vazyme, Nanjing, China). Each transfected group contained four multiple pores, and dual luciferase reporter assay kit (Cat# E1910, Promega, Madison, WI, USA) was used to detect the luciferase activity after transfection for 48 hours in GloMax 20/20 Luminometer (E5311, Promega, Madison, WI, USA) following the manufacturer’s instructions. Luciferase values were normalized to those of Renilla luciferase.
Detection of cytokines
Concentrations of IFI16, IL-17, IL-1β, and IL-18 in tissue or serum from mice were measured using Enzyme-Linked Immune Sorbent Assay (ELISA) kits (Jianglai Bio, Shanghai, China) following the manufacturer’s instructions. Each sample was tested in triplicate using SpectraMax Plus 384 system (Molecular Devices, San Jose, CA, USA).
Analysis of Lactate Dehydrogenase (LDH)
The cell culture media were transferred into microcentrifuge tubes, and centrifuged at 3,000 g for 15 min at 40 to remove cell debris. The cleared media were transferred into fresh tubes. Lactate Dehydrogenase (LDH) levels in media were measured using the LDH assay kit (Cat# BC0685, Solarbio, Beijing, China) following the manufacturer’s instructions. Each sample was tested in triplicate.
Assay of glutathione transaminase activity
Mice were anesthetized after ConA-induced AIH for 8 hours. Blood from orbital sinus was collected into microcentrifuge tubes and kept at room temperature for 30 min, followed by standing on ice for 1 hour. Serum was separated at 3,000 g for 10 min at 40 and used for the measurement of glutathione transaminase activities using Hitachi 7600 Automatic Analyzer.
Histopathology assessments
Liver tissues from mice were fixed in 4% paraformaldehyde fixative solution. The tissue sections were stained with Hematoxylin and Eosin (H&E) and observed under a light microscopy. Liver injury was evaluated based on the inflammation of the portal area, lobule and fusion necrosis. The degree of hepatic pathological damage was scored using a double blind method.
Score-0: No pathological change.
Score-1: Hepatocyte edema, inflammatory cell infiltration, spotty necrosis, no focal necrosis area.
Score-2: Increased inflammatory cell infiltration, visible focal necrosis area.
Score-3: Massive focal necrosis area, disorder of the liver tissue structure.
Transmission Electron Microscopy (TEM)
Liver tissue (<1 mm3) were seeded in 6-well plates and sequentially treated with 2.5% glutaraldehyde, 1% osmium tetroxide, and increasing gradient of ethanol and acetone. The tissue was fixed, dehydrated and embedded with Spurr’s resin. After slicing in the Electron Microscope (EM) UC6 ultramicrotome (Leica Microsystems, Wetzlar, Germany), the samples were adhered to uncoated copper grids and stained with 4% uranyl acetate. Then, the stained samples were observed under the transmission electron microscopy (TECNAI 10, Philips, Netherlands).
Statistical analysis
Statistical analyses were performed using the statistical package GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, United States). All data were displayed as mean ± Standard Deviation (SD) for at least three independent experiments per each group. Statistical comparisons were made using one-way ANOVA or Student’s t-test. A P value<0.05 was considered statistically significant.
Results
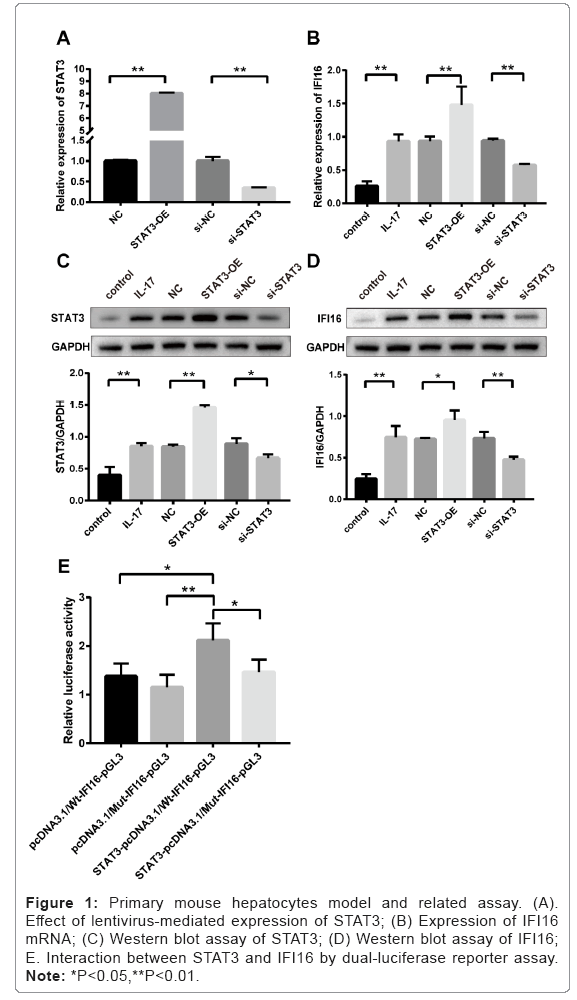
Effect of lentivirus-regulated STAT3 in mouse primary hepatocytes
Expression level of STAT3 was significantly up-regulated in cells treated with STAT3-OE lentivirus compared to that in NC group (p<0.01, STAT3-OE versus NC), and down-regulated in cells treated with si-STAT3 lentivirus compared to that in si-NC group (p<0.01, si-STAT3 versus si-NC). As shown in Figure 1A, the results indicated that the effectiveness of lentivirus-regulated STAT3 in mouse primary hepatocyte
Increased mRNA levels of IFI16 in mouse primary hepatocytes treated with IL-17 and STAT3-OE lentivirus
As shown in Figure 1B, the mRNA level of IFI16 was significantly increased in mouse primary hepatocytes treated with IL-17 compared to that in Control group without IL-17 treatment (p<0.01, IL-17 versus Control). The expression of IFI16 mRNA was further elevated in hepatocytes treated with STAT3-OE lentivirus, compared to that in NC group without lentivirus treatment (p<0.01, STAT3-OE versus NC). In contrast, the mRNA level of IFI16 was significantly reduced in hepatocytes treated with si-STAT3 lentivirus compared to that in si- NC group treated with scrambled shRNA lentivirus (p<0.01, si-STAT3 versus si-NC).
Binding interaction between STAT3 and IFI16
Dual-luciferase reporter assay showed that the ratio of luc/Rluc from the co-transfected Wt-IFI16 plasmid and STAT3-pcDNA3.1 plasmid is the highest among the other three paired groups (Figure 1E), and the relative luciferase activity is statistically significant difference between STAT3-pcDNA3.1/Wt-IFI16-pGL3 and the other three paired groups.
Figure 1: Primary mouse hepatocytes model and related assay. (A). Effect of lentivirus-mediated expression of STAT3; (B) Expression of IFI16 mRNA; (C) Western blot assay of STAT3; (D) Western blot assay of IFI16; E. Interaction between STAT3 and IFI16 by dual-luciferase reporter assay. Note: *P<0.05,**P<0.01.
p<0.05; STAT3-pcDNA3.1/Wt-IFI16-pGL3 versus pcDNA3.1/ Wt-IFI16-pGL3; p<0.01: STAT3-pcDNA3.1/Wt-IFI16-pGL3 versus pcDNA3.1/Mut-IFI16-pGL3; p<0.05: STAT3-pcDNA3.1/Wt-IFI16- pGL3 versus STAT3-pcDNA3.1/Mut-IFI16-pGL3.
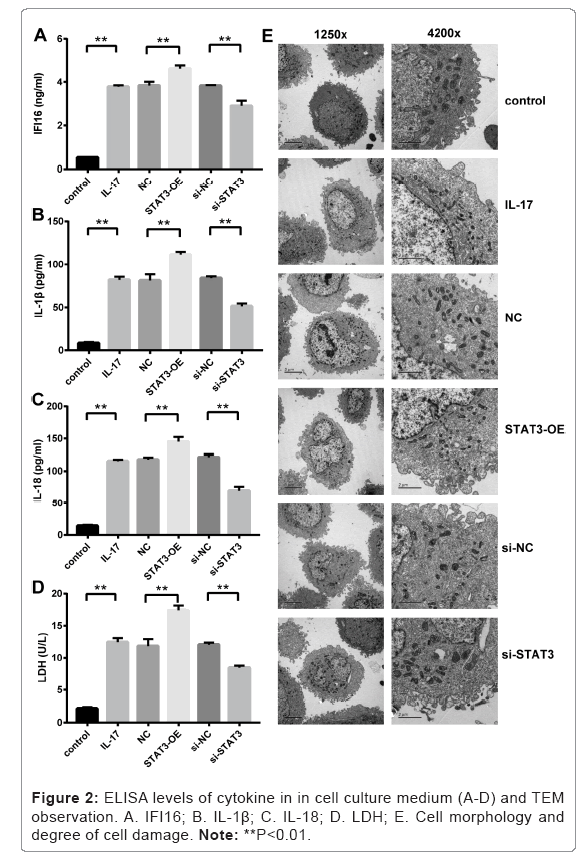
Increased levels of cytokines in mouse primary hepatocytes treated with IL-17 and STAT3-OE lentivirus
ELISA assay of cytokines in culture medium showed that IFI16, IL- 1β and IL-18 were significantly increased in IL-17 treated hepatocytes compared to that in Control group without IL-17 treatment as shown in Figures 2A-2C (p<0.01, IL-17 versus Control). The levels of those cytokines were further elevated in hepatocytes treated with STAT3-OE lentivirus compared to that in NC group without lentivirus treatment (p<0.01, STAT3-OE versus NC). In contrast, the expression levels of those cytokines were significantly decreased after the hepatocytes were treated with si-STAT3 lentivirus compared to that in si-NC group treated with scrambled shRNA lentivirus (p<0.01, si-STAT3 versus si- NC).
Increased levels of LDH in mouse primary hepatocytes treated with IL-17 and STAT3-OE lentivirus
The levels of LDH in culture medium were significantly increased in IL-17 treated hepatocytes compared to that in Control group without IL-17 treatment as shown in Figure 2D (p<0.01, IL-17 versus Control).
The levels of LDH were further elevated in hepatocytes treated with STAT3-OE lentivirus compared to that in NC group without lentivirus treatment (p<0.01, STAT3-OE versus NC). In contrast, the levels of LDH were significantly reduced after the hepatocytes were treated with si-STAT3 lentivirus compared to that in si-NC group treated with scrambled shRNA lentivirus (p<0.01, si-STAT3 versus si-NC).
Observed pyroptosis in mouse primary hepatocytes treated with IL-17 and STAT3-OE lentivirus
The changes of cell morphology and degrees of cell damage in different conditions were observed by TEM (Figure 2E). Compared to the Control group, the IL-17 treated hepatocytes appeared swelling, increased protrusion and vesicles, and deformed organelles. Severe cell damage occurred in hepatocytes treated with STAT3-OE lentivirus (i.e. STAT3-OE group) compared to that in NC group without STAT3-OE lentivirus treatment. The degrees of cell damage became alleviated in hepatocytes treated with si-STAT3 lentivirus (i.e. si-STAT3 group) compared to that in si-NC group treated with scrambled shRNA lentivirus.
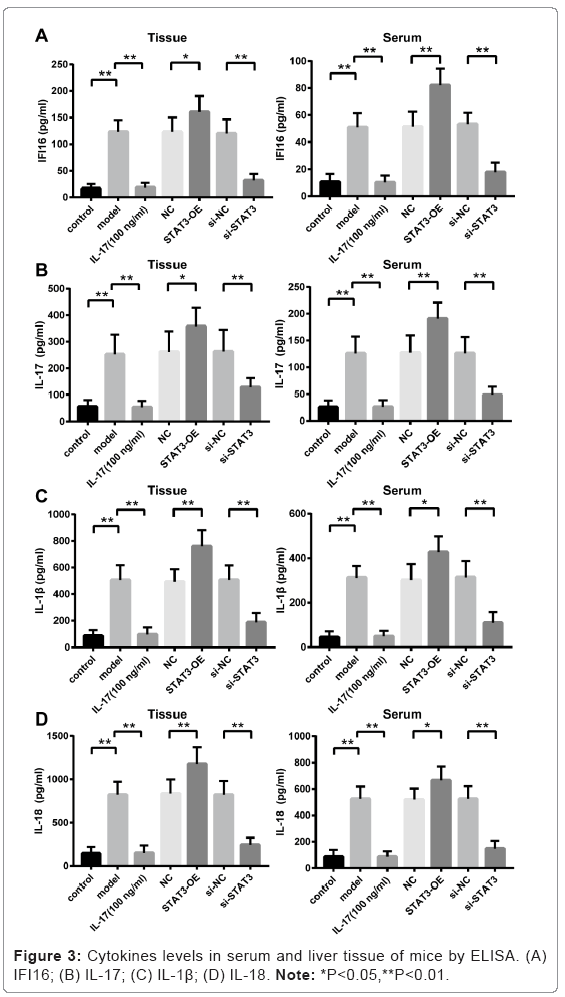
Elevated levels of cytokines in serum and tissue from mice treated with ConA and STAT3-OE lentivirus
The levels of cytokines (IFI16, IL-17, IL-1β and IL-18) in both serum and tissue were significantly increased in ConA-induced AIH in mice (i.e. the Model group) compared to that in Control group without ConA treatment (p<0.01, Model versus Control) as shown in Figures 3A-3D.
The levels of cytokines were further elevated in mice injected with STAT3-OE lentivirus compared to that in NC group (p<0.01, STAT3- OE versus NC). However, the levels of cytokines were significantly reduced in mice injected with si-STAT3 lentivirus compared to that in si-NC group injected with scrambled shRNA lentivirus (p<0.01, si- STAT3 versus si-NC). Furthermore, the ConA-induced liver damage was alleviated and levels of cytokines were remarkable reduced in mice administrated with anti-IL-17 neutralizing antibody (p<0.01, IL-17 versus Model).
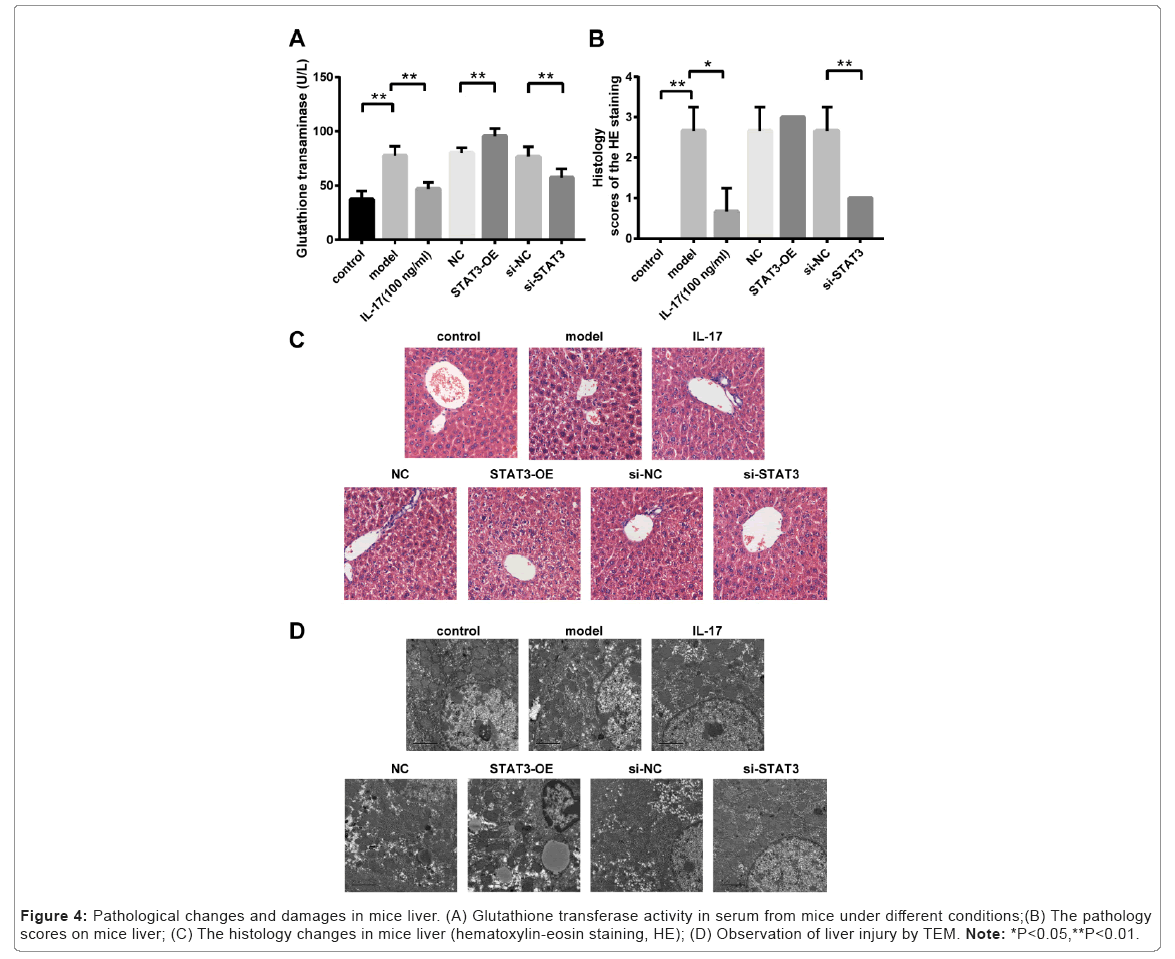
Increased glutathione transaminase activity in mice treated with ConA and STAT3-OE lentivirus
Glutathione transaminase activities in serum were significantly increased in ConA-induced AIH mice (i.e. the Model group) compared to that in Control group without ConA treatment (p<0.01, Model versus Control) as shown in Figure 4A. There was no significant difference in glutathione transaminase activity among Model group, NC group and si-NC group (Figure 4A). However, glutathione transaminase activities were significantly increased in mice treated with STAT3-OE lentivirus compared to that in NC group (p<0.01, STAT3-OE versus NC). However, glutathione transaminase activities were significantly reduced in mice treated with si-STAT3 lentivirus compared to that in si-NC group treated with scrambled shRNA lentivirus (p<0.01, si- STAT3 versus si-NC). In addition, glutathione transaminase activities were significantly decreased in mice administrated with anti-IL-17 neutralizing antibody compared to that in Model group (p < 0.01, IL-17 versus Model)..
Figure 4: Pathological changes and damages in mice liver. (A) Glutathione transferase activity in serum from mice under different conditions;(B) The pathology scores on mice liver; (C) The histology changes in mice liver (hematoxylin-eosin staining, HE); (D) Observation of liver injury by TEM. Note: *P<0.05,**P<0.01.
Histopathological changes in liver from mice treated with ConA, STAT3-OE lentivirus and anti-IL-17 neutralizing antibody
The liver pathology scores and histology changes were observed as shown in Figures 4B and 4C. The pathology scores were significantly increased in ConA induced AIH mice (i.e. the Model group) compared to that in Control group without ConA treatment (p<0.01, Model versus Control). There was no significant difference in pathology scores among Model group, NC group and si-NC group. Although the pathology scores were additionally increased in mice treated with STAT3-OE lentivirus compared to that in NC group, there was no significant difference between these two groups (i.e. STAT3-OE versus NC). The pathology scores were significantly decreased in mice treated with si-STAT3 lentivirus compared to that in si-NC group injected with scrambled shRNA lentivirus (p<0.01, si-STAT3 versus si-NC). Furthermore, elevated pathology scores caused by ConA induced liver injury was greatly alleviated in mice administrated with anti-IL-17 neutralizing antibody compared to that in Model group (p<0.01, IL-17 versus Model).
Liver injury in mice treated with ConA, STAT3-OE lentivirus and anti-IL-17 neutralizing antibody
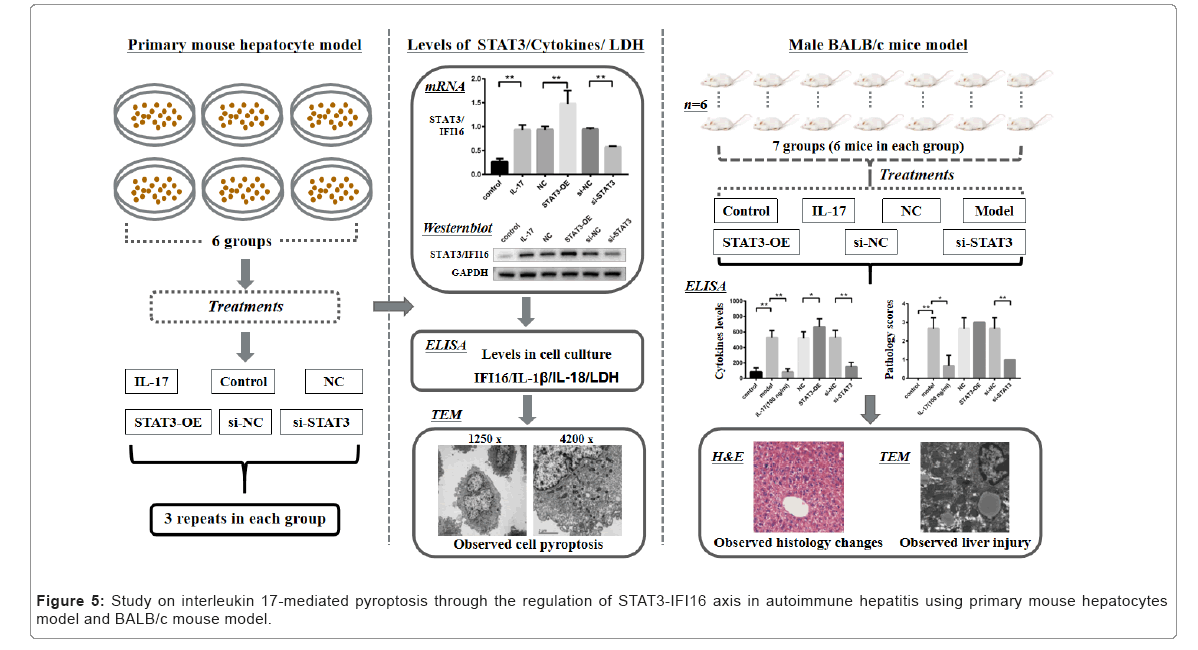
TEM analysis showed that cytoplasmic looseness of hepatocytes, cystic dilatation of rough endoplasmic reticulum, and shedding of partial ribosomes from the surface of nuclear membrane appeared in the hepatic tissue from ConA induced AIH mice (i.e. the Model group) compared to that in Control group (Figure 4D). In addition, the degree of pathological damage of hepatocytes was aggravated in mice treated with STAT3-OE lentivirus (i.e. the STAT3-OE group). However, severity of pathological damage in liver was markedly reduced in mice treated with si-STAT3 lentivirus (i.e. the si-STAT3 group) or anti IL-17 neutralizing antibody (i.e. the IL-17 group) (Figure 5).
Discussion
IL-17 mediated immune response has been demonstrated to play a critical role in immune-mediated liver injury. It stimulates the activation of T lymphocytes and induces the differentiation of Th17 cells, resulting in immune cell infiltration, hepatic inflammation, and autoimmune hepatic disease [19-21]. IL-17 promotes the release of proinflammatory cytokines such as IL-1b, IL-18, TNF-a and IFN-g, and consequently activates the STAT3 pathway. STAT3 may interact with IFI16, which promoted the initiation of inflammation and linked to the pathogenesis of AIH. However, the role of IL-17 in AIH is still unclear, and there is a lack of comprehensive study on the mechanism of IL-17-STAT3- IFI16 axis in AIH. In the present study, we investigated the impact of IL-17-STAT3-IFI16 pathway on the pathogenesis of AIH using the IL- 17 treated primary mouse hepatocytes and ConA-induced AIH mice, respectively. AIH caused pathological damages with apparent pyroptosis morphology from the primary mouse hepatocytes and mice liver were observed. Cell pyroptosis, known as cell inflammatory necrosis, is a novel discovered programmed inflammatory cell death with a distinct cell morphology changes featured by constant cell expansion, membrane rupture, and release of proinflammatory cytokines. Various inflammatory cytokines released during pyroptosis might contribute to the activation of immunoresponse in infectious diseases such as AIH. It was found that the degree of liver injury can be alleviated, and the production of inflammation cytokines can be reduced by inhibiting the occurrence of IL-17 induced pyroptosis in inflammatory hepatic disease [25]. Our study indicated that the levels of pyroptosis-related cytokines including IL-1β and IL-18 were significantly changed in primary mouse hepatocytes, mice liver and serum after either adding IL-17 recombinant protein to the cell culture or injecting anti IL-17 neutralizing antibody to the mice. The TEM results showed that IL- 17 promoted cell swelling with increased protrusion and vesicles, and deformed organelles. In addition, the histopathological damage of hepatocytes was aggravated in mice challenged with ConA compared to that in mice treated with anti IL-17 neutralizing antibody before challenged with ConA. This further confirmed our finding that IL-17 may play an important role in promoting the pathological changes in AIH-related pyroptosis of hepatocytes.
The formation of inflammasomes is a key feature during pyroptosis, and IFI16 is an important component of inflammasomes. IFI16 is involved in progression of inflammation processes. Several other studies has reported that the abnormal expression of IFI16 was found in autoimmune diseases [26-27]. IL-17, IL-18, IL-1b and TNF-a may up-regulate the expression of IFI16, however the exact mechanism still remains unclear. Based on the preliminary results from dual-luciferase reporter assay, we found that there was certain binding interaction between STAT3 and IFI16 promoter region and it may help to further explored the underlining mechanism of IFI16 in promoting AIH- related pyroptosis. However, the interaction between STAT3 and IFI16 needs to be further investigated through the CO-IP and GST-pull down experiments in future study. STAT3, as an important transcription factor, plays a critical role in autoimmune disease. Abnormal activation of STAT3 pathway has been confirmed in AIH [22]. The results from our multiple assays indicated that the expression of IFI16 was increased in primary mouse hepatocytes treated with IL-17 recombinant protein, and was further elevated in hepatocytes treated with STAT3-OE lentivirus, whereas the expression of IFI16 was decrease in hepatocytes treated with si-STAT3 lentivirus. In addition, the cell morphology, levels of pyroptosis related effectors (IL-1β, IL-18, and LDH) were significantly changed in cells treated with either STAT3-OE lentivirus or si-STAT3 lentivirus. Our results revealed that IL-17 may promote AIH-related pyroptosis through the STAT3-regulated expression of IFI16.
We employed the ConA-induced AIH mice for further evaluating the function of IL-17-STAT3-IFI16 axis in AIH-related pyroptosis. Our results showed that the expression levels of inflammatory cytokines (IFI16, IL-1β, IL-18 and IL-17) were significantly elevated, and the activity of serum glutathione transaminase was also significantly enhanced in ConA-induced AIH mice compared to that in Control group. Consistently, the pathological injury was observed in liver tissue by H&E and TEM assays. We found that mice in STAT3-OE group showed even more aggravated liver damage compared to the mice in si-NC group, together with higher levels of inflammatory cytokines and increased activity of serum glutathione transaminase. Thus, our results indicated a promoted role of STAT3 on AIH-associated hepatocyte pyroptosis, and this was further illustrated in mice from si-STAT3 group with alleviated liver injury and reduced inflammatory cytokines and transferase activity. In addition, anti IL-17 neutralizing antibody significantly reduced hepatocyte pyroptosis and alleviated histopathological changes in ConA-induced AIH in mice. Our data suggest that IL-17 played a critical role in IL-17-STAT3-IFI16 pathway, and contributed to the pathogenesis of hepatocyte pyroptosis in AIH disease. Other researchers have also reported that the inflammation- related hepatic injury can be alleviated by inhibiting IL-17 [21]. In this light, IL-17 can be a potential therapeutic target for AIH disease.
Conclusion
Our study found that AIH-related pyroptosis was triggered by IL- 17 mediated STAT3 activation and transcriptional regulation of IFI16. Thus, a better understanding of the IL-17-STAT3-IFI16 axis underlined the autoimmune attack to the liver cells in AIH-related pyroptosis may suggest new therapeutic strategies for AIH patients. In addition, our preliminary results have demonstrated that the histological hepatic damages and serum aminotransferase levels were dramatically decreased in AIH mice administrated with anti IL-17 neutralizing antibody. Thus, the development of novel treatments that target the IL-17-STAT3-IFI16 axis deserves to be further investigated. It is thus that IL-17-STAT3-IFI16 pathway may be the potential therapeutic target for AIH disease. In summary, our study revealed that IL-17 may play a key role in promoting the hepatocyte pyroptosis in AIH through regulating IFI16 by activated STAT3. The development of novel interventions targeting the IL-17-STAT3-IFI16 axis that worth to be further investigated
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
Not applicable.
Contributions
All authors contributed to the study conception and design. Material preparation, experiments, data collection and analyses were performed by YW, CJ, WZ, JC, XC, and JG. WX, XC, WZ and YW contributed to reagents or analytic tools. The first draft of the manuscript was written by WX and HW. All authors read and approved the final manuscript.
Corresponding Author
Correspondence to Wenfang Xu and Hong Wang.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A (2022). Cancer statistics, 2022. CA Cancer J Clin 72:7-33.
[Crossref] [Google scholar] [PubMed]
- Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, et al. (2021) Hepatocellular carcinoma. Nat Rev Dis Primers 7:6.
[Crossref] [Google scholar] [PubMed]
- Fujiwara N, Friedman SL, Goossens N, Hoshida Y (2018). Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol 68:526-549.
[Crossref] [Google scholar] [PubMed]
- Sharma R, Verna EC, Simon TG, Söderling J, Hagström H, et al. (2022) Cancer risk in patients with autoimmune hepatitis: A nationwide population-based cohort study with histopathology. Am J Epidemiol 191:298-319.
[Crossref] [Google scholar] [PubMed]
- Doherty DG (2016). Immunity, tolerance and autoimmunity in the liver: A comprehensive review. J Autoimmun 66:60-75.
[Crossref] [Google scholar] [PubMed]
- Horst AK, Neumann K, Diehl L, Tiegs G (2016). Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol Immunol 13:277-292.
[Crossref] [Google scholar] [PubMed]
- Dos Santos IP, de Assunção MT, Mauch RM, Sandy NS, Nolasco da Silva MT, et al. (2022) Patients with treated autoimmune hepatitis and persistent suppression of plasmacytoid dendritic cells: A different point of view. Int J Immunopathol Pharmacol 36:1-8.
[Crossref] [Google scholar] [PubMed]
- Primer NRD (2018). Autoimmune hepatitis. Nat Rev Dis Primers 4:18018.
[Crossref] [Google scholar] [PubMed]
- Rigopoulou EI, Dalekos GN (2021). Current trends and characteristics of hepatocellular carcinoma in patients with autoimmune liver diseases. Cancers 13:102.
[Crossref] [Google scholar] [PubMed]
- Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, et al. (2020) Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the Study of Liver Diseases. Hepatology 72:671-722.
[Crossref] [Google scholar] [PubMed]
- European Association for the Study of the Liver (2015). EASL Clinical Practice Guidelines: autoimmune hepatitis. J Hepatol 63:971-1004.
[Crossref] [Google scholar] [PubMed]
- Dalekos GN, Arvaniti P, Gatselis NK, Samakidou A, Gabeta S, et al. (2022) First results from a propensity matching trial of mycophenolate mofetil vs. azathioprine in treatment-naive AIH patients. Front Immunol 12:798602.
[Crossref] [Google scholar] [PubMed]
- Lohse AW, Sebode M, Jørgensen MH, Ytting H, Karlsen TH, et al. (2020) European Reference Network on Hepatological Diseases (ERN RARE-LIVER); International Autoimmune Hepatitis Group (IAIHG). Second-line and third-line therapy for autoimmune hepatitis: A position statement from the European Reference Network on Hepatological Diseases and the International Autoimmune Hepatitis Group. J Hepatol 73:1496-1506.
[Crossref] [Google scholar] [PubMed]
- Ikeda A, Aoki N, Kido M, Iwamoto S, Nishiura H, et al. (2014) Progression of autoimmune hepatitis is mediated by IL-18-producing dendritic cells and hepatic CXCL9 expression in mice. Hepatology 60:224-236.
[Crossref] [Google scholar] [PubMed]
- Zhao R, Zhou H, Su SB (2013). A critical role for interleukin-1b in the progression of autoimmune diseases. Int Immunopharmacol 17:658-669.
[Crossref] [Google scholar] [PubMed]
- Zhao L, Tang Y, You Z, Wang Q, Liang S, et al. (2011) Interleukin-17 contributes to the pathogenesis of autoimmune hepatitis through inducing hepatic interleukin-6 expression. PLoS One 6:e18909.
[Crossref] [Google scholar] [PubMed]
- Nakayama S (2013). Autoimmune Hepatitis Triggered by Anti-TNF-aTherapy. Case Rep Med 2013:561748.
[Crossref] [Google scholar] [PubMed]
- Jaruga B, Hong F, Kim WH, Gao B (2004). IFN-gamma/STAT1 acts as a proinflammatory signal in T cell-mediated hepatitis via induction of multiple chemokines and adhesion molecules: a critical role of IRF-1. Am J Physiol Gastrointest Liver Physiol 287:G1044-1052.
[Crossref] [Google scholar] [PubMed]
- Hammerich L, Heymann F, Tacke F (2011). Role of IL-17 and Th17 cells in liver diseases. Clin Dev Immunol 2011:345803.
[Crossref] [Google scholar] [PubMed]
- Singh B, Schwartz JA, Sandrock C, Bellemore SM, Nikoopour E (2013). Modulation of autoimmune diseases by Interleukin (IL)-17 producing regulatory T helper (Th17) cells. Indian J Med Res 138:591-594.
[Google scholar] [PubMed]
- Beringer A, Miossec P (2018). IL-17 and IL-17-producing cells and liver diseases, with focus on autoimmune liver diseases. Autoimmun Rev 17:1176-1185.
[Crossref] [Google scholar] [PubMed]
- Lorenzini T, Dotta L, Giacomelli M, Vairo D, Badolato R (2017). STAT mutations as program switchers: turning primary immunodeficiencies into autoimmune diseases. J Leukoc Biol 101:29-38.
[Crossref] [Google scholar] [PubMed]
- Yasumi Y, Takikawa Y, Endo R, Suzuki K (2007). Interleukin-17 as a new marker of severity of acute hepatic injury. Hepatology Research 37:248-254.
[Crossref] [Google scholar] [PubMed]
- Yu H, Huang J, Liu Y, Ai G, Yan W, et al. (2010) IL-17 contributes to autoimmune hepatitis. J Huazhong Univ Sci Technolog Med Sci 30:443-446.
[Crossref] [Google scholar] [PubMed]
- Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, et al. (2016) Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535:153-158.
[Crossref] [Google scholar] [PubMed]
- Lei L, Sun J, Han J, Jiang X, Wang Z, et al. (2021) Interleukin-17 induces pyroptosis in osteoblasts through the NLRP3 inflammasome pathway in vitro. Int Immunopharmacol 96:107781.
[Crossref] [Google scholar] [PubMed]
- Caneparo V, Cena T, De Andrea M, Dell'oste V, Stratta P, et al. (2013) Anti-IFI16 antibodies and their relation to disease characteristics in systemic lupus erythematosus. Lupus 22:607-613.
[Crossref] [Google scholar] [PubMed]
- De Andrea M, De Santis M, Caneparo V, Generali E, Sirotti S, et al. (2020) Serum IFI16 and anti-IFI16 antibodies in psoriatic arthritis. Clin Exp Immunol 199:88-96.
[Crossref] [Google scholar] [PubMed]
- Matsuda T, Muromoto R, Sekine Y, Togi S, Kitai Y, et al. (2015) Signal transducer and activator of transcription 3 regulation by novel binding partners. World J Biol Chem 6:324-332.
[Crossref] [Google scholar] [PubMed]
Citation: Xu W, Wang Y, Jin C, Zhang W, Chen J, et al. (2023) The Role of IL-17 Imbalance in Promoting the Pyroptosis in Immune-Mediated Liver Injury through STAT3-IFI16 Axis. Diagnos Pathol Open S13:006. DOI: 10.4172/2476-2024.8.S13.006
Copyright: © 2023 Xu W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 739
- [From(publication date): 0-2023 - Nov 21, 2024]
- Breakdown by view type
- HTML page views: 672
- PDF downloads: 67