The Role of GABAA Receptor δ Subunit and its Agonist THIP in Thermal Hypersensitivity in a Mouse Model of Neuropathic Pain
Received: 16-Jan-2018 / Accepted Date: 19-Jan-2018 / Published Date: 23-Jan-2018 DOI: 10.4172/2167-0846.1000308
Abstract
Decreased gamma-aminobutyric acid (GABA)-mediated phasic inhibitory transmission in the spinal cord is thought to be responsible for the development of neuropathic pain. However, the role of GABAergic tonic current in substantia gelatinosa (SG) neurons remains to be fully elucidated. Using real-time polymerase chain reaction, we investigated the expression of the GABAA receptor δ subunit, which contributes to tonic current in the SG, in chronic constriction injury (CCI; a well-known model of neuropathic pain) and naive mice. The expression of the δ subunit mRNA was reduced by 40% in the ipsilateral SG of the dorsal horn of CCI mice compared to naive mice. We also performed behavioral experiments to assess the effect of the δ subunit-preferring agonist 4,5,6,7- tetrahydroisoxazolo(5,4-c)pyridine-3-ol (THIP) on thermal hypersensitivity with the Hargreaves test. Intrathecal administration of THIP significantly improved thermal thresholds of the ipsilateral hindpaw (4.55 ± 0.78 to 6.56 ± 1.09 s from baseline to after injection, respectively, P<0.005), while normal saline did not (4.41 ± 0.86 to 4.46 ± 1.1 s, P>0.1). GABAA receptor δ subunit-mediatedtonic current contributes to thermal hypersensitivity of CCI mice, and THIP may represent a therapeutic tool to improve thermal hypersensitivity.
Keywords: GABAA receptors; Hyperalgesia; Neuralgia; Substantia gelatinosa
Abbreviations
GABA: Gamma-Amino Butyric Acid; CCI: Chronic Constriction Injury; CNS: Central Nervous System; PCR: Polymerase Chain Reaction; NS: Normal Saline; SG: Substantia Gelatinosa; THIP: 4,5,6,7-Tetrahydroisoxazolo(5,4-c)pyridin-3-ol
Introduction
Neuropathic pain is defined as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system” [1]. The mechanisms of neuropathic pain have been investigated but remain largely unclear. Gamma-aminobutyric acid (GABA)-mediated inhibitory transmission has antinociceptive and sedative effects in the central nervous system (CNS) and peripheral neurons [2,3]. Dysfunctional signaling in the spinal pain processing sites due to reduced GABAergic inhibition is one mechanism underlying chronic pain development [3]. There are two types of inhibitory transmission via GABAA receptors (GABAARs): Tonic and phasic. Phasic (synaptic) inhibition is mediated by activation of postsynaptic receptors by saturating concentrations of vesicular GABA [4].
Conversely, tonic inhibition results from activation of extrasynaptic GABAARs by low concentrations of ambient GABA [5-7]. In the spinal cord, GABAAR-mediated phasic currents of dorsal neurons have been shown to be decreased after sciatic nerve chronic constriction injury (CCI) in rats [8]. Electrophysiological experiments have revealed the presence of GABAergic tonic inhibitory currents mediated by GABAARs containing the α5, δ, and ε subunits in a subset of substantia gelatinosa (SG) neurons [9-12]. However, the physiological role of GABAergic tonic current and its relationship to neuropathic pain in SG neurons remains largely unknown. In a previous study, we found that GABAergic tonic current-positive cells were reduced in SG neurons of the spinal dorsal horn in CCI mice [13]. CCI mice also showed less expression of δ subunits in SG neurons. These results suggested that the decline of tonic currents mediated by δ subunitcontaining GABAAR (δGABAAR) in SG neurons is associated with thermal and/or mechanical hypersensitivities of CCI mice. 4,5,6,7-Tetrahydroisoxazolo(5,4-c)pyridin-3-ol (THIP) is a GABAAR agonist with preference for the δ subunit. Bonin et al. reported that increasing δGABAAR activity using THIP dose-dependently inhibited acute thermal nocifensive behaviors in a formalin model of pain in mice [12]. However, the effect of THIP on neuropathic pain is unknown.
To clarify the role of δ subunit-mediated GABAergic tonic current in neuropathic pain, we investigated the change of δ subunit expression in CCI mice using real-time polymerase chain reaction (PCR). We also assessed the effect of intrathecally administered THIP on induced neuropathic pain in CCI mice.
Materials and Methods
Animals
One hundred and fifteen specific pathogen-free strain ddY mice (Japan SLC, Shizuoka, Japan) were used. All animals were housed in groups of three in polypropylene cages with paper chip bedding and access to food and water ad libitum in a temperature-controlled room with a 12:12 light:dark cycle. All animal procedures and study protocols were approved by the Institute of Experimental Animal Sciences Faculty of Medicine, Osaka University.
Surgical procedures
All surgeries were carried out under aseptic conditions. Sciatic nerve cuffing was induced using the polyethylene cuff method [14]. Male ddY mice (5 weeks old) were operated on to induce CCI under general anesthesia. After confirmed adequate anesthesia by 4% sevoflurane (Maruishi, Osaka, Japan) with O2 (1 L/min), as this promotes quick recovery, the common branch of the right sciatic nerve was exposed. A 2 mm long split section of polyethylene tubing (inner diameter=0.38 mm, external diameter=1.09 mm; PE-20, Imamura Co, Ltd, Tokyo, Japan) was placed around the nerve to induce CCI (CCI group). After confirming that there was no bleeding, the wound was closed with sutures. Age-matched naive mice were used as a control group (naive group). After surgery, mice were single-housed in a cage until they completely recovered from anesthesia and started to drink water.
Behavioral tests with mechanical and thermal stimulations
To measure pain thresholds, we evaluated the mechanical paw withdrawal thresholds and the latency of thermal stimulation. Thermal hypersensitivity was assessed with a thermal stimulus apparatus, as described in detail by Hargreaves and colleagues [15]. Briefly, mice were placed in clear Plexiglas cages on a glass surface. An infrared beam of the radiant heat source was applied to the plantar surface of each hindpaw. The cut-off point to prevent damage to the skin was set at 20 s. Three measurements of latency per side were recorded at 1-min intervals and averaged.
The mechanical threshold for hindpaw withdrawal was determined using an automated electronic von Frey system (37450 Dynamic Plantar Aesthesiometer; Ugo Basile, Comerio, Italy). Mice were placed in plastic cages with a wire mesh floor and were allowed to habituate for 15 min before testing. A filament (0.5-mm diameter) was then applied to the plantar surface of each hindpaw. The applied force was initially below the detection threshold, as it was increased from 0 to 10 g at a rate of 0.5 g/s. The applied force necessary to elicit a reflex removal of the hindpaw was determined through this method, according to the three measurements obtained at 1 min intervals.
Real-time PCR
To observe the change of the δ subunit mRNA expression in the SG, we performed real-time PCR experiments at 7, 14, 21, and 28 days after surgery. Total RNA extraction from SG tissues of CCI (n=6 for each period) and naive mice (n=6 for each period) was performed using the RNeasy Rapid Mini Kit (QIAGEN, Tokyo, Japan) according to the manufacturer’s instructions. To collect SG tissue, we dissected the entire spinal column of mice and trimmed the L4 input, including an area of 1 cm radius around it, and then separated the right side of the SG by longitudinal halving. Next, we isolated the SG area with the help of cuspidate borosilicate glass rods (WPI, Sarasota, FL). Products from two mice were mixed in one tube and homogenized, because the quantity of mRNA purified from one mouse was insufficient. The lysis reagent was the QIAzol included in the RNeasy Rapid Mini Kit. The concentration of RNA was measured by spectroscopy with an expected A260/280 ratio close to 1.8-2.0.
All mRNA samples were diluted to 45 ng/μL before being synthesized, after which 450 ng of mRNA from each sample was reverse transcribed in a volume of 10 μL to produce cDNA using the High Capacity RNA to cDNA Kit (Thermo Fisher, Yokohama, Japan) according to the manufacturer’s protocol. The thermal cycler conditions were as follows: 37°C for 60 min, 95°C for 5 min, and 4°C. Subsequently, PCR amplification was performed using the TaqMan Gene Expression Assay (Thermo Fisher) for the δ subunit and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the relative internal control gene with Fast Master Mix (Thermo Fisher) in 96-well plates (Thermo Fisher). Three samples from each specimen were amplified and the mean values were analyzed. We used the cerebellum of naive mice as the calibration curve for the δ subunit and GAPDH.
Intrathecal injection
To examine the effect of THIP on the pain threshold, intrathecal injections were performed 7 days after surgery. All mice were injected intrathecally 15 min before behavioral testing. Intrathecal injections were administered without general anesthesia to avoid its sedative and anti-nociceptive effects [16]. Briefly, one person held the mouse, while another made bone contact with a 30G needle connected to a Hamilton syringe (Hamilton LT type) perpendicularly on the mouse’s back at the lumbar level between L5 and L6 and felt the intervertebral spaces (usually in the middle of spinous processes). We tilted the syringe forward 30°, then pushed the syringe slowly and injected the drug. A characteristic tail flick indicates penetration of the needle in the intrathecal space and ensures success for the delivery of the drug. CCI mice in the THIP group (n=15) were injected with THIP (0.7 μg in 5 μL) and CCI mice in the control group (n=12) were injected with the same volume of normal saline (NS). The experimenters and data analyst were blinded to the pharmacological intervention. In addition, to examine the duration of action of THIP, we evaluated the post thresholds 15, 30, 60, and 120 min after intrathecal THIP injection.
Statistics
All statistical analyses were performed using IBM SPSS V24 (IBM Co., Armonk, NY). Numerical data of the threshold changes of CCI and naive mice are expressed as the mean ± SEM. Statistical differences were tested using paired Student’s t-test for two-group comparisons. Paw laterality (CCI left, right, or naive) and the time-course were treated as within-subjects factors with repeated measures analysis of variance (ANOVA). Significant differences were assessed using Bonferroni post-hoc comparison.
Results
Threshold of thermal and mechanical stimulation of CCI mice
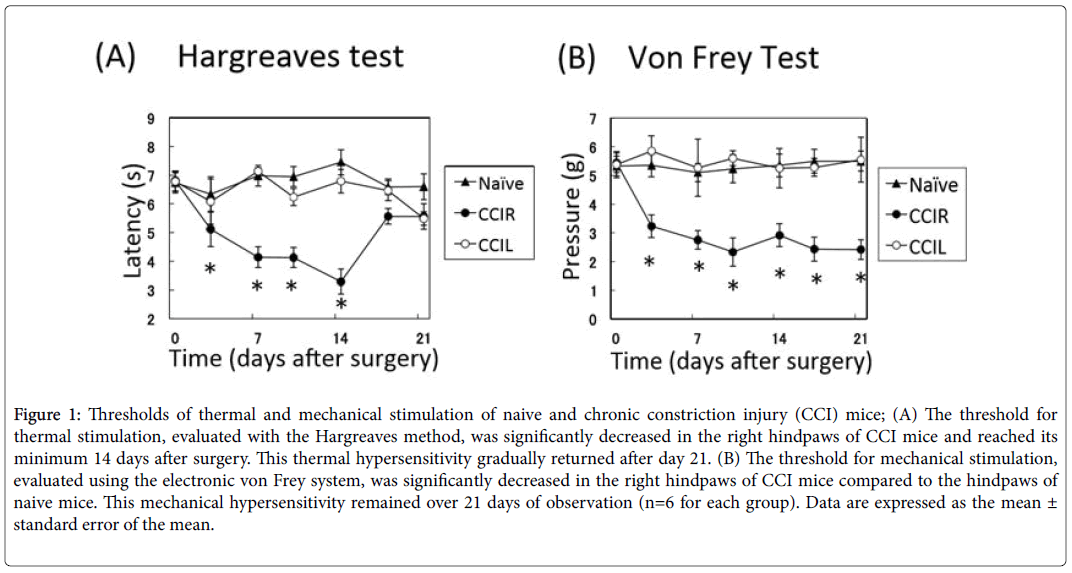
Unilateral cuff implantation induced both thermal and mechanical hypersensitivities in CCI mice. The threshold for thermal stimulation was significantly decreased in the right hindpaws of CCI mice (n=6) compared to the left hindpaws of CCI mice and the hindpaws of naive mice (n=6) and reached its minimum at day 14 (3.29 ± 0.44 s) after surgery (paw laterality × time interaction, F12.90=5.11, P<0.001, Bonferroni: P<0.01, n=6). This thermal hypersensitivity gradually returned and at 21 days after surgery, the significance of the threshold disappeared (Figure 1A), as reported previously by Benbouzid et al. [14]. The sensitivity to mechanical stimulation in the right hindpaws of the CCI group was decreased compared to the left hindpaws and naive mice (paw laterality × time interaction: F12.90=1.98, P<0.05, Bonferroni: P<0.01). Mechanical hypersensitivities remained 21 days after surgery (Figure 1B).
Figure 1: Thresholds of thermal and mechanical stimulation of naive and chronic constriction injury (CCI) mice; (A) The threshold for thermal stimulation, evaluated with the Hargreaves method, was significantly decreased in the right hindpaws of CCI mice and reached its minimum 14 days after surgery. This thermal hypersensitivity gradually returned after day 21. (B) The threshold for mechanical stimulation, evaluated using the electronic von Frey system, was significantly decreased in the right hindpaws of CCI mice compared to the hindpaws of naive mice. This mechanical hypersensitivity remained over 21 days of observation (n=6 for each group). Data are expressed as the mean ± standard error of the mean.
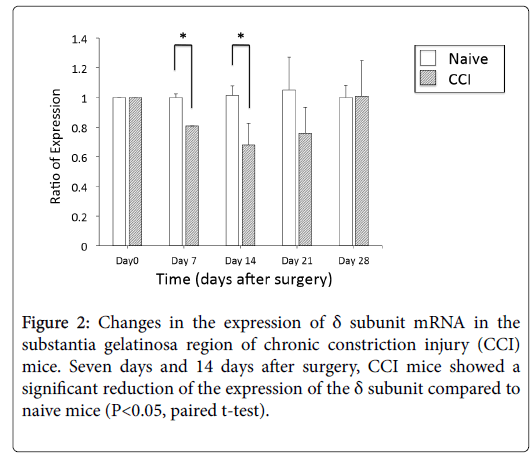
Change in the expression of δ subunit mRNA in the SG of CCI mice
Using real-time PCR, we observed the expression changes of the δ subunit mRNA in the SG at 7, 14, 21, and 28 days after surgery. The expression of the δ subunit in the SG of CCI mice was significantly reduced (20%) at 7 days after surgery compared to the expression in the SG of naive mice. At 14 days after surgery, CCI mice also showed 40% less expression than naive mice. However, 21 days after surgery, this significant difference disappeared. At 28 days after surgery, mRNA expression of the δ subunit recovered to a level comparable to that of naive mice (Figure 2).
CCI mice showed both thermal and mechanical hypersensitivities 7 days after surgery. The mechanical hypersensitivity continued until 28 days after surgery, but thermal hypersensitivity remained for only 14 days after surgery (Figure 1). This change of thermal thresholds was similar to the time-passage change of mRNA expression of the δ subunit.
Effect of THIP on thermal hypersensitivity in CCI mice
Since the transition of δ subunit mRNA expression was similar to the change of the threshold of thermal stimulation, we next examined the effect of THIP on thermal hypersensitivity in CCI mice.
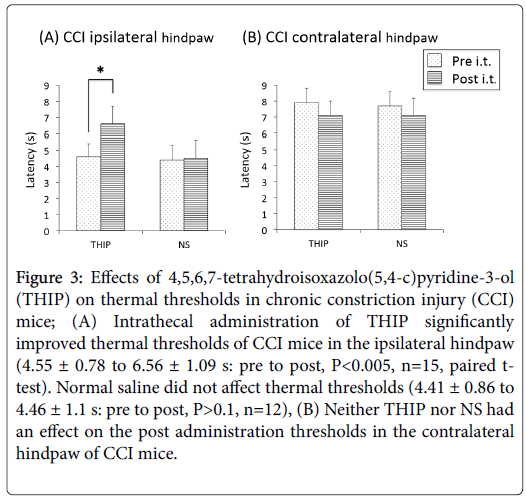
Intrathecal administration of THIP significantly improved thermal hypersensitivity of the ipsilateral hindpaw 15 min after injection (4.55 ± 0.78 to 6.56 ± 1.09 s: pre to post, P<0.005, n=15, paired t-test) (Figure 3A). NS did not have aneffect on the thermal thresholds of the ipsilateral hindpaw (4.41 ± 0.86 to 4.46 ± 1.1 s: pre to post, P>0.1, n=12) (Figure 3A).
Figure 3: Effects of 4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridine-3-ol (THIP) on thermal thresholds in chronic constriction injury (CCI) mice; (A) Intrathecal administration of THIP significantly improved thermal thresholds of CCI mice in the ipsilateral hindpaw (4.55 ± 0.78 to 6.56 ± 1.09 s: pre to post, P<0.005, n=15, paired ttest). Normal saline did not affect thermal thresholds (4.41 ± 0.86 to 4.46 ± 1.1 s: pre to post, P>0.1, n=12), (B) Neither THIP nor NS had an effect on the post administration thresholds in the contralateral hindpaw of CCI mice.
The thermal threshold of the contralateral hindpaw was not changed by either THIP (7.86 ± 0.89 to 7.14 ± 0.86 s, P>0.05, n=15) or NS (7.66 ± 0.95 to 7.11 ± 1.12 s, P>0.05, n=12) (Figure 3B).
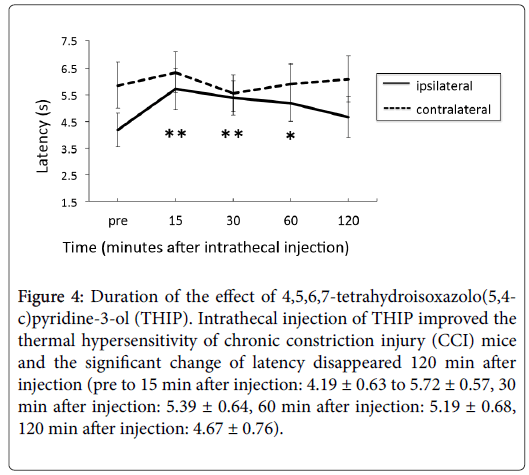
Figure 4 shows the time course of post thresholds after injection. The contralateral thresholds did not change significantly compared to the pre thresholds. On the other hand, ipsilateral thresholds 15, 30, 60 min after injection were significantly improved (pre to 15 min after injection: 4.19 ± 0.63 to 5.72 ± 0.57 s, 30 min after injection: 5.39 ± 0.64, 60 min after injection: 5.19 ± 0.68 s), but 120 min after the injection the effect of THIP disappeared.
Figure 4: Duration of the effect of 4,5,6,7-tetrahydroisoxazolo(5,4- c)pyridine-3-ol (THIP). Intrathecal injection of THIP improved the thermal hypersensitivity of chronic constriction injury (CCI) mice and the significant change of latency disappeared 120 min after injection (pre to 15 min after injection: 4.19 ± 0.63 to 5.72 ± 0.57, 30 min after injection: 5.39 ± 0.64, 60 min after injection: 5.19 ± 0.68, 120 min after injection: 4.67 ± 0.76).
Discussion
In this study, we revealed that the period at which CCI mice showed thermal hypersensitivity coincided with a reduced expression of GABAAR δ subunit mRNA. Intrathecal administration of THIP attenuated the thermal hypersensitivity of CCI mice. Relationship between reduced δ subunit mRNA expression and CCI-induced neuropathic pain.
δGABAAR is one of the receptors that mediate tonic inhibitory current. This tonic current is thought to be essential to alcohol sensitivity, the sedative effect of neurosteroids, and the mechanism underlying epilepsy [17-19]. In a previous electrophysiological study, we revealed that the number of tonic current-positive SG neurons is reduced. Moreover, CCI mice show a reduction of δ subunit expression in SG neurons 2 weeks after injury on real-time PCR and western blot experiments [13]. These data suggested that the δGABAAR-mediated tonic current plays an important role in CCI-induced neuropathic pain. CCI mice show both thermal and mechanical hypersensitivities, but the time course is different. Therefore, it is unclear whether δGABAAR-mediated tonic current contributes to CCI-induced thermal and/or mechanical hypersensitivity.
In the present study, we attempted to elucidate the impact of δGABAAR-mediated tonic current on CCI-induced neuropathic pain. Real-time PCR showed that the expression of δ subunit mRNA in CCI mice was significantly reduced at 7 to 21 days after surgery, but it returned to baseline at 28 days. Interestingly, the time-course of the change of mRNA expression was similar to the change of the threshold of thermal stimulation. However, mechanical hypersensitivity continued for longer than the period of the reduction of δ subunit expression. This suggested that δ subunit-mediated tonic current participates in thermal hypersensitivity, but not mechanical hypersensitivity, in CCI mice. Traumatic brain injury has been found to induce a reduction of THIP-induced tonic current in dentate granule cells [20,21].
Changes in functional GABAAR signaling have been reported to reflect rearrangements in GABAAR subunit composition in the CNS [22-25]. Taken together, our results indicate that CCI can induce the same rearrangement in GABAAR subunit composition and lead to disruption of GABAergic inhibitory circuits in the spinal cord.
THIP is a GABAAR agonist with preference for the δ subunit
Past electrophysiological experiments have revealed that lowconcentration THIP increases δGABAAR tonic current in dentate granule cells [26,27] and vagal motor neurons [28]. SG neurons of the spinal cord play an important role in the transmission of pain sensation [29]. The δ subunit contributes to GABAergic tonic currents in SG neurons of the spinal cord [9,12]. Han et al. found that the stress-related neurosteroid 3α,5α-tetrahydrodeoxycorticosterone (THDOC), another δGABAAR powerful endogenous positive allosteric modulator, enhanced tonic currents in SG neurons of the spinal trigeminal nucleus pars caudalis [10]. These results indicated that intrathecal administration of THIP attenuates CCI-induced hypersensitivity through potentiating δGABAAR mediated tonic current of SG neurons. Bonin et al. revealed that intrathecal administration of THIP increased the mean latency of hot plate response in wild-type mice [12]. They also showed that intrathecal administration of THIP improved formalin-induced phase I response, but not phase II response. Those data demonstrated the analgesic effect of THIP on acute nociception. Our data suggest the possibility that THIP can improve not only acute pain but also CCI-induced neuropathic pain.
How THIP might affect CCI-induced hypersensitivity
In a previous study, we revealed that the number of tonic current positive SG neurons was reduced in CCI mice. The expression of the δ subunit mRNA and protein in the SG region of CCI mice was also reduced. In the current study, we used real-time PCR to reveal the time-dependent change of the expression of δ subunit mRNA in the SG region. Our data suggest that intrathecal administration of THIP potentiated the tonic current, which was reduced after CCI in the SG, and attenuated the thermal hypersensitivities. The expression of the δ subunit in the dorsal horn is not high, but δ GABAARs contribute to tonic current in SG neurons [30]. Tonic current results from persistent activation of high affinity extrasynaptic GABAARs; thus, the agonist can cause a greater increase in the absolute charge transfer associated with the tonic current compared with the phasic current. Findings that nonsteroidal anti-inflammatory drugs have no effect on CCI-induced neuropathic pain indicated that the mechanism of CCI-induced neuropathic pain is not related to inflammatory pain, which supports our theory [14].
Other possible effects of THIP on CCI-induced hypersensitivity
Low-dose THIP (at the nM order) has been proposed as a selective ligand for extrasynaptic GABAARs, especially α4β3δ and α6β3δ [31]. On the other hand, relatively high doses of THIP (at the μM order) can potentiate other GABAARs, such as α5GABAAR [32]. The α5GABAARs are expressed in the superficial dorsal horn where they contribute to tonic current in a subset of SG neurons [9,33-35]. Thus, there is a possibility that the effect of THIP on CCI-induced thermal hypersensitivity occurred through α5GABAAR-mediated tonic current. Indeed, Bonin et al. proved that THIP attenuated formalininduced phase II response in δGABAAR knock-out mice at a dose similar to that used in our experiment [12]. Recently, Perez-Sanchez et al. showed that α5GABAAR knock-out mice showed no difference of acute nociception compare to WT but the hypersensitivity after complete Freund’s adjuvant injection was longer lastingand the mice exhibited increased late phase sensitization to formalin [35].
In addition, Bravo-Hernandez et al. reported an increase in α5GABAAR expression several days after formalin injection [36]. Taken together, the effect of THIP on phase II responses of δGABAAR knock-out mice may occur through the enhancement of α5GABAAR. On the other hand, our previous experiment revealed no significant difference in the expression of mRNA of α5GABAARs between naive mice and CCI mice at 2 weeks after injury. This might be due to the differences in the pain model (CCI versus formalin test), species (mouse versus rat), and time (2 weeks versus 3-6 days). Taken together, these results suggest the effect of THIP on induced thermal hyperalgesia in CCI mice occurred via enhancement of attenuated δGABAAR. We administrated THIP intrathecally. Therefore, there is a possibility that THIP altered upper transmission above the spinal cord, showing a sedative or anesthetic effect on the brain that could explain pain relief in CCI mice. Using the Rotarod treadmill test, Bonin et al. showed that low-dose THIP (0.35 μM) has no effect on motor coordination [12]. Furthermore, THIP showed no effect on the contralateral thresholds of CCI mice. If THIP affected the brain, showing an antinociceptive effect, the normal threshold could be changed. Note that it remains possible that THIP-induced attenuation may have occurred from the sedative effect on spontaneous motor function, which modified the response latency in the thermal nociception. The role of δ GABAAR-mediated tonic current in neuropathic pain.
In naive rats, potentiation of δGABAAR by intrathecal administration of low-dose THIP (1 μg) did not affect hot plate latency at 10-60 min after injection [37]. On the other hand, our data suggest that THIP attenuated CCI-induced thermal hypersensitivity. Thus, spinal disinhibition through the attenuation of δGABAAR-mediated tonic current may participate in the thermal hypersensitivity of CCI mice. Bonin et al. reported that δGABAAR knock-out mice showed similar latency value for the hot-plate test response under control conditions [12]. They also reported no difference in formalin-induced phase I response between δGABAAR knock-out mice and WT mice, despite an increased phase II response [12]. Those data imply that δGABAAR plays a role in restraining central sensitization. In this study, we focused on the δ subunit of the GABAA receptor, which contributes to the tonic current of SG neurons. Our data suggest that reduced δGABAAR-mediated tonic current in the SG is a complex mechanism of neuropathic pain. SG neurons are heterogeneous and the characteristics of cells expressing the δ subunit remain to be determined. The presence of tonic inhibition in SG is related to the expression of GABAA receptors containing δ and α5 subunits [9,12,35].
Recently, α5GABAAR-mediated tonic inhibition in the spinal cord dorsal horn has been reported to play an important role in chronic inflammatory pain and neuropathic pain [35,36]. On the other hand, our data suggest that δGABAAR-mediated tonic inhibition in SG plays a role in the mechanism of CCI-induced neuropathic pain, especially thermal hyperalgesia. Further studies to define cell-specific expression of δGABAAR will more conclusively demonstrate the role of this receptor in nociceptive processing. In summary, our results suggest that δGABAAR-mediated tonic inhibition in SG neurons plays a role in the mechanism underlying CCI-induced neuropathic pain, especially thermal hyperalgesia. δGABAAR can be a possible therapeutic tool to improve thermal hypersensitivity.
Acknowledgment
The authors thank Yoko Ishida for her assistance with the real-time PCR method.
Author contributions
Ayako Takahashi, Saya Hakata, and Akira Iura conceived and designed the study. Ayako Takahashi, Yoichi Matsuda, and Yuji Fujino supervised the work. Ayako Takahashi, Saya Hakata, Seiichi Osako, Akira Iura, and Hironobu Uematsu carried out the experiments. All authors edited the manuscript and helped with experimental design. Ayako Takahashi and Saya Hakata performed data analysis. Ayako Takahashi and Saya Hakata wrote the manuscript.
Funding
This work was supported JSPS KAKENHI Grant Number JP16K20094 and JSPS KAKENHI Grant Number JP25861374.
Conflict of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, et al. (2008) Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology 70: 1630-1635.
- Besson M, Matthey A, Daali Y, Poncet A, Vuilleumier P, et al. (2015) GABAergic modulation in central sensitization in humans: A randomized placebo-controlled pharmacokinetic-pharmacodynamic study comparing clobazam with clonazepam in healthy volunteers. Pain 156: 397-404.
- Jergova S, Gajavelli S, Varghese MS, Shekane P, Sagen J (2016) Analgesic effect of recombinant GABAergic cells in a model of peripheral neuropathic pain. Cell Transplant 25: 629-643.
- Mtchedlishvili Z, Lepsveridze E, Xu H, Kharlamov EA, Lu B, et al. (2010) Increase of GABAA receptor-mediated tonic inhibition in dentate granule cells after traumatic brain injury. Neurobiol Dis 38: 464-475.
- Rossi DJ, Hamann M (1998) Spillover-mediated transmission at inhibitory synapses promoted by high affinity alpha6 subunit GABA receptors and glomerular geometry. Neuron 20: 783-795.
- Mody I, Pearce RA (2004) Diversity of inhibitory neurotransmission through GABA A receptors. Trends Neurosci 27: 569-575.
- Stell BM, Mody I (2002) Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J Neurosci 22: RC223 1-5.
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, et al. (2002) Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci 22: 6724-6731.
- Takahashi A, Mashimo T, Uchida I (2006) GABAergic tonic inhibition of substantia gelatinosa neurons in mouse spinal cord. Neuroreport 17: 1331-1335.
- Han SM, Youn D (2008) GABAA receptor-mediated tonic currents in substantia gelatinosa neurons of rat spinal trigeminal nucleus pars caudalis. Neurosci Lett 441: 296-301.
- Maeda A, Katafuchi T, Oba Y, Shiokawa H, Yoshimura M (2010) Enhancement of GABAergic tonic currents by midazolam and noradrenaline in rat substantia gelatinosa neurons in vitro. Anesthesiology 113: 429-437.
- Bonin RP, Labrakakis C, Eng DG, Whissell PD, De Koninck Y, et al. (2011) Pharmacological enhancement of d-subunit-containing GABAA receptors that generate a tonic inhibitory conductance in spinal neurons attenuates acute nociception in mice. Pain 152: 1317-1326.
- Iura A, Takahashi A, Hakata S, Mashimo T, Fujino Y (2016) Reductions in tonic GABAergic current in substantia gelatinosa neurons and GABA A receptor δ subunit expression after chronic constriction injury of the sciatic nerve in mice. Eur J Pain 20: 1678-1688.
- Benbouzid M, Choucair-Jaafar N, Yalcin I, Waltisperger E, Muller A, et al. (2008) Chronic, but not acute, tricyclic antidepressant treatment alleviates neuropathic allodynia after sciatic nerve cuffing in mice. Eur J Pain 12: 1008-1017.
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1998) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32: 77-88.
- Fairbanks CA (2003) Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev 55: 1007-1041.
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, et al. (2007) A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci 10: 40-48.
- Romo-Parra H, Blaesse P, Sosulina L, Pape HC (2015) Neurosteroids increase tonic GABAergic inhibition in the lateral section of the central amygdala in mice. J Neurophysiol 113: 3421-3431.
- Pandit S, Jeong JA, Jo JY, Cho HS, Kim DW, et al. (2013) Dual mechanisms diminishing tonic GABAA inhibition of dentate gyrus granule cells in Noda epileptic rats. J Neurophysiol 110: 95-102.
- Gupta A, Elgamal FS, Proddutur A, Santhakumar V (2012) Decrease in tonic inhibition contributes to increase in dentate semilunar granule cell excitability after brain injury. J Neurosci 32: 2523-2537.
- Bulter CR, Boychuk JA, Smith BN (2016) Differential effects of rapamycin treatment on tonic and phasic GABAergic inhibition in dentate granule cells after focal brain injury in mice. Exp Neurol 280: 30-40.
- Gibbs JW, Sombati S, DeLorenzo RJ, Coulter DA (1997) Physiological and pharmacological alterations in postsynaptic GABAA receptor function in a hippocampal culture model of chronic spontaneous seizures. J Neurophysiol 77: 2139-2152.
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA (1998) Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med 4: 1166-1172.
- Cossart R, Tyzio R, Dinocourt C, Esclapez M, Hirsch JC, et al. (2001) Presynaptic kainate receptors that enhance the release of GABA on CA1 hippocampal interneurons. Neuron 29: 497-508.
- Leroy C, Poisbeau P, Keller AF, Nehlig A (2004) Pharmacological plasticity of GABAA receptors at dentate gyrus synapses in a rat model of temporal lobe epilepsy. J Physiol 557: 473-487.
- Marowsky A, Vogt KE (2014) Delta-subunit-containing GABAA-receptors mediate tonic inhibition in paracapsular cells of the mouse amygdala. Front Neural Circuits 8: 27.
- Medrihan L, Ferrea E, Greco B, Baldelli P, Benfenati F (2015) Asynchronous GABA release is a key determinant of tonic inhibition and controls neuronal excitability: A study in the synapsin II-/- mouse. Cereb Cortex 25: 3356-3368.
- Gao H, Smith BN (2010) Tonic GABAA receptor-mediated inhibition in the rat dorsal motor nucleus of the vagus. J Neurophysiol 103: 904-914.
- Yoshimura M, Jessell TM (1989) Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J Neurophysiol 62: 96-108.
- Klinger F, Bajric M, Salzer I, Dorostkar MM, Khan D, et al. (2015) δ Subunit-containing GABAA receptors are preferred targets for the centrally acting analgesic flupirtine. Br J Pharmacol 172: 4946-4958.
- Meera P, Wallner M, Otis TS (2011) Molecular basis for the high THIP/gaboxysadol sensitivity of extrasynaptic GABAA receptors. J Neurophysiol 106: 2507-2064.
- Storustovu SI, Elbert B (2006) Pharmacological characterization of agonists at delta containing GABAA receptors: Functional selectivity for extrasynaptic receptors is dependent on the absence of gamma2. J Pharmacol Exp Ther 316: 1351-1359.
- Knabl J, Witschi R, Hösl K, Reinold H, Zeilhofer UB, et al. (2008) Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature 451: 330-334.
- Paul J, Zeilhofer HU, Fritschy JM (2012) Selective distribution of GABA(A) receptor subtypes in mouse spinal dorsal horn neurons and primary afferents. J Comp Neurol 520: 3895-3911.
- Perez-Sanchez J, Lorenzo LE, Lecker I, Zurek AA, Labrakakis C, et al. (2017) α5GABAA receptors mediate tonic inhibition in the spinal cord dorsal horn and contribute to the resolution of hyperalgesia. J Neurosci Res 95: 1307-1318.
- Bravo-Hernández M, Corleto JA, Barragán-Iglesias P, González-RamÃrez R, Pineda-Farias JB, et al. (2016) The α5 subunit-containing GABAA receptors contribute to chronic pain. Pain 157: 613-626.
- Hammond DL, Drower EJ (1984) Effects of intrathecally administered THIP, baclofen and muscimol on nociceptive threshold. Eur J Pharmacol 103: 121-125.
Citation: Hakata S, Takahashi A, Iura A, Osako S, Uematsu H, et al. (2018) The Role of GABAA Receptor δ Subunit and its Agonist THIP in Thermal Hypersensitivity in a Mouse Model of Neuropathic Pain . J Pain Relief 7: 308. DOI: 10.4172/2167-0846.1000308
Copyright: ©2018 Hakata S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5183
- [From(publication date): 0-2018 - Apr 26, 2025]
- Breakdown by view type
- HTML page views: 4331
- PDF downloads: 852