The Role of Epidermal Growth Factor in Early Tissue Healing After Surgical Periodontal Therapy
Received: 05-Apr-2021 / Accepted Date: 19-Apr-2021 / Published Date: 26-Apr-2021 DOI: 10.4172/2332-0702.1000278
Abstract
The aim of the current study was to investigate the potential association of the Epidermal Growth (EGF) Factor levels of mRNA expression in oral epithelium with early healing response after surgical periodontal therapy. The study included forty-three patients undergoing surgical periodontal treatment by means of access flap in maxilla. A gingival tissue fragment was collected from the hard palate adjacent to the surgical field and was further processed for the evaluation of EGF expression in oral epithelial cells using real-time PCR. The trauma left, was followed up for the evaluation of the healing response at 1week (T1), 2 weeks (T2) and 2 months (T3). The modified Early Healing [M-EHI] and the Master Scar Proforma (M-MSPI) Indexes were used for the visual assessment of healing after intraoral photographs were taken. The effect of smoking in the healing period was also assessed. The results showed that EGF expression did not affect the healing response, as the association among the studied variables did not reach statistical significance at any time point. The results were similar even when smoking was considered. In conclusion, our study did not show any statistically significant association between the mRNA expression levels of EGF and the healing response.
Keywords: Periodontics; Epidermal growth factor; Wounds and injuries; Messenger RNA
Introduction
Growth factors are a large family of polypeptidic molecules that regulate key cellular events, including cell proliferation, chemotaxis and differentiation after binding to specific receptors [1]. Research on growth factors has undergone substantial steps during the last decades due to the pivotal role of those molecules in tissue healing, repair and regeneration [1]. Wound healing involves several stages that have been recognized as the inflammatory reaction, the granulation tissue stage and the tissue- remodeling stage [1]. During these events, cell function is regulated by a few elements, counting cell–matrix interactions and soluble molecules such as, cytokines and growth factors [2]. The sequence of cell activation by growth factors includes events that have not yet been elucidated. Platelet-derived growth factors (PDGFs), vascular endothelial growth factor (VEGF), insulin-like growth factors (IGF), fibroblast growth factors (FGFs), epidermal growth factor (EGF), transforming growth factor beta (TGF-β) and bone morphogenetic proteins (BMPs) have been extensively studied in order to understand their roles in wound healing [3]. Current evidence indicates the presence of growth factors in several stages of the healing cascade after trauma. Their release starts immediately after the disruption of blood vessels from local platelets during blood clot formation, they regulate inflammatory cell chemotaxis and proliferation of fibroblasts and monocytes and enhance vascularization [4–6].
Healing of oral mucosal trauma is a particularly interesting phenomenon because gingival tissues heal surprisingly fast when compared with other tissues [7]. Possible explanations of this phenomenon are the presence of saliva in the oral cavity and the high vascularization of the oral mucosa [8,9]. Healing after non-surgical and surgical periodontal therapy follows similar pathways including blood clot formation and tissue remodeling. Research on growth factors on periodontal wound healing and regeneration has shown the potential ability of growth factors, combined or not with bone grafts, to regenerate the periodontal attachment apparatus [1]. Interestingly PDGF-BB combined with β-tricalcium phosphate (β-TCP) has been approved by Federal Drug Administration (FDA) for use in periodontal regenerative surgeries [10].
However, when periodontal wound healing is considered, smoking is a critical constraint. Smoking disturbs healing with the effects of nicotine on cell function and hypoxic effects of carbon monoxide [11–13]. In addition it provokes vasoconstriction and reduces the rate of neoangiogenesis [12]. In periodontology smoking has been recognized as one of the major risk factors of periodontitis [14] and of course attenuates the healing response after surgical and no surgical periodontal treatment [15–17].
The role of EGF in periodontal wound healing has not been clearly described yet. EGF is a small polypeptide growth factor found to be involved in control of epithelial growth and differentiation of periodontal tissues [18]. EGF has been found in milk, urine, amniotic fluid and saliva [19–21] and is involved in several events including embryonic development [22] tissue regeneration [23–25] and healing response [26,27]. Both animal and human trials indicated its role in healing after trauma and ulceration [28–30]. Clinical studies have shown that EGF enhances the proliferation of epithelial cells and fibroblasts [31]. In addition, it has been found in elevated levels in saliva after surgical periodontal therapy [32] and topical application of the growth factor has shown signs of rapid healing [33], indicating its potential role in the repair of oral tissues.
The scope of the current study was to investigate the potential association among the pre-surgical levels of EGF-mRNA in gingival tissues with the early healing rate. In addition, the role of smoking in the previous association was examined.
Materials And Methods
Study design and sample size calculation
The study was designed as a cross-sectional study including patients attending the Postgraduate Clinic of the department of Periodontology and Implant biology of Dental School, Aristotle University of Thessaloniki, for periodontal therapy. All participant’s undergone periodontal surgery between September 2018 and May 2019.
The study protocol was approved by the ethics committee of The Faculty of Dentistry of the Aristotle University of Thessaloniki (protocol number, 4/13.02.2019). All participants received written information regarding the aim and the procedures of the study, and they were asked to sign a consent form.
The association among the pre-surgical EGF-mRNA levels and the rate of healing was examined with the Spearman’s rank correlation coefficient. The statistical significance level was set at p-value ≤ 0.05, thus the sample size calculation resulted in 38 patients.
Inclusion exclusion criteria
Patients included in the study were referred to the postgraduate periodontology clinic for periodontal treatment. The participants were planned to receive surgical periodontal therapy (access flap). Only systemically healthy adult patients were considered eligible for the study. All participants had to sign their inform consent. Participants were divided in 2 separate groups concerning smoking. Exclusion criteria were set as systemic disease which would not allow surgical periodontal therapy, systemic medication or drugs affecting gingival tissue healing (insulin, calcium channel blockers, bisphosphonates, immune suppressants and anticonvulsants), use of antimicrobials during the last 6 months, pregnancy and lactation and acute inflammation in the oral cavity the previous 3 months.
Surgical process
All surgical procedures were carried out by postgraduate students of the department of Periodontology and implant biology. The main surgical scheme was an access flap for periodontal debridement in the maxilla, with osteotomy or osteoplasty was indicated. The surgical process included local application of anaesthesia, elevation of full-thickness flaps, scaling and root-planing with removal of the granulation tissue, osteoplasty, when needed, and suturing.
Collection of gingival tissue
In each surgical procedure an incision 2 mm in length was made 2mm palataly of the gingival margin and 3mm in depth. From each incision a small fragment of epithelial and connective tissue (0,5 mm) was collected and stored in RNA later (Sigma) at -80° C until RNA isolation.
RNA isolation and reverse transcription
Total RNA was isolated from the tissue fragment using the Nucleospin RNA Kit (Macherey Nagel) with modifications. Briefly, the tissue fragment was removed from the RNA later solution and was ground in lysis buffer in a 2 mL tube containing a mix of 1.4 mm and 2.8 mm zirconium oxide beads (CK mix, Bertin Instruments). Three cycles of homogenization at full speed for 30 s were performed in a Minilys homogenizer (Bertin Instruments) with a 60 s rest on ice. After homogenization, the samples were treated as described in the Nucleospin RNA kit protocol. RNA concentration in each sample was measured using a Genova Plus spectrophotometer (Jenway). 200 μg RNA from each sample were reversibly transcripted to cDNA using the PrimerScript II 1st strand cDNA synthesis kit [Takara] following manufacturer’s protocol, using random hexamer primers. The cDNA samples were stored at -20°C until further processing. Quantitative real-time PCR [RT-PCR] was performed in 96- well PCR plates in triplicate. Each 20 μl reaction contained 2 μL cDNA, 1 μL of each primer [10 μM], 10 μL 2x Luna ® qPCR Master Mix (New England Biolabs) and 6 μl RNAse free water. The primers used for EGF expression were ΕGF-Forward: 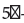 CAACCAGTGGCTGGTGAGGA
CAACCAGTGGCTGGTGAGGA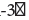 , and EGF-Reverse:
, and EGF-Reverse: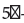 GAGCCCTTATCACTGGATACTGGAA
GAGCCCTTATCACTGGATACTGGAA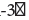 [34]. The gene encoding for the human β-actin protein was used as a reference gene to normalize target gene expression. Actin primers were Forward: 5’ CATGGATGATGATATCGCCGCG 3’ and Reverse: 5’ACATGATCTGGGTCATCTTCTCG 3’ [35]. The thermal cycler protocol was comprised of an initial denaturation step at 95° C for 3 min, followed by 40 cycles of heating at 95° C for 15 s, then 60° C for 30 s followed by a plate read. A melting curve was constructed between 95° C and 60° C with plate reads every 0.5° C to verify the amplified products. A threshold cycle (Ct) was set according to the standard curves constructed for each gene. Relative gene expression analysis was achieved according to the double delta Ct analysis (ΔΔCt) method [36].
[34]. The gene encoding for the human β-actin protein was used as a reference gene to normalize target gene expression. Actin primers were Forward: 5’ CATGGATGATGATATCGCCGCG 3’ and Reverse: 5’ACATGATCTGGGTCATCTTCTCG 3’ [35]. The thermal cycler protocol was comprised of an initial denaturation step at 95° C for 3 min, followed by 40 cycles of heating at 95° C for 15 s, then 60° C for 30 s followed by a plate read. A melting curve was constructed between 95° C and 60° C with plate reads every 0.5° C to verify the amplified products. A threshold cycle (Ct) was set according to the standard curves constructed for each gene. Relative gene expression analysis was achieved according to the double delta Ct analysis (ΔΔCt) method [36].
Evaluation of the healing response
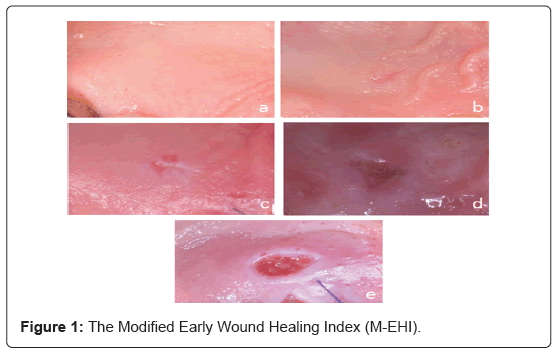
Post-surgical gingival tissue healing evaluation included the examination of intraoral photographs at 3 different time points after the surgical procedure [1week (1), 2 weeks (T2), 2 months (T3)]. All photos were assessed independently by two different examiners (S.P., E.D.). Two different indexes were used for the assessment of early healing. The early wound healing-index (EHI) proposed by Wachtel and co-workers [37] was modified and used for the evaluation of the healing response during the 1st (T1) and 2nd (T2) week of healing (Figure 1). More specifically the modified Wachtel index (M-EHI) includes.
1: Complete trauma closure – no fibrin line present between the wound edges (Figure 1a).
2: Complete trauma closure – fine fibrin line present wound edges (Figure 1b).
3: Complete trauma closure – fibrin clot in the area wound edges (Figure 1c).
4: Incomplete trauma closure – partial necrosis of the tissue wound edges (Figure 1d).
5: Incomplete trauma closure – complete necrosis of the tissue wound edges (Figure 1e).
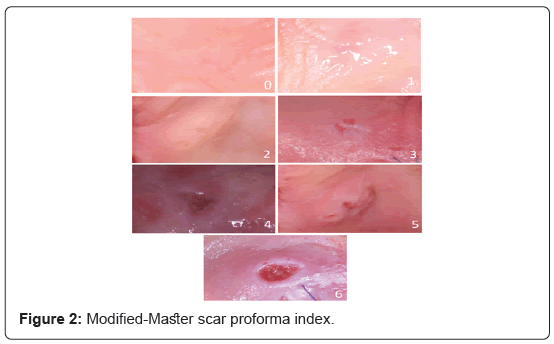
The “Manchester scar Proforma” index [38] was modified (M-MSPI) and used for the evaluation of the scar formation in the 1st (T1), 2nd (T2) and 3rd (T3) week of healing. The modified index included a 0-6 scale depending on the color, distortion and contour of the tissues (Table 1). The lower the final score, the better healing capacity.
| (A) Color (Compared With Surrounding Skin) | (B) Contour | (C) Distortion |
|---|---|---|
| 0 Perfect | 0 Flush with surrounding skin | 0 None |
| 1 Slight mismatch | 1 Slightly proud or indented | 1 Mild or moderate |
| 2 Obvious or gross mismatch | 2 Hypertrophic | 2 Severe |
Table 1: Modified Manchester Scar Proforma index (M-MSPI) for clinical scar assessment (score range: 0–6; lower values represent improved healing quality, i.e., less scar), Adapted from Wong et al, 2009.
Statistical analysis
The association among the pre-surgical EGF-mRNA levels and the rate of response was examined with the Spearman’s rank correlation coefficient. Linear regression model was used for the investigation of the potential association among EGF levels and the expression of healing with the Wachtel and Master Scar Proforma Indexes in each time point. Multiple linear regression models were used for the examination of the effects of smoking on the previous association. For the statistical analysis IBMM SPSS Statistics 20 was used. The statistical significance level was set at p-value ≤ 0.05.
Results
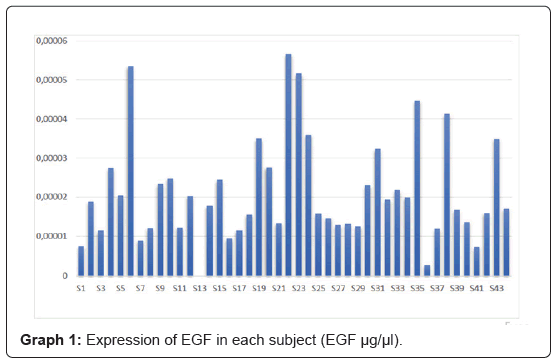
The study included 44 patients according to the established inclusion criteria, however, one patient had to be excluded from the final analysis due to zero amplification of EGF and β-actin, resulting in a final sample of 43 subjects. The mean age of the patients was 52.8 years (Table 2). Among the included participants 62.79% [27] were nonsmokers and 37.21% (16) smokers. The expression of EGF mRNA levels in each subject is illustrated in Graph 1 (Figure 2).
| N (%) | |
|---|---|
| Sex | |
| Men | 21 (48.84) |
| Women | 22 (51.16) |
| Smoking | |
| Non-Smoker | 27 (62.79) |
| Smoker | 16 (37.21) |
| Age (Mean ± S.D.) | 52.79 ± 12.07 |
Mean ± S.D., Mean, Standard Deviation
Table 2: Demographic characteristics of the participants.
Evaluation of the healing response
The mean values of the M-EHI during the 1st week (T1) were 2.08 ± 1.05. Among the 42 patients attending the T1 evaluation, 16 presented a mean value of 1, 12 a mean M-EHI of 2, 10 a mean value of 3 while 3 patients had a mean value of 4. Only 1 patient presented a mean value of 5 accompanied with partial necrosis of the wound. In the 2nd week examination (T2), the mean values of M-EHI were 1.53 ± 0.91 with most of the participants presenting a value of 1 or 2 (76% of patients). The mean values and the standard deviation for the M-EHI in each examination (T1, T2) are depicted in Table 3.
| Index | Value (Mean ± S.D) |
|---|---|
| M-EHI – T1 | 2.08 ± 1.05 |
| M-EHI – T2 | 1.53 ± 0.91 |
| M-MSPI – T1 | 2.55 ± 1.72 |
| M-MSPI – T2 | 1.74 ± 1.31 |
| M-MSPI – T3 | 0.89 ± 1.32 |
Mean ± S.D., Mean, Standard Deviation
M-EHI, Modified Early Healing Index
M-MSPI, Modified Master Scar ProForma Index
Table 3: Healing response indexes mean values.
The M-MSPI, used for the evaluation of scar formation, had a mean value of 2.55 ± 1.72 at T1, 1.74 ± 1.31 at T2 and 0.89 ± 1.32 at T3 (Table 3). More specifically during the first two weeks of healing (T1, T2) most of the patients presented a value of 1 or 2 (59% of patients at T1, 86% at T2) while only 3 and 6 patients presented a value of 0 at T1 and T2 respectively. In the second month of healing (T3) most of the participants presented a value of 0 or 1 (69% of patients), while none of the patients had a value of 6.
Association of EGF expression in gingival tissue and early healing response
The association of the EGF pre-surgical mRNA levels and the M-EHI values are depicted in Table 4. During the 1st week of healing (T1) the increase of EGF expression was accompanied with a trend towards a reduction in the M-EHI values, however the later association did not reach a statistical significance (p-value, 0.813>0.05). Smokers had an increased value of M-EHI (+0.29) in comparison with non-smokers, however the difference was not statistically significant. In the second week (T2) (Table 4), the trend was reversed, as increasing EGF levels were accompanied with an increase of M-EHI values. Nor this association was significant, however (p-value, 0.541>0.05). Again, smokers and non-smokers did not differ in the M-EHI values. In addition, smoking did not affect EGF expression in any time points (p-value: 0.382 at T1, 0.174 at T2).
| Single Variable Analysis | Multi Variable Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Evaluation | 95% CI | p-value | Evaluation | 95% CI | p-value | |
| T1 | EGF | -3031.65 | -28705.35, 22642.06 | 0.813 | -4513.6 | -30512.83, 21485.63 | 0.727 |
| Smoking | |||||||
| Non-Smoker | - | - | - | - | |||
| Smoker | 0.29 | -0.40, 0.97 | 0.404 | 0.3 | -0.40, 1.00 | 0.388 | |
| T2 | EGF | 6735.93 | -15349.68, 28821.54 | 0.541 | 6638.59 | -15975.74, 29252.91 | 0.556 |
| Smoking | |||||||
| Non-Smoker | - | - | |||||
| Smoker | 0.04 | -0.55, 0.63 | 0.883 | 0.02 | -0.58, 0.62 | 0.952 |
CI, Confidence interval
Table 4: Linear Regression Model of The Association of M-EHI with smoking and EGF expression in the 1st and 2nd week of healing.
As for the M-MSPI statistical analysis revealed a trend for a reduction in the indexes levels as the EGF expression increased, both in T1 and T2 (Table 5). This association was not significant (p-value, 0.271 at T1, 0.417 at T2). The trend was reversed in the second month of healing (T3) but neither at this time the association did not reach a statistical significance. Smoking did not affect either the EGF expression or the association among the variables at any time point (Table 5).
| Single Variable Analysis | Multi Variable Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Evaluation | 95% CI | p-value | Evaluation | 95% CI | p-value | |
| T1 | EGF | -22935.24 | -64425.06, 18554.58 | 0.271 | -23491.19 | -65893.27, 18910.88 | 0.269 |
| Smoking | |||||||
| Non-Smoker | - | - | |||||
| Smoker | 0.03 | -1.10, 1.16 | 0.958 | 0.11 | -1.03, 1.25 | 0.842 | |
| T2 | EGF | 12884.74 | -18859.55, 44629.03 | 0.417 | 13598.86 | -18866.33, 46064.06 | 0.402 |
| Smoking | |||||||
| Non-Smoker | - | - | |||||
| Smoker | -0.08 | -0.93, 0.77 | 0.847 | -0.13 | -1.00, 0.73 | 0.756 | |
| T3 | EGF | 3560.47 | -29411.57, 36532.51 | 0.828 | 5319.38 | -28656.83, 39204.58 | 0.752 |
| Smoking | |||||||
| Non-Smoker | - | - | |||||
| Smoker | -0.25 | -1.18, 0.69 | 0.593 | -0.28 | -1.24, 0.69 | 0.565 |
CI, Confidence interval
Table 5: Linear Regression Model of The Association of M-MSPI with smoking and EGF expression in the 1st, 2nd week and 2nd month of healing.
Discussion
EGF is a potent mitogenic factor, induces epithelial cell proliferation and division, improves collagen construction and has also chemotactic effects on vascular endothelial cells and fibroblasts [39]. The role of EGF in oral wound healing has been investigated mainly in studies using saliva [40,41]. A temporal increase in EGF protein concentration in saliva has been found after 3rd molar extraction, tumor removal from parotitis gland and after surgical periodontal therapy [32], while local application of the factor in skin grafted sites seems to promote the healing ability [33]. Furthermore, EGF has been found to be expressed in gingival tissues and periodontal ligament cells [34,42]. Application of human gingival fibroblasts in cutaneous radiation burns accelerated wound healing by modulating expression of FGF7, EGF and VEGF [43]. Moreover, mucosal transplants accelerated wound repair in rats’ abdomen compared to skin transplants by upregulating EGF and VEGF expression [39]. Those findings suggest a potential role of EGF in early healing of mucosal trauma.
In the current study the expression of EGF mRNA in keratinized oral epithelium was examined, while the association between mRNA levels and the healing ability was also studied. It is known that early wound healing is influenced by a variety of factors such as surgical experience, tissue management, primary or secondary wound closure wound and patients’ post-operative behavior, such as oral hygiene performance and smoking habits. Consequently, in order to eliminate all these critical factors for the wound healing response, we decided to examine wound closure in a small tissue fragment 2 × 2 × 3 mm was removed from the hard palate and the wounded area was closed with 2 interrupted sutures. For the clinical evaluation of the early healing response the Wachtel early healing index [37] was modified (M-EHI) to assess trauma closure and during the first two weeks of healing. Moreover the <<Manchester Scar Proforma>> index [38] was used for the evaluation of scar tissue formation at 1 and 2 weeks, and at 2 months of healing.
The results of the study unveiled a trend towards a better healing response in the first week of healing as EGF mRNA levels increased and as evaluated by the two clinical indexes. This association did not reach statistical significance.
Surprisingly in in the second week and second month of healing the later association was reversed, suggesting a compromised healing response in the cases with increased EGF levels. However, neither this association was significant. Smokers and non-smokers were evaluated in a separate analysis and smoking did not seem to affect healing at any time point. It is possible that EGF might be implicated in the quality of tissue response, but its expression increases after the initiation of trauma. Similarly with this hypothesis, a previous study examining the EGF levels after mucosa grafting in rats’ abdomen showed a substantial increase in EGF mRNA and protein expression 3 days after grafting and this increase was higher in mucosal compared to dermal grafts [39]. As a result, it was impossible to detect any association at the time of tissue harvesting. If we could harvest tissue at later time points, we might have seen an association between a better healing response and increased EGF mRNA levels.
Another possible explanation is that an increase in any protein expression does not necessarily mean an increase in mRNA levels, since there are various post-transcriptional mechanisms involved before and during translation [44]. One of those common mechanisms is RNA silencing, which means RNA degradation by small double stranded RNAs [45]. Moreover, only EGF secreted in saliva and not the one present in gingival epithelial cells or fibroblasts might be implicated in tissue healing response.
In addition, EGF might play a role in wound healing only in the presence of inflammation. It was previously shown that one of the virulence factors of Porphyromonas gingivalis (The peptidylarginine deiminase PPAD), was inhibition of the ability of EGF to accelerate cell proliferation [46], affecting the function of many different signals. It is possible that our study did not find an association between EGF and wound healing, because we examined only healthy gingival tissues. Finally, we can also speculate that since early wound healing is a complicated mechanism, other growth factors are important for the quality of healing response.
Conclusion
The current study suggests a potential role of EGF in early gingival healing, although the results did not reach statistical significance. Future studies could include a larger sample and correlation of both EGF mRNA and protein levels at different time points after gingival injury.
Acknowledgements
The authors would like to thank Apostolos Angelidis, Department of Food Hygiene and Technology of Food of Animal Origin, for providing the laboratory equipment for the mRNA isolation. Also Persefoni Talimtzi for helping in the statistical analysis of the results.
Authors’ Contributions
The conception of the study belongs to Makris Georgios and Aikaterini-Elisavet Doufexi, who also designed part of the acquisition of the study. Aikaterini-Elisavet Doufexi supervised the clinical work, took part in the analysis of the data and was responsible for the final approval for the work to be published. Stergoula Papamanoli worked on all the needed clinical processes for the acquisition of the sample, followed up the clinical examinations of the patients and performed the mRNA extractions. Ioannis Fragkioudakis did part of the clinical work, drafted and revised the manuscript. Mary Kalamaki designed and performed the gene expression experiments, analyzed the data and revised the text before the final submission.
Declaration of Interest
The authors certify that they have no commercial or associative interest that represents a conflict of interest in connection with the manuscript.
Funding
This study was funded by the department of Periodontology and Implant Biology, Dental School, Aristotle University of Thessaloniki.
References
- Smith PC, MartÃnez C, Cáceres M, MartÃnez J (2015) Research on growth factors in periodontology. J Periodontol 67:234–50.
- Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. 453:314–21.
- Abraham JA, Klagsbrun M (1998) Modulation of Wound Repair by Members of the Fibroblast Growth Factor Family. The Molecular and Cellular Biology of Wound Repair, Springer: 195–248.
- Clark RAF (1993) Regulation of fibroplasia in cutaneous wound repair. Am J Med Sci 306:42–8.
- Heldin CH, Westermark B (1999) Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79:1283–316.
- Gale NW, Yancopoulos GD (1999) Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev 13:1055–66.
- Larjava H, Wiebe C, Gallant-Behm C, Hart DA, Heino J, et al. (2011) Exploring scarless healing of oral soft tissues. J Can Dent Assoc 77:b18.
- Zelles T, Purushotham KR, Macauley SP, Oxford GE, Humphreys-Beher MG (1995) Concise Review: Saliva and Growth Factors: The Fountain of Youth Resides in Us All. J Dent Res 74:1826–32.
- Schapher M, Wendler O, Gröschl M (2011) Salivary cytokines in cell proliferation and cancer. Clin Chim Acta 412:1740–8.
- Nevins M, Giannobile WV, McGuire MK, Kao RT, Mellonig JT, et al. (2005) Platelet-Derived Growth Factor Stimulates Bone Fill and Rate of Attachment Level Gain: Results of a Large Multicenter Randomized Controlled Trial. J Periodontol 76:2205–15.
- Sherwin MA, Gastwirth CM (1990) Detrimental effects of cigarette smoking on lower extremity wound healing. J Foot Surg 29:84–7.
- Nolan J, Jenkins RA, Kurihara K, Schultz RC (1985) The acute effects of cigarette smoke exposure on experimental skin flaps. Plast Reconstr Surg 75:544–9.
- Mosely LH, Finseth F (1977) Cigarette smoking: Impairment of digital blood flow and wound healing in the hand. Hand 9:97–101.
- Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, et al. (2018) Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol 45:S162–70.
- Grossi SG, Goodson JM, Gunsolley JC, Otomo-Corgel J, Bland PS, et al. (2007) Mechanical Therapy With Adjunctive Minocycline Microspheres Reduces Red-Complex Bacteria in Smokers. J Periodontol 78:1741–50.
- Heasman L, Stacey F, Preshaw PM, McCracken GI, Hepburn S, et al. (2006) The effect of smoking on periodontal treatment response: A review of clinical evidence. J Clin Periodontol 33:241–53.
- Preber H, Bergström J (1990) Effect of cigarette smoking on periodontal healing following surgical therapy. J Clin Periodontol 17:324–8.
- Nordlund L, Hormia M, Saxén L, Thesleff I (1991) Immunoshistochemical localization of epidermal growth factor receptors in human gingival epithelia. J Periodontal Res 26:333–8.
- Carpenter G, King L, Cohen S (1978) Epidermal growth factor stimulates phosphorylation in membrane preparations in vitro. Nature 276:409–10.
- Fisher DA, Lakshmanan J (1990) Metabolism and effects of epidermal growth factor and related growth factors in mammals. Endocr Rev 11:418–42.
- Dvorak B (2010) Milk Epidermal Growth Factor and Gut Protection. J Pediatr 156:S31-5.
- Wei Z, Park KW, Day BN, Prather RS (2001) Effect of epidermal growth factor on preimplantation development and its receptor expression in porcine embryos. Mol Reprod Dev 60:457–62.
- Suzuki Y, Yanagisawa M, Yagi H, Nakatani Y, Yu RK (2010) Involvement of β1-integrin up-regulation in basic fibroblast growth factor- and epidermal growth factor-induced proliferation of mouse neuroepithelial cells. J Biol Chem 285:18443–51.
- Tamama K, Kawasaki H, Wells A (2010) Epidermal Growth Factor (EGF) treatment on Multipotential Stromal Cells (MSCs). Possible enhancement of therapeutic potential of MSC. J Biomed Biotechnol 795385.
- Soeda A, Inagaki A, Oka N, Ikegame Y, Aoki H, et al. (2008) Epidermal growth factor plays a crucial role in mitogenic regulation of human brain tumor stem cells. J Biol Chem 283:10958–66.
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M (2008) Growth factors and cytokines in wound healing. Wound Repair Regen 16:585–601.
- Sari Kiliçaslan SM, Coşkun Cevher Ş, Güleç Peker EG (2013) Ultrastructural changes in blood vessels in epidermal growth factor treated experimental cutaneous wound model. Pathol Res Pract 209:710–5.
- Niall M, Ryan GB, O’Brien BM (1982) The effect of epidermal growth factor on wound healing in mice. J Surg Res 33:164–9.
- Olsen PS, Poulsen SS, Kirkegaard P, Nexø E (1984) Role of submandibular saliva and epidermal growth factor in gastric cytoprotection. Gastroenterology 87:103–8.
- MacNeil S, Dawson RA, Crocker G, Barton CH, Hanford L, et al. (1988) Extracellular calmodulin and its association with epidermal growth factor in normal human body fluids. J Endocrinol 118:501–9.
- Kim JM, Bak EJ, Chang JY, Kim S-T, Park W-S, et al. (2011) Effects of HB-EGF and epiregulin on wound healing of gingival cells in vitro. Oral Dis 17:785–93.
- Oxford GE, Nguyen KH, Alford CE, Tanaka Y, Humphreys-Beher MG (1998) Elevated salivary EGF levels stimulated by periodontal surgery. J Periodontol 69 :479–84.
- Brown GL, Nanney LB, Griffen J, Cramer AB, Yancey JM, et al. (1989) Enhancement of Wound Healing by Topical Treatment with Epidermal Growth Factor. N Engl J Med 321:76–9.
- Teramatsu Y, Maeda H, Sugii H, Tomokiyo A, Hamano S, et al. (2014) Expression and effects of epidermal growth factor on human periodontal ligament cells. Cell Tissue Res 357:633–43.
- Kalamaki M, Zempila M, Doufexi V (2018) PR336: The role of Prx2 transcription factor, tenascin-C and TGF-β1 in promoting scarless healing ability οf free soft tissue gingival grafts. J Clin Periodontol: 233–233.
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, et al. (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–22.
- Wachtel H, Schenk G, Böhm S, Weng D, Zuhr O,et al. (2003) Microsurgical access flap and enamel matrix derivative for the treatment of periodontal intrabony defects: a controlled clinical study. J Clin Periodontol 30:496-504.
- Wong JW, Gallant-Behm C, Wiebe C, Mak K, Hart DA, et al. (2009) Wound healing in oral mucosa results in reduced scar formation as compared with skin: Evidence from the red duroc pig model and humans. Wound Repair Regen 17:717–29.
- Qi S, Yang C, Zhu M, Chen H (2019) Effect of oral mucosal transplantation on the expression of EGF and VEGF-C during skin wound repair. Exp Ther Med: 320–5.
- Campreciós G, Navarro M, Soley M, RamÃrez I (2009) Acute and chronic adrenergic stimulation of submandibular salivary glands. Effects on the endocrine function of epidermal growth factor in mice. Growth Factors 27:300–8.
- Hormia M, Thesleff I, Perheentupa J, Pesonen K, Saxén L (1993) Increased rate of salivary epidermal growth factor secretion in patients with juvenile periodontitis. Scand J Dent Res 101:138–44.
- Irwin CR, Schor SL, Ferguson MW (1991) Expression of EGF-receptors on epithelial and stromal cells of normal and inflamed gingiva. J Periodontal Res 26:388–94.
- Linard C, Tissedre F, Busson E, Holler V, Leclerc T, et al. (2015) Therapeutic potential of gingival fibroblasts for cutaneous radiation syndrome: Comparison to bone marrow-mesenchymal stem cell grafts. Stem Cells Dev 24:1182–93.
- Greenbaum D, Colangelo C, Williams K, Gerstein M (2003) Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 4:117.
- Podolska K, Svoboda P (2011) Targeting genes in living mammals by RNA interference. Brief Funct Genomics 10:238–47.
- Pyrc K, Milewska A, Kantyka T, Sroka A, Maresz K, et al. (2013) Inactivation of epidermal growth factor by Porphyromonas gingivalis as a potential mechanism for periodontal tissue damage. Infect Immun 81:55–64.
Citation: Papamanoli S, Fragkioudakis I, Kalamaki MS, Makris G, Doufexi AE (2021) The Role of Epidermal Growth Factor in Early Tissue Healing After Surgical Periodontal Therapy. J Oral Hyg Health 9: 278. DOI: 10.4172/2332-0702.1000278
Copyright: © 2021 Papamanoli S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
11th International Conference on Complementary & Alternative Medicine
Zurich, Switzerland
9th World Conference on Nursing Education & Nursing Practice
Toronto, CanadaRecommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2589
- [From(publication date): 0-2021 - Dec 03, 2024]
- Breakdown by view type
- HTML page views: 1959
- PDF downloads: 630