The Role of Diffusion Weighted Imaging and Magnetic Resonance Imaging Scoring System in Assessing the Effectiveness of Treatment with Hypothermia in Neonates with Hypoxic-ischemic Encephalopathy
Received: 19-Aug-2017 / Accepted Date: 12-Sep-2017 / Published Date: 21-Sep-2017 DOI: 10.4172/2572-4983.1000135
Abstract
Background: The aim was to assess whether magnetic resonance imaging (MRI) that included diffusion weighted imaging (DWI) with apparent diffusion coefficient (ADC) values and morphological MRI scoring system ultimately correlated with neurologic assessment at 1 month of age in neonates with hypoxic-ischemic encephalopathy (HIE) treated with hypothermia.
Objective: With ADC values and MRI scoring system we can predict the efficiency of treatment with hypothermia at 1 month of age for neonates with hypoxic-ischemic brain injury and we can set the threshold ADC values.
Materials and methods: The retrospective study enrolled 25 neonates with HIE treated with hypothermia. All neonates had MRI within 9 days of life and an Amiel-Tison neurologic assessment at 1 month of age. The level of injury was evaluated by Mary Rutherford scoring system and ADC values were measured. Data were analyzed by Mann-Whitney U test and ROC curve. Results Sixteen neonates had well and 9 neonates had poor clinical outcome. We confirmed a statistically significant difference of ADC values between both groups in the posterior limb of the internal capsule/thalamus (PLIC/T) and in the deep white matter (WM). The threshold ADC value measured in the PLIC/T was set at 920 × 10-6 mm2/s and the threshold ADC value measured in the WM was set at 1153 × 10-6 mm2/s. Statistically, significant differences by the morphological scoring system were confirmed in the basal ganglia/ thalamus (BG/T) and in the cortex.
Conclusion: DWI and MRI have an important role in predicting clinical outcome at 1 month of age for neonates with HIE treated with hypothermia.
Keywords: Hypoxic-ischemic encephalopathy; Neonate; Diffusion weighted imaging; Morphological scoring system; Hypothermia; Clinical outcome
27966Introduction
Hypoxic-ischemic encephalopathy (HIE) is one of the most important reasons for perinatal mortality and morbidity [1]. It affects 1-3/1000 live born neonates [2]. HIE develops due to a deprivation of oxygen before, during or shortly after birth. This is called perinatal asphyxia. Which part of the neonatal brain will be affected the most and how severe the damage will be, depends on the velocity and range of oxygen deprivation [3,4]. In acute near-total asphyxia, the most affected parts of the brain are basal ganglia (BG), thalamus (T) and posterior limb of the internal capsule (PLIC) [3]. In prolonged partial asphyxia, the most affected parts are deep white matter (WM) and cortex [4]. The most effective known therapy for HIE is hypothermia [5]. Whole-body cooling has to be initiated within 6 hours of birth. Body temperature is maintained between 33.5 and 34.5°C for 72 hours. This is followed by slow re-warming over 4 hours, until the neonate’s rectal temperature reaches from 36.5 to 37°C [6-9]. Review of the 11 randomized controlled trials by Jacobs, et al. showed that hypothermia reduces mortality in term and late preterm neonates with HIE without increasing major disability in survivors. The review emphasized that the benefits of hypothermia outweigh the short-term adverse effects, such as sinus bradycardia and thrombocytopenia [10].
HIE brain lesions are at their most visually obvious on conventional T1 and T2 weighted magnetic resonance imaging (MRI) sequences between 1 and 2 weeks from delivery. The diffusion weighted imaging (DWI) appearances of brain lesions can be seen during the first 24 hours of infarction and last for 7-14 days [3]. Bednarek, et al. showed that the time course of the apparent diffusion coefficient (ADC) reduction is delayed in neonates with HIE treated with hypothermia. Pseudonormalization of the ADC values was slower in the group of hypothermic neonates, which occurred after the tenth day, as compared to the group of normothermic neonates, where pseudonormalization occurred after 6–8 days [11]. A recent study by Alderliesten, et al. demonstrated the association between low ADC values of the BG and T and adverse outcome in neonates with HIE treated with hypothermia [12]. Goergen, et al. found that the lowest value for the ADC in the group with good clinical outcome at 2 years of age was 1020 ×10-6 mm2/s for the BG and 960 × 10-6 mm2/s for the T. All neonates had MRI between days 3 and 7. No neonate in the study was treated with hypothermia [1].
In the study of Twomey, et al. all neonates had MRI within 10 days of birth, however none of them was treated with hypothermia. All neonates with an ADC value in the PLIC of -6 mm2/s had an unfavorable outcome at 2 years of age. Neonates with an ADC value of >860 × 10-6 mm2/s in the PLIC combined with an ADC value of >1070 × 10-6 mm2/s in the BG had a favorable outcome. ADC values in the WM were also predictive of outcome [13]. Vermeulen, et al. showed that all neonates with HIE and poor clinical outcome at 2 years of age had lower mean ADC values in the PLIC, T, BG, parietal cortex (PC) and hippocampus, in comparison to neonates with a good clinical outcome. ADC values in the PLIC lower than 850 × 10-6 mm2/s were predictive of a poor clinical outcome. MRI was done between 1 and 45 days after birth [14]. The primary object of the present study was to assess the value of DWI in the evaluation of the efficiency of treatment with hypothermia for neonates with hypoxic-ischemic brain injury, secondarily to assess which region of ADC value measurement is statistically associated with clinical outcome at 1 month of age for neonates with HIE treated with hypothermia and tertiary to determine if ADC values and scores given by the morphological MRI scoring system are equivalent in predicting clinical outcome at 1 month of age.
Materials And Methods
All neonates with HIE enrolled in our study were treated with whole body hypothermia. They underwent MRI within 9 days of birth and had satisfied the inclusion criteria defined by the Department of Pediatric Surgery and Intensive Care, University Medical Centre Ljubljana.
The inclusion criteria were a gestational age of 36 weeks or more, with at least one of the additional criteria, such as an Apgar score under 5 at 10 minutes, reanimation or acidosis with pH<7.0, neurological signs of moderate or severe HIE, at least 30 minutes long EEG recording showing abnormal brain activity or seizures. Patients with congenital malformations due to chromosomopathy or malformations connected to severe brain anomaly and patients older than 6 hours at the time of hypothermia were excluded from the study. Clinical characteristics of the neonates with HIE enrolled in our study are represented in Table 1.
| Infant | Sex | Gestational age (weeks) | Birth weight (grams) | Apgar score (1., 5., 10. minute) | Initiation of hypothermia (hours) | Arterial cord pH |
|---|---|---|---|---|---|---|
| 1 | Male | 41 | 2800 | 1, 4, 5 | 5 | 6,82 |
| 2 | Male | 41 | 3610 | 4, 7, 8 | 4 | 6,73 |
| 3 | Male | 40 | 3400 | 1, 6, 8 | 6 | 6,83 |
| 4 | Female | 38 | 3580 | 1, 1, 1 | 3,5 | 6,80 |
| 5 | Female | 41 | 3865 | 1, 4, 4 | 4,5 | 6,70 |
| 6 | Female | 39 | 4010 | 3, 4, 4 | 6 | 6,81 |
| 7 | Male | 39 | 2880 | 1, 6, 7 | 3 | 7,12 |
| 8 | Male | 40 | 3030 | 2, 5, 6 | 2,5 | 7,00 |
| 9 | Male | 40 | 3315 | 0, 0, 4 | 3 | 6,70 |
| 10 | Male | 37 | 2720 | 1, 2, 3 | 3 | 6,60 |
| 11 | Male | 39 | 3000 | 3, 5, 6 | 3 | 7,23 |
| 12 | Female | 38 | 3460 | 1, 3, 5 | 3,5 | 7,44 |
| 13 | Female | 40 | 3100 | 1, 1, 2 | 3 | 7,18 |
| 14 | Male | 36 | 2420 | 0, 4, 6 | 4,5 | 6,94 |
| 15 | Female | 40 | 2500 | 0, 4, 7 | 3 | 7,46 |
| 16 | Male | 40 | 3500 | 2, 5, 7 | 2,5 | 7,09 |
| 17 | Male | 38 | 3180 | 1, 6, 7 | 4 | 6,89 |
| 18 | Female | 40 | 3450 | 1, 1, 3 | 2,5 | 6,88 |
| 19 | Male | 37 | 3100 | 0, 4, 5 | 3,5 | 7,25 |
| 20 | Male | 39 | 2950 | 3, 4, 6 | 4,5 | 6,90 |
| 21 | Female | 36 | 2580 | 0, 0, 0 | 3 | 6,57 |
| 22 | Female | 36 | 2680 | 1, 5, 8 | 3,5 | 7,15 |
| 23 | Female | 41 | 3300 | 1, 2, 3 | 5 | 6,97 |
| 24 | Female | 38 | 3300 | 6, 1, 1 | 4 | 6,99 |
| 25 | Female | 38 | 2320 | 1, 4, 6 | 2,5 | 7,19 |
Table 1: Clinical characteristics of the neonates with HIE treated with hypothermia.
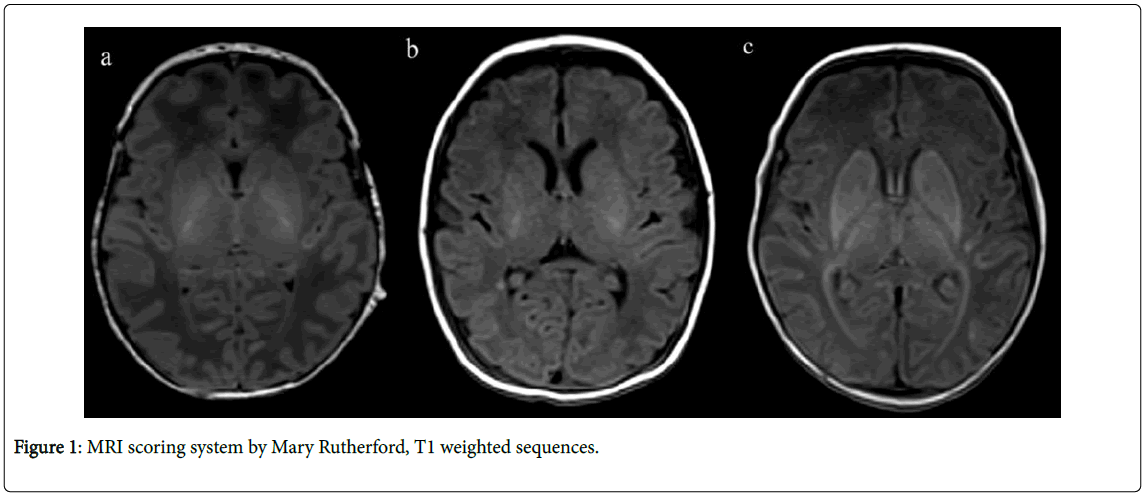
The level of injury seen on T1 and T2 weighted sequences was evaluated by the scoring system introduced by Mary Rutherford [5]. PLIC, BG/T, WM and cortex were evaluated in the transversal plane for each infant in the same way (Figure 1).
In the area of PLIC, the given scores ranked from 0 to 2, in the areas of BG/T, WM and cortex the given scores ranked from 0 to 3. If the signal intensity was normal, the given score was 0. If there was a mild signal abnormality, for example focal signal abnormality in the BG/T, the given score was 1. If there was a moderate signal abnormality, such as multifocal abnormal signal intensity in the BG/T, the given score was 2. And if there was a severe signal abnormality, such as widespread abnormal signal intensity in the BG/T, the given score was 3 [5].
Figure 1A shows normal signal intensity in the PLIC, the given score is 0. Figure 1B shows reduced, asymmetrical signal intensity in the PLIC, the given score is 1. There is also a punctate hyperintense ischemic lesion in the deep white matter near the atrium trigone of the right lateral ventricle. Figure 1C shows abnormal signal intensity in the PLIC bilaterally, with a total loss of signal on T1 weighted sequences, the given score is 2.
ADC maps were used for measuring ADC values. Regions of interest (ROI) were selected manually in the form of a circle size 0.20 cm2. ADC values were measured in the left and right hemispheres, in the following regions of interest: PLIC/T, BG, WM, insular cortex (IC) and PC. The lowest ADC value from each region of interest, right or left, was chosen for the statistical analysis.
Clinical outcome was based on the Amiel-Tison neurologic assessment at 1 month of age. The Amiel-Tison neurologic assessment classifies affected neonates into three groups according to their neurological clinical examination. Neonates are divided into groups with minor, moderate or severe neurological deficit. For the purpose of statistical analysis, neonates determined to have a good clinical outcome included those with a minor or moderate neurological deficit, such as the presence of abnormalities of tone and excitability with or without central nervous system (CNS) depression and up to two isolated seizures. Neonates considered to have a poor clinical outcome included those with severe neurological deficit, such as the presence of repeated seizures, lasting more than 30 minutes, associated with overt CNS depression from lethargy to coma [15].
Data were analyzed using IBM SPSS Statistics version 20.0 (SPSS Inc., Chicago, IL, USA, 2011). The statistically significant difference between ADC values in the group with a good versus a poor clinical outcome, for each of the 5 measured regions, and the statistically significant difference between 4 brain regions evaluated by the scoring system introduced by Mary Rutherford on T1 and T2 weighted sequences were calculated by the Mann-Whitney U test with Bonferroni correction. P values less than 0.01 were considered significant.
ROC curve analysis was used to check at which ADC values, sensitivity and specificity for predicting clinical outcome are the best. Taking into account the Bonferroni correction, p values less than 0.01 were regarded as significant.
Results
Of the 25 neonates included in the study, 9 had minor and 7 had moderate neurological deficit. All of these 16 (64%) neonates were categorized in the good clinical outcome group. Nine (36%) neonates had severe neurological deficit and were categorized in the poor clinical outcome group. The mean gestational age was 39 weeks (range 36-41). MRI was done at a mean age of 5 days (range 3-9). Mean ADC values for 5 regions of interest are in Table 2. MRI examinations were reviewed and scored by the scoring system introduced by Mary Rutherford. As shown in Table 3, the most frequently given score (mode) in the group with a good clinical outcome in the PLIC was 0, in the BG/T 2, in the WM 0 and in the cortex 0. The most frequently given score in the group with a poor clinical outcome in the PLIC was 1, in the BG/T 3, in the WM 3 and in the cortex 3.
| PLIC/T (10-6 mm2/s) | BG (10-6 mm2/s) | WM (10-6 mm2/s) | IC (10-6 mm2/s) | PC (10-6 mm2/s) | |
|---|---|---|---|---|---|
| Good outcome | 1003 (895-1079) | 1121 (1006-1304) | 1425 (597-1727) | 1147 (922 -1376) | 1090 (748-1251) |
| Poor outcome | 730 (506-973) | 865 (601-1258) | 977 (438-1436) | 858 (506-1284) | 817 (460-1223) |
ADC apparent diffusion coefficient, PLIC/T posterior limb of the internal capsule and thalamus, BG basal ganglia, WM white matter, IC insular cortex, PC parietal cortex.
Table 2: Mean ADC values with highest and lowest values for 5 regions of interest.
| PLIC | BG/T | WM | Cortex | |
|---|---|---|---|---|
| Good outcome | 0 | 2 | 0 | 0 |
| Poor outcome | 1 | 3 | 3 | 3 |
PLIC posterior limb of the internal capsule, BG/T basal ganglia and thalamus, WM white matter
Table 3: The most frequently given score (mode) by the MRI scoring system for each of the 4 anatomical areas.
Statistically significant differences between 4 brain regions evaluated by the scoring system introduced by Mary Rutherford in groups with a good versus a poor clinical outcome were confirmed in the BG/T (p=0.006) and in the cortex (p=0.001). Scores given in the PLIC (p=0.149) and in the WM (p=0.018) were not statistically associated with clinical outcome.
Statistically significant differences between ADC values in groups with a good versus a poor clinical outcome were confirmed in the PLIC/T (p<0.001) and in the WM (p=0.004). ADC values measured in the BG (p=0.024), in the IC (p=0.048) and in the PC (p=0.042) were not statistically associated with clinical outcome. ROC curve analysis showed that the threshold ADC value measured in the PLIC/T was 920 × 10-6 mm2/s, with 94% sensitivity and 89% specificity. The threshold ADC value measured in the WM was 1153 × 10-6 mm2/s, with 94% sensitivity and 67% specificity.
Analysis of AUC values of the ROC curve for PLIC/T (AUCPLIC/ T=0.951) and WM (AUCBM=0.854) also showed a strong capacity for differentiating between the two groups.
Discussion
The most effective current treatment for neonatal HIE is hypothermia. Therapeutic hypothermia has been shown to cause a reduction in cerebral lesions seen on MRI [5] and has a neuroprotective effect in neonates with HIE [6-10].
MRI has an important role in the assessment of neonates with HIE. The imaging protocol should include at least T1 and T2 weighted sequences and DWI with ADC mapping [3]. Based on the known facts that DWI visual appearances of infarcted areas can be seen during the first 24 hours of infarction and last for 7-14 days and that the time course of the ADC reduction is delayed in neonates with HIE treated with hypothermia, the study included 25 neonates with HIE treated with hypothermia who had MRI done within 9 days of life [3,11]. ADC values were measured in the PLIC/T, BG, WM, IC and in the PC. As can be seen from Table 2, mean ADC values that were measured in each of the 5 areas are higher in the group with a good clinical outcome than in the group with a poor clinical outcome.
We found that ADC values measured in the PLIC/T (p<0.001) and in the WM (p=0.004) were significantly associated with clinical outcome at 1 month of age but ADC values measured in the BG, IC and PC were not (p>0.01). Our findings were similar to those quoted by Twomey, et al. and by Vermeulen, et al. [13,14]. Twomey has found that ADC values measured in the PLIC and in the BG are significantly related to clinical outcome. ADC values measured in the WM are also predictive of outcome but not as much as ADC values measured in the PLIC and in the BG [13]. Vermeulen has also found that ADC values measured in the PLIC and in the T are statistically associated with outcome. Furthermore, he has shown that values measured in the BG, in the PC and in the hippocampus are also associated with clinical outcome [14]. The main difference between our study and their two studies was that our neonates with HIE had been treated with hypothermia.
Our threshold value for measured ADC in the PLIC/T of 920 × 10-6 mm2/s, above which 94% of neonates had a good and below which 89% of neonates had a poor clinical outcome, was higher compared to the threshold values quoted by Twomey, et al. of 860 × 10-6 mm2/s and by Vermeulen, et al. of 850 × 10-6 mm2/s [13,14]. Among the mentioned studies above, only neonates in the present study were treated with hypothermia. Because therapeutic hypothermia is associated with fewer grey and white matter abnormalities we suggested that this was one of the main reasons why our ADC values were among the highest. Higher ADC values after treatment with hypothermia indicate less hypoxic-ischemic brain injury [14].
We also found that ADC values measured in the WM were significantly associated with clinical outcome. Our threshold value for ADC in the WM of 1153 × 10-6 mm2/s, above which 94% of neonates had a good and below which 67% of neonates had a poor clinical outcome, was lower than the threshold value quoted by Twomey, et al. of 1290 × 10-6 mm2/s, even though infants in the study by Twomey had not been treated with hypothermia [13].
Twomey, et al. has quoted the threshold ADC value above which all neonates have a good outcome and not the threshold ADC value with the highest sensitivity and specificity for predicting clinical outcome. If we had decided to represent the ADC value above which all neonates had a good clinical outcome, the value would be 1436 × 10-6 mm2/s. As can be seen, threshold ADC values that are quoted in studies also depend on the presentation of the results.
Based on comparison between ADC values of the present and ADC values of the previous studies measured in the PLIC/T and WM, it can be concluded that the ADC values of the present study were among the highest. Neonates in the present study were treated with hypothermia whereas neonates of the previous studies mentioned in this paper have not been. Therapeutic hypothermia causes a reduction in cerebral lesions seen on MRI and has a neuroprotective effect [5-10]. It can therefore be concluded that threshold ADC values represented in the present study were among the highest because neonates had been treated with hypothermia.
We evaluated and scored the level of injury seen on T1 and T2 weighted sequences, for each of the 25 neonates. Table 3 shows the most frequently given scores in the group with a good and that with a poor clinical outcome. Considering all of the evaluated areas together, it can be seen that the most frequently given score in the group with a good clinical outcome was 0, only in the BG/T was the most frequently given score 2. In the group with a poor clinical outcome, the most frequently given score overall was 3, only in the PLIC was the most frequently given score 1.
We found that scores given by the morphological MRI scoring system in the area of BG/T (p=0,006) and cortex (p=0,001) were significantly associated with clinical outcome at 1 month of age but scores given in the PLIC and WM were not (p>0.01). It can be seen that there are some differences in association between clinical outcome and the results obtained by ADC values and the MRI scoring system. We showed that ADC values measured in the PLIC/T and in the WM were most associated with clinical outcome, but scores given by the morphological MRI scoring system in these areas were not. Instead we have shown association between clinical outcome and MRI scores given in the area of BG/T and cortex. Differences may be present due to the fact that the morphological MRI scoring system is fairly subjective and depends on the person evaluating the image. At this point we would like to emphasize that evaluation of hypoxic-ischemic lesions in the BG/T by the morphological MRI scoring system is more difficult compared to cortex. Therefore ADC values have a greater role and are more objective in evaluation of lesions of the deep brain areas.
The first limitation of our study was the number of enrolled neonates. Low number of neonates with HIE treated with hypothermia at the national level was the main reason for that. Our second limitation was that we correlated our results with Amiel-Tison neurologic assessment at 1 month of age. We did so because we had a complete neurologic assessment for all treated neonates at this early age. Comparison of our results with clinical outcome at 2 years of age will be evaluated in our future studies. Our third limitation was that we did not have a control group. Because hypothermia is currently the best known treatment for neonates with HIE, it would had been unethical to have a control group of untreated neonates with HIE for comparison.
Conclusion
The present study has shown statistically significant association between DWI with ADC mapping and clinical outcome and between morphological MRI scoring system and clinical outcome in neonates with HIE at 1 month of age. ADC values measured in the PLIC/T and in the WM were shown to have the best association with clinical outcome. In comparison to threshold ADC values from previous studies, values set in the present study were among the highest. We predicted that the main reason for higher ADC values was because neonates in the present study had been treated with therapeutic hypothermia. MRI scores given in the area of BG/T and cortex have shown the strongest association with clinical outcome.
References
- Goergen SK, Ang H, Wong F, Carse EA, Charlton M, et al. (2014) Early MRI in term infants with perinatal hypoxic-ischaemic brain injury: Interobserver agreement and MRI predictors of outcome at 2 years. Clinical Radiology 69: 72-81.
- Lai MC, Yang SN (2011) Perinatal Hypoxic-Ischemic Encephalopathy. Journal of Biomedicine and Biotechnology 2011.
- Rutherford MA, Malamateniou C, McGuinness A, Allsop J, Biarge MM, et al. (2010) Magnetic resonance imaging in hypoxic-ischaemic encephalopathy. Early Human Development 86: 351-360.
- Martinez-Biarge M, Bregant T, Wusthoff CJ, Chew AT, Diez-Sebastian J, et al. (2012) White Matter and Cortical Injury in Hypoxic-Ischemic Encephalopathy: Antecedent Factors and 2-Year Outcome. The Journal of Pediatrics 161: 799-807.
- Rutherford MA, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, et al. (2010) Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol 9: 39-45.
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, et al. (2005) Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 365: 663-670.
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, et al. (2005) Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 353: 1574-1584.
- Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, et al. (2009) Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 361: 1349-1358.
- Zhou WH, Cheng GQ, Shao XM, Liu XZ, Shan RB, et al. (2010) Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr 157: 367-372.
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, et al. (2013) Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 1:CD003311.
- Bednarek N, Mathur A, Inder T, Wilkinson J, Neil J, et al. (2012) Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology 78: 1420-1427.
- Alderliesten T, de Vries LS, Staats L, Van Haastert LC, Weeke L, et al. (2016) MRI and spectroscopy in (near) term neonates with perinatal asphyxia and therapeutic hypothermia. Dis Child Fetal Neonatal Ed 102: 147-152.
- Twomey E, Twomey A, Ryan S, Murphy J, Donoghue VB (2010) MR imaging of term infants with hypoxic-ischaemic encephalopathy as a predictor of neurodevelopmental outcome and late MRI appearances. Pediatr Radiol 40: 1526-1535.
- Vermeulen RJ, van Schie PEM, Hendrikx L, Barkhof F, Van Weissenbruch M, et al. (2008) Diffusion-weighted and Conventional MR Imaging in Neonatal Hypoxic Ischemia: Two-year Follow-up Study. Radiology 249: 631-639.
- Paro-Panjan D, Neubauer D, Kodric J, Bratanic B (2005) Amiel-Tison Neurological Assessment at term age: clinical application, correlation with other methods, and outcome at 12 to 15 months. Dev Med Child Neurol 47: 19-26.
Citation: Cimpersek M, Meglic NP, Panjan DP, Skofljanec A, Popovic KS (2017) The Role of Diffusion Weighted Imaging and Magnetic Resonance Imaging Scoring System in Assessing the Effectiveness of Treatment with Hypothermia in Neonates with Hypoxic-ischemic Encephalopathy. Neonat Pediatr Med 3: 135. DOI: 10.4172/2572-4983.1000135
Copyright: © 2017 Cimpersek M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4159
- [From(publication date): 0-2017 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 3350
- PDF downloads: 809