The Role of CC Chemokine Receptor Six (Ccr6) in Prospective ImmuneModulation Therapy in Ibd
Received: 29-Dec-2020 / Accepted Date: 12-Jan-2021 / Published Date: 19-Jan-2021 DOI: 10.4172/jmir.1000128
Description
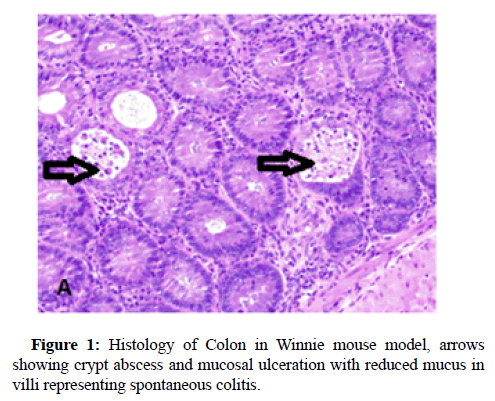
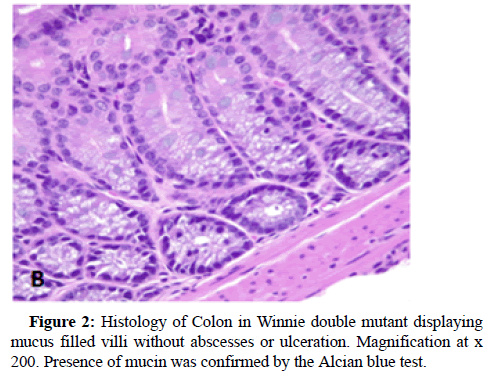
Inflammatory Bowel Disease (IBD) comprises of a gut disease complex primarily consisting of Crohn’s Disease and Ulcerative Colitis, both of which are caused by immune-deficiency combined with infectious microbial penetration of the intestinal epithelium. This disease is multi-factorial and affects both children and adults, particularly in the age groups of 15-30 and 60-70 years, which debilitates the digestive system causing a multitude of problems such as anxiety, depression, financial and productivity loss due to the inability to continue in education or employment and weakening of gut-mucosal immunity and there by running a high risk of contracting colorectal cancer. A plethora of environmental factors have been named as the perpetrators of IBD development although the clinical diagnosis is principally attributed to immune dysfunction which drives innate and adaptive immune processes in the human body into serious disarray. IBD has also being named as a consequence of poverty, illiteracy and low socio-economic background and their food habits and addictions such as smoking and consumption of fast food. Some of its major symptoms are uncontrollable bowel movements resulting in intermittent diarrhoea, nausea, general malaise and abdominal pain which has no permanent cure and the worse case scenarios require surgery such as colectomy. Early signs of this disease could however be identified by abdominal scans, colonoscopy, faecal occult tests, monitoring weight loss and testing for biomarkers such as faecal calprotectin and C -reactive protein in blood. The major difficulty in diagnosis and treatment is the absence of a specific biomarker and unavailability of targeted therapeutics although no particular immunemodulatory drug which could successfully treat this acutely debilitating disease, has been found up to date. The recently discovered cytokine therapy has focused on Tumour Necrosis Factor (TNF) alpha antagonist known as Infliximab and several such biosimilars, although their use has been riddled with unpleasant sideeffects. Hence our mucosal immunology research group, headed by A/ Prof Rajaraman Eri at the University of Tasmania, have been optimistically trying to focus on natural immune modulatory therapies of the human immune system, which could yield natural immunological resistance to curb and manipulate the acute immunedeficiency that stems once a patient is afflicted with IBD. One despondent fact that was revealed by my preclinical research studies associated with IBD was that not only the digestive system is chronically affected, but also almost every other organ system is at risk of developing inflammation due to this disease complex because histological evidence has shown increased concomitant inflammation in the liver and spleen as well. Chemokines are small natural proteins produced by the human immune system which are chemoattractant biochemicals that act as immune-modulatory envoys which are responsible for mass-scale lymphocyte trafficking into the gut mucosal system in response to microbial infections and it is considered as the major cause of upregulating inflammation in the gut. Chemokines are present in receptor-ligand pairs and there are around 50 chemokine receptors in the human body and several receptors can bind with either one or multiple ligands. However, we utilised the CC Chemokine Receptor Six abbreviated as CCR6 which has only one ligand identified as CC chemokine ligand 20 (CCL20) whose titre conspicuously increases in the gut epithelium simultaneously in response to infectious microbial penetration, usually via Microfold (M) -cell conduits which attract the antigen-sampling dendritic cells and lymphocyte repertoires to the tissue sites of entry. The varied lymphocyte cohorts congregate in the gut mucosa which stimulate the pro- inflammatory cytokines such as interferon-gamma, tumour necrosis factor alpha, interleukins (IL-4,13,6,9,5,21,22,23,17 A-F), thus promoting inflammation followed by tissue injury, ulceration and destruction of the gut epithelium and the mucosal tissue found beneath in the bowel walls. Among the lymphocytes which accumulate in the gut mucosa belong to the T- lymphocyte subdivisions identified as Thelper cells (TH1, TH2, TH17 and Regulatory T cells (Treg cells) and growth factors and allied molecules such as the Transforming Growth Factor Beta (TGF-beta), which play an important role in determining the immunological outcome that produces chronic inflammation which is highly symptomatic of IBD. Various studies have indicated that an imbalance exists between the TH17 and Treg cell groups and the tilt of TH17 leads to tissue inflammation while IL-10 stimulated by the release of TGF-beta and associated cytokines serve to upregulate tissue restitution and repair and/or regeneration of the bowel walls that would lead to a sustainable recovery. Our attempts were mainly focused on the mass-scale migration of the lymphocytes, both B and T cells, which is grossly dependent on CCR6 which drives lymphocyte mobility towards the infected gut wall tissue. CCL20 is a well-known inflammatory biomarker which promotes inflammation and therefore attract the immune cell types up holding CCR6 including dendritic cells, macrophages, natural killer cells and Innate Lymphoid Cells (ILC) in addition to the lymphocytes which decide the immunological response towards IBD. Thus, CCR6 was targeted as a potential therapeutic modality which could serve to decrease gut tissue inflammation by restricting the migratory flow of these immune cells to the bowel wall. The deployment of immune cells arises in the mesenteric lymph nodes after the dendritic cells present the microbial antigens to the naïve lymphocytes which are then transformed into primed lymphocytes which are attracted to the gut mucosa which stimulate the release of inflammatory cytokines. Therefore, our rationale behind blocking the immune cell migration was meant to alleviate the inflammation in the bowel tissue which results in lessening tissue damage leading to reduced disease severity in the gut. Our chosen pre-clinical IBD model was Winnie, well known to represent a spontaneous colitis mouse model which produces gut inflammation by 6 weeks of age and is considered the best available model, because no strong chemicals are required to induce the disease such as the DSS or TNB or Oxazolone mouse models and genetically modified (transgenic) mouse models which do not produce natural immune responses. Winnie is a natural colitis model because it carries a point mutation in MUC2 gene which has decreased mucin 2 biosynthesis leading to aggravated microbial penetration. Therefore, we produced a Winnie double mutant which carried a natural gene deletion of MUC2 as well as targeted deletion of Ccr6 in which CCR6 -deficiency was genetically induced in the presence of colitis, from which we investigated clinical, histological (Figures 1 and 2) and immunological parameters, all of which have indicated attenuation of gut mucosal inflammation during chronic colitis. Thus, further clinical validation is required to ascertain the therapeutic potential of CCR6 in down regulating inflammation and as a prospective immune modulatory target in the treatment of IBD [1-15].
References
- Ranasinghe R, Eri R (2019) CCR6–CCL20 Axis in IBD: What Have We Learnt in the Last 20 Years? Gastrointest Disord 1(1):57-74.
- Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, et al. (2018)Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 390(10114):2769-2778.
- Plevinsky J, Gumidyala A, Fishman L (2015) Transition experience of young adults with inflammatory bowel diseases (IBD): a mixed methods study. Child 41(5):755-761.
- Park K, Ehrlich OG, Allen JI, Meadows P, Szigethy EM, et al. (2020) The Cost of Inflammatory Bowel Disease: An Initiative From the Crohn’s & Colitis Foundation. Inflammat Bowel Dis 26(1): 1-10.
- Sewitch, Maida J, Abrahamowicz G, Michal P; Bitton, Alain MD, Donald MD, Gary E.
- Hrabe JE, Byrn JC, Button AM, Zamba GK Kapadia MR, et al. (2014) A matched case-control study of IBD-associated colorectal cancer: IBD portends worse outcome. J Surg Oncol 109(2): 117-121.
- Basheer W, Kunde D, Eri R (2013) Role of Chemokine Ligand CCL20 and its Receptor CCR6 in lntestinal Inflammation. Immunol Infect Dis 1(2): 30-37.
- Ranasinghe R, Eri R. (2019) CCR6–CCL20-Mediated Immunologic Pathways in Inflammatory Bowel Disease. Gastrointest Disord 1(1): 15-29.
- Basheer W (2018) Genetic Ablation of CCR6 Confers Differential Exacerbation in a Spontaneous Colitis Model.
- Lee A, Eri R, Lyons AB, Grimm M, Korner H (2013) CC chemokine ligand 20 and its cognate receptor CCR6 in mucosal T cell immunology and inflammatory bowel disease: Odd couple or axis of evil? Front Immunol 4: 194.
- Ranasinghe R, Eri R (2018) Modulation of the CCR6-CCL20 Axis: A Potential Therapeutic Target in Inflammation and Cancer. Medicina 54(5): 88.
- Mateer SW, Cardona J, Marks E, Goggin BJ, Hua S, et al. (2016) Ex vivo intestinal sacs to assess mucosal permeability in models of gastrointestinal disease. J Visual Exper 108: e53250.
- Turner JE, Paust HJ, Steinmetz OM, Peters A, Riedel J, et al. (2010) CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J Am Soc Nephrol 21(6): 974-985.
- Welsh-Bacic D, Lindenmeyer M, Cohen CD, Draganovici D, Mandelbaum J, et al. (2010) Expression of the chemokine receptor CCR6 in human renal inflammation. Nephr. Dialysis Transplant 26(4):1211-1220.
- Oo YH, Banz V, Kavanagh D, Liaskou E, Withers DR, et al. (2012) CXCR3-dependent recruitment and CCR6-mediated positioning of Th-17 cells in the inflamed liver. J Hepat 57(5): 1044-1051.
Citation: Ranasinghe R (2021) The Role of Cc Chemokine Receptor Six (Ccr6) in Prospective Immune Modulation Therapy in Ibd. J Mucosal Immunol Res 5: 128. DOI: 10.4172/jmir.1000128
Copyright: © 2021 Ranasinghe R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2472
- [From(publication date): 0-2021 - Dec 04, 2025]
- Breakdown by view type
- HTML page views: 1596
- PDF downloads: 876