The Role and Function of Mucins and Its Relationship to Inflammatory Bowel Disease
Received: 04-Jul-2022 / Manuscript No. jcidp-22-70268 / Editor assigned: 06-Jul-2022 / PreQC No. jcidp-22-70268 / Reviewed: 20-Jul-2022 / QC No. jcidp-22-70268 / Revised: 25-Jul-2022 / Manuscript No. jcidp-22-70268 (R) / Published Date: 30-Jul-2022 DOI: 10.4172/2476-213X.1000156
Abstract
Recently, numerous studies have shown that disruption of the mucus barrier plays an important role in the exacerbation of inflammatory bowel disease, particularly in ulcerative colitis. Alterations in the mucus barrier are well supported by published data and are widely accepted. The use of fluorescence in situ hybridization and Carnoy’s fixation has revealed the importance of the mucus barrier in maintaining a mutualistic relationship between host and bacteria. Studies have raised the possibility that modulation of the mucus barrier may provide therapies for the disease, using agents such as short-chain fatty acids, prebiotics and probiotics. This review describes changes in the mucus barrier of patients with inflammatory bowel disease and in animal models of the disease. We also review the involvement of the mucus barrier in the exacerbation of the disease and explore the therapeutic potential of modifying the mucus barrier with short-chain fatty acids, prebiotics, probiotics, fatty acid synthase, H2S, neutrophil elastase inhibitor and phophatidyl choline.
Keywords
Mucus barrier, inflammatory bowel disease, bacterial penetration, nutrients, immune.
Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic relapsing disorder characterized by inflammation and mucosal tissue damage of the gastrointestinal tract. It has been suggested that IBD may result from: (1) an imbalance of intestinal microbiota characterized by changes in the composition, quantity and diversity of the microbiota; (2) an altered mucosal barrier structure and function; (3) an aberrant innate immune response; and (4) an imbalanced of T cell responses.
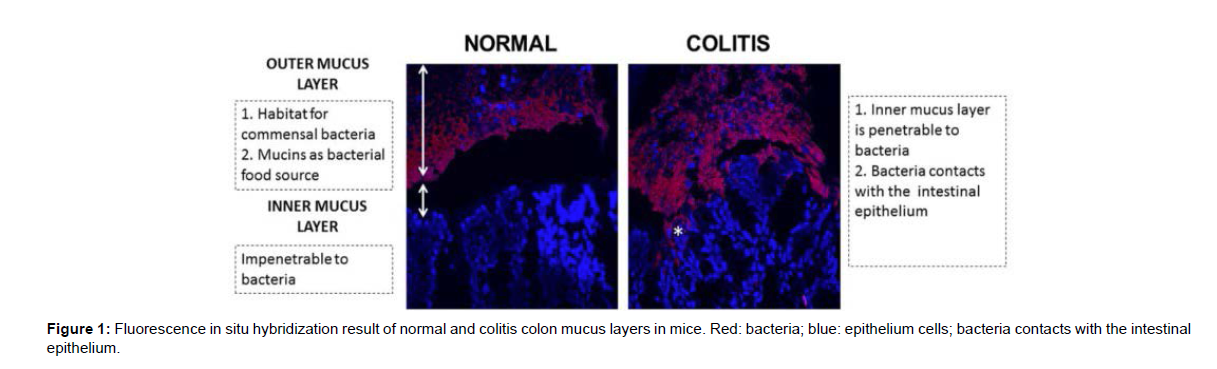
The primary defence against microbe and pathogen penetration of the lamina propria is the single layer of epithelial cells and its associated protective mucus layer. Increasing evidence suggests that an imbalanced mucus barrier may play an important role in the disease progression of IBD [1]. As the first anatomical site of the intestinal barrier, the mucus barrier forms a matrix preventing particles the size of bacteria from penetrating the epithelium. Additionally, as an important part of the innate immune system, the mucus barrier helps to maintain the immune mutualistic relationship between host and bacteria and to reduce the activation of sub epithelial lymphocytes. In addition, the well-accepted animal models for IBD, dextran sulfate sodium (DSS) induced mice and Il10{´ mice, reveal bacterial penetration into the inner mucus layer and epithelial crypt (Figure 1), implying dysfunction in the mucus barrier in the exacerbation of IBD. Aberrations in the mucus barrier most likely reduce the ability of the intestinal barrier to cope with bacteria and may contribute to the susceptibility to developing colitis.
Colonic mucus consists of two layers: an inner “firmly” adherent mucus layer forms the physical barrier against bacteria, and outer “loose” nonadherent mucus layer generates the preferred habitat for the commensal microbes. Goblet cells secrete mucus into the inner mucus layer through so-called compound exocytosis, and the inner layer transforms into the outer layer. An estimate of the turnover of the inner mucus layer in live murine distal colonic tissue is fast, approximately 1–2 h, which is important for maintaining this inner mucus layer free from bacteria. The secretion of mucin from the apical surface is normally constitutive but increases in response to a variety of external stimuli, which helps to reinforce the barrier and flush bacteria from the normally sterile crypts [3]. It was reported that mucin secretion was markedly increased in mice during infection compared to uninfected controls. Goblet cells in the upper crypt do not seem to synthesize enough mucin to meet a constant stimulus because the replenishment of new goblet cells is too slow (longer than 4–5 h) to replace or refill these mucin filled cells. This suggests that continuous stress will limit mucin availability and result in mucus defects.
In addition to composition and quantity, the quality of the mucus barrier is also crucial for the function of the barrier. MUC2, as the main secreted mucin in the intestine, is a large and heavily O-glycosylated gel-forming mucin that forms enormous polymeric nets by C-terminal dimerization and N-terminal trimerization. Mucus quality is associated with correct glycosylation, sialylation and sulfation of mucins. Glycans cover the protein backbone and thus protect the mucin from proteolytic enzymes. The normal degradation of mucin glycans is relatively slow as one monosaccharide is removed at a time. If the glycans become short, the degradation process will be too rapid and not only the outer mucus layer but also the inner can be degraded to allow bacteria to reach the epithelium [4]. Gouyer V. et al., reported that delivery of a mucin domain enriched in cysteine residues into the gut lumen can alter the O-glycosylation and strengthen the intestinal mucous barrier. In addition, sulfation and sialylation play a role in the resistance of mucin to bacterial degradation. Increased sulfation of mucin was found to yield a gel with a higher viscosity, which is predicted to be more resistant to physical erosion.
Changes in the Mucus Barrier in Inflammatory Bowel Disease
Ulcerative Colitis
Previous observations and earlier studies on UC patients showed bacteria directly in contact with the epithelium. A number of specific changes in the mucus barrier have been reported in UC patients, with both the adherent mucus layer and the whole mucus layer being thinner, more variable and partly denuded, compared to controls. Histologic analysis of UC patients often shows depletion of recognizable goblet cells, decreased MUC2 synthesis and decreased MUC2 secretion in the colonic epithelium. The mucus layer of UC patients also has reduced mucin glycosylation and shortening of the oligosaccharide side chains of mucin. In addition, decreased sulfation of mucin, which is associated with decreased viscosity and increased susceptibility to erosion and colonic inflammation, was observed in UC patients. It has been shown that in UC patients, the degree of mucosal inflammation correlates significantly with a decrease in MUC2 synthesis and secretion, implying that the thickness of the mucus gel is affected by the severity of UC [5]. During active inflammation, the mucus layer thickness is reduced, the goblet cell population is depleted, and individual goblet cells contain less mucin than in healthy controls. Theodossi A et al., found that during periods of disease remission both the number and appearance of goblet cells return to normal. In addition, the disease course also influences the mucus barrier. Rectal biopsies of 59 UC patients showed that there was no global change of mucus protection until severe UC. As a consequence of large regions lacking mucus, the mucus layer was less effective due to decreased thickness, a loss of goblet cells and decreased secretory potential. Larsson and colleagues found significant alterations in MUC2 O-glycosylation with the most severe patient phenotype and that the glycan pattern reverted to normal when in remission. In active disease, there was a marked shift towards smaller glycans, but the MUC2 glycosylation patterns were similar in controls and UC patients in remission, which indicated that the magnitude of this shift of mucus quality was also significantly correlated with both the degree of inflammation and disease course [6].
Crohn’s Disease
In contrast to UC, the mucus layer is thicker in CD subjects compared with controls. This was mirrored by the yields of mucin obtained from whole-gut lavage, which were low in UC but high in CD. In addition, detectable MUC2 protein is increased in CD, irrespective of inflammation. CD, unlike UC, is deep seated, therefore cytokines may initially stimulate mucus secretion, increase the mucus layer thickness [7], which may explain why MUC2 protein increases in CD patients, but it begins to impair mucus production when the inflammation becomes more extensive. The quality of the mucus barrier is also changed, and the oligosaccharide chain length is reduced by 50%, yet sialylation is increased. Therefore, increased MUC2 probably does not reflect increased synthesis, but rather decreased post transcriptional sulfation and glycosylation along with altered viscoelastic properties of mucus. Quantity and quality changes of mucus in IBD are shown in (Table 1).
| Ulcerative Colitis | Crohn’s Disease | |
|---|---|---|
| Mucus thickness | Decreased | Increased |
| Goblet cell numbers | Decreased | Unchanged/Increased |
| MUC 2 protein | Decreased | Increased |
| Glycosylation | Decreased | Unknown |
| Sulfation | Decreased | Unchanged |
| Sialylation | Increased | Increased |
Table 1: Quantity and quality changes of mucus in irritable bowel disease (IBD)
The Role of Mucus Barrier Dysfunction in the Exacerbation of Inflammatory Bowel Disease
Commensal bacteria lining the mucus surface that maintains gut homeostasis are called biofilms. These benefit the host by digesting substrates inaccessible to host enzymes, modulating immunity, and conferring resistance against transient enteropathogens. The relationship between the mucus barrier and the biofilms is dynamic. Although mucins are constitutively secreted, infection of mucosal surfaces can result in a rapid release of stored mucin granules to bolster the barrier and exclude pathogens. The importance of mucus for clearance has been shown in a mouse infected with Trichuris muris; this species is closely related to Trichuris trichiura, which infects the human colon. Mucus barrier defects allow bacteria to penetrate and reach the epithelia. These defects have been observed in mouse strains with genetic loss or defects in the mucin, MUC2, as well as in molecules that are involved in the formation of the MUC2 mucin polymer [8]. MUC2 deficiency leads to exacerbated disease by the attaching and effacing (A/E) pathogen, Citrobacter rodentium, characterized by an increased rate of pathogen colonization and an inability to clear pathogen burdens through increased mucus secretion. Thus, mucus barrier dysfunction could influence the effects of bacteria on the colonic mucosa and be instrumental in the development of colitis. Patients with IBD exhibit a dysbiosis of gut microbiota, characterized by mucus heavily loaded with bacteria in the intestine, some of which adhere to, or even invade, the epithelial surface. The abundance of the mucolytic bacterium, Ruminococcus torques, which has a strong mucin degrading ability, was increased ~100-fold. In contrast, mucolytic bacteria present in healthy controls, such as Akkermansia muciniphila, which has weak mucin degrading abilities, was reduced many fold in macroscopically and histologically normal intestinal epithelium of both CD and UC. In addition, certain enteric pathogens have evolved strategies to circumvent the mucus barriers. Bacteria can secrete not only carbohydrate degrading enzymes but also proteases [9]. Examples are the toxin released from Bacteroides fragilis that has been shown to be a proteolytic enzyme and the protease secreted by the oral bacteria, Porphyromonas gingivalis that has been shown to be able to cleave MUC2.
More recently, several E. coli family proteases with similar properties have been identified. High levels of sulfate in mucin decrease its susceptibility to bacterial glycosidases and limit the rate and extent of degradation. Therefore, it has been proposed that reduced mucin sulfation might be closely correlated with the increase in bacterial translocation in murine models of gut disease. Approximately 1% of normal colonic bacteria secrete glycosidases and sulphatases capable of degrading mucin oligosaccharides, allowing the enteric microflora to exploit mucin carbohydrates as an energy source. Under these conditions, the mucus barrier remains intact. Some virulent bacteria secrete sulphatases that remove the sulfate ester and thus render the mucin molecule susceptible to degradation by bacterial glycosidases [10]. Bacteria that are capable of cleaving sulfate and utilizing it as a metabolite have been found to be overrepresented in the colitic colon, which may offer an explanation for the reduced sulfated content of mucin in the colitic colon. In UC, there is also increased bacterial sulphatase activity, which may mirror disease activity.
The Mucus Barrier and Sub epithelial Immune System
Il10´{´ mice kept in a special pathogen free (SPF) environment, which display only minor signs of histological inflammation, still have a mucus layer that can be penetrated by both beads and bacteria. This argues for a link between mucus properties, the immune system and the cytokines produced. As the important element of innate immunity, the mucus barrier is impaired in IBD. Bacterial products and dietary antigens penetrate the mucus layer, cross the epithelium and enter the lamina propria. Most of the immune system resides in the sub epithelial compartment, and the antigen-presenting cells (APCs) are ready to take up and present antigens (such as bacteria) to T cells for action or tolerance development. The cytokines from APCs regulate the abnormal differentiation of T cells, which secrete a large number of pro-inflammatory mediators. The active adaptive immune responses are protective for the host in normal conditions [11], but the response is constantly amplified in IBD. Both host innate and adaptive immunity can regulate the differentiation of goblet cells, the glycosylation of mucins, and the production rates of antimicrobial molecules and cell surface mucins. For many years, it has been assumed that IBD is a T cell-mediated disease. Th1 cells and a novel subset of IL-17-producing CD4+ Th cells, Th17 cells, have more recently been implicated in the pathogenesis of CD.
In contrast, UC was reported to be associated with up-regulation of Th2 cells. It was reported that cytokines produced by Th2 cells (such as IL-4, IL-13) in response to parasitic infections, can promote goblet cell hyperplasia and substantially increased mucus production in the intestine. In addition, interferon-γ (IFN-γ) and IL-17, which are classically produced by Th1 cells and Th17 cells in response to intracellular and extracellular pathogens, respectively, affect goblet cells by increasing mucin production. T cell cytokines also mediate changes in mucin glycosylation, probably representing an attempt by the host to alter the pattern of glycosylation that fail to prevent infection by a pathogen or parasite.
Implications for Clinical Utility
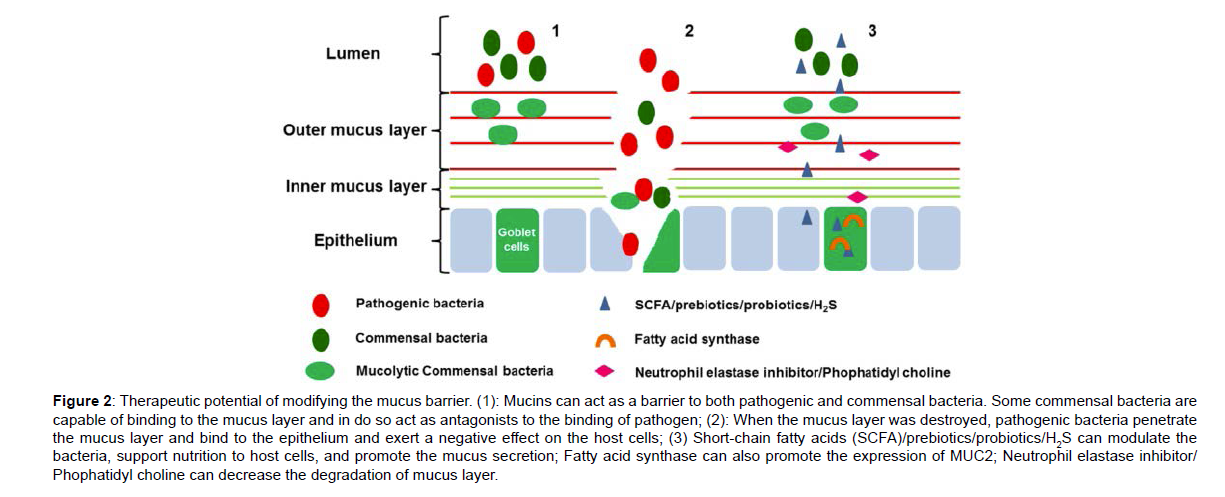
Mucosal healing as a therapeutic aim has become a common endpoint in clinical trials in addition to traditional subjective clinical indices. The mucus barrier, as the first anatomical site of the mucosal barrier, contacts with numerous luminal microbiota and with the sub mucosal immune system [12]. Disequilibrium of the mucus barrier plays an important role in delaying healing of the damaged tissue of IBD. In addition, changes in the mucus barrier are clearly associated with disease activity and severity, which promotes the potential of the mucus barrier as a target for therapeutics of IBD (Figure 2).
Conclusions
The mucus layer has long been recognized as an important ingredient
Figure 2: Therapeutic potential of modifying the mucus barrier. (1): Mucins can act as a barrier to both pathogenic and commensal bacteria. Some commensal bacteria are capable of binding to the mucus layer and in do so act as antagonists to the binding of pathogen; (2): When the mucus layer was destroyed, pathogenic bacteria penetrate the mucus layer and bind to the epithelium and exert a negative effect on the host cells; (3) Short-chain fatty acids (SCFA)/prebiotics/probiotics/H2S can modulate the bacteria, support nutrition to host cells, and promote the mucus secretion; Fatty acid synthase can also promote the expression of MUC2; Neutrophil elastase inhibitor/ Phophatidyl choline can decrease the degradation of mucus layer.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pandolfi F, Cianci R, Pagliari D, Landolfi R, Cammarota G (2009) Cellular mediators of inflammation: Tregs and TH17 cells in gastrointestinal diseases. Mediat Inflamm.13: 245-247.
- Geremia A, Biancheri P, Allan P, Corazza GR, Sabatino A (2014) Innate and adaptive immunity in inflammatory bowel disease. Autoimmune 13: 3–10.
- Boltin D, Perets TT, Vilkin A, Niv Y (2013) Mucin function in inflammatory bowel disease. J Clin Gastroenterol. 47: 106–111.
- Johansson ME, Stovall H, Hansson GC (2013) The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 10: 352–361.
- Chassaing B, Darfeuille-Michaud A (2011) The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology 140: 1720–1728.
- Bergstrom KS, Kissoon-Singh V, Gibson DL, Montero M, Sham, Huang T et al. (2010) Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 6: 148-150.
- Johansson ME, Gustafsson JK, Holmen-Larsson J, Jabbar KS, Xia L et al. (2014) Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. 63: 281–291.
- Schwerbrock NM, Makkink MK, Buller HA, Einerhand AW, Sartor RB et al. (2004) Interleukin 10-deficient mice exhibit defective colonic muc2 synthesis before and after induction of colitis by commensal bacteria. Inflamm Bowel Dis. 10: 811–823.
- Atuma C, Strugala V, Allen A, Holm L (2001) The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol. 280: 922–929.
- Ermund A, Schütte A, Johansson ME, Gustafsson JK, Hansson GC et al. (2013) Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am J Physiol Gastrointest Liver Physiol. 305: 341–347.
- Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X et al. (2011) The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 334: 255–258.
- Johansson ME, Larsson JM, Hansson GC (2011) The two mucus layers of colon are organized by the muc2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci. 108: 4659–4665.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Ogawa Y (2022) The Role and Function of Mucins and Its Relationship to Inflammatory Bowel Disease. J Clin Infect Dis Pract, 7: 156. DOI: 10.4172/2476-213X.1000156
Copyright: © 2022 Ogawa Y. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 1867
- [From(publication date): 0-2022 - Nov 20, 2025]
- Breakdown by view type
- HTML page views: 1361
- PDF downloads: 506