The Response of Biogas Production and Methanogenic Community to the Variation of Intermediate Vfas Produced During the Anaerobic Digestionof Food Waste
Received: 09-Aug-2017 / Accepted Date: 08-Sep-2017 / Published Date: 11-Sep-2017 DOI: 10.4172/2155-6199.1000411
Abstract
This study focused on the effect of the variation of intermediate volatile fatty acids (VFAs) caused by the change of organic load on methane production and methanogenic community shift during anaerobic digestion (AD). Eight groups with different F/I (VSfood wastes:VSsludge) were conducted, while the inoculation sludge had a same amount. Operational performance for every groups was monitored by assessing biological activity, methane production, concentration of VFAs. The major intermediate VFAs of anaerobic digestion were acetate, propionate, iso-butyrate, n-butyrate, n-valerate and iso-valerate; and acetate and n-butyrate were the most abundant components. The increasing of F/I changed the predominant VFAs type from acetic acid to n-butyric acid, while the total VFAs concentration increased. Besides, the methane production increased and then decreased, and reached the maximum (170.3 ml/g added VS-1) at F/I of 1. The methanogenic diversity was screened using the high-throughput sequencing, which shown VFAs had a significant impact on the archaeal community as the dominant archaea shifted from Methano bacterium to Methano sarcina.
Keywords: Food waste; Anaerobic digestion; Volatile fatty acid; Methane; Methanogens
Introduction
Due to the rapid growth of urban populations and changes in consumption patterns, the amount of food waste (FW) produced in China has quickly increased yearly, which accounts for about 60% of the total municipal solid waste (MSW) collected and transported in China in 2006 [1,2]. FW with great producing mount creates many challenges in terms of environmental protection and public health [3,4]. However, FW contains a large amount of volatile organic compounds mainly in terms of single sugars, starch and protein, which are nutrient-rich organic material to produce biogas and other energy-rich organic compounds as end-products by anaerobic digestion (AD)[5]. AD has been a desirable solution for waste management owing to its low emission of secondary pollutants, its utilization as a renewable energy source, and its high economic feasibility [6,7].
AD is a complex biochemical process for the utilization of organic materials, which involved a series of metabolic reactions such as hydrolysis, acidogenesis, and methanogenesis [8]. And the biggest drawback for the AD is that the methanogens are highly sensitive to environmental change, especially the variation of pH. A sudden change of pH could lead to an entire system failure, which could be mainly attributed to the activity loss of acid-sensitive glycolytic enzymes [9]. On the other hand, acetogens producing some volatile fatty acids (VFAs) are more robust to environmental change than methanogens[10, 11]. Considering the metabolic pathway of anaerobic processes, the concentrations of VFAs could be considered as good indicators of anaerobic reactor performances, specifically in the activity of acetogens and methanogens. Because of the character of high volatile organic compounds for FW, the accumulation of VFAs easily occur during the AD process, which would result in a severe acidification with a sharp pH drop [10,11]. To stabilize the anaerobic process and increase methane yields, it is necessary to study the effects of intermediate VFAs produced on the methane production and methanogenic community shift during the anaerobic digestion.
So far, most of previous studies have centered on analyses of the effects of pH on anaerobic hydrolysis and acidification of food waste, but few no systematic studies focused on the effect of intermediate VFAs produced on diversity methanogenic community shift. Nowadays, comprehensive understanding depth of the microbial community is impeded by the low sequencing depth. Many traditionally molecule technology approaches, such as denaturing gradient gel electrophoresis (DGGE), single-strand conformation polymorphism (SSCP), and terminal restriction fragment length polymorphism (T-RFLP), are limited to reveal higher microbial diversity in the environment due to the limitation in number of DNA templates sequenced[12]. Based on the analysis 16S rRNA gene, high-throughput sequencing (HTS) technologies (e.g., HiSeq 2000, HiSeq 2500, MiSeq) have been an attractive option to fully explore the microbial composition and diversity in the environment, which are lower cost, rapid analysis, and higher accuracy[12,13]. This method could support many unrevealed details about the variation of methanogenic community structure with VFAs inhibition caused by different FW dosage during AD.
To achieve a stable maximum methane production, this study was performed to study the effects of intermediate VFAs on biogas production and methanogenic community shift during anaerobic digestion with FW as the sole substrate under different organic loading rate (OLR) represented by F/I (VSfoodwastes:VSsludge) with 0.2L inoculum. The variations of VFAs (including formic acid, acetic acid, propionic acid, and butyric acid), Dehydrogenase activity and coenzyme F420 (7, 8-didemethyl-8-hydroxy-5-deazariboflavin derivative) were analyzed and compared. And methane production potential, was also assessed. At the end of reaction, the methanogenic community were analyzed by the high-throughput sequencing to reveal the shift of methanogenic community structure.
Materials And Methods
Inoculum and substrates
Anaerobic digester sludge (ADS) was used as the inoculum for initiating the digestion reactions in each test. The ADS were taken from an anaerobic digester in Municipal Engineering Ltd., Wuhan University in Wuhan. FW was collected from the canteen in Wuhan University, Wuhan. And the properties of ADS and FW were shown in the table1. The indigestible compositions FW, such as plastic and chopsticks were selected out before they were crushed and homogenized. The homogenized substrates were kept in a −20°C fridge till for anaerobic digestion.
| Inoculum sludge | Food waste | |
|---|---|---|
| pH | 7.20 ± 0.00 | 6.90 ± 0.01 |
| TS (%,) | 3.86 ± 0.01 | 26.84 ± 0.01 |
| VS (%,) | 1.44 ± 0.01 | 29.36 ± 0.01 |
| The moisture content(%) | 96.14 ± 0.01 | 73.16 ± 0.01 |
| C/N | 7.12 ± 0.01 | 20.46 ± 0.01 |
Table 1: The characteristics of inoculum sludge and food waste.
Anaerobic digestion of food waste
The batch digestion in lab-scale was carried out in 1L conical flasks with working volume of 0.8 L at temperature of 37 ± 1°C. ADS added was 0.2L for every conical flask, and was diluted with MQ-water to 0.8L working volume. To study the variation of intermediate VFAs produced during the anaerobic digestion of food waste on biogas production and methanogenic community, the difference OLRs
represented by F/I (VSfoodwastes:VSsludge) were set to 0, 0.5, 1.0, 2.0, 4.0, 8.0, 12.0 and 20.0 with 0.2L ADS, which were used in experimental groups H0, H1, H2 , H3, H4, H5, H6 and H7, respectively. Before the start of reaction, the headspace of each bottle was purged with N2 for 20 min. The bottles were shaken by hand with shaking frequency of 2 times per day and 2 min for every shaking. No alkalinity or buffering agent was added into the system before the reaction, and the pH value was not adjusted during the process. Three parallel reactions were set for the experiment.
Analytical methods
Biogas samples were collected from the headspaces of the bottles using aluminum gas collecting bags. The concentrations of CH4, H2 and CO2 were quantified by a GC (GC-2030, Tet Instrument, China) equipped with a thermal conductivity detector (TCD) and a 2m stainless column packed with Porapak TDS201 (60/80 mesh). The operating temperatures of the column and detector were kept at 80°C and 110°C, respectively. High purity nitrogen was used as the carrier gas at the flow rate of 500ml.min−1. At each time interval, the total volume of biogas production was measured by drainage method.
The method to analysis of VFAs was similar to that described in Liu et al. [14] and Li et al. [15]with minor modifications. Samples were harvested by centrifugation at 14,000×g for 10 min, the supernatant was lyophilized. After lyophilization, the samples were redissolved the sample in chloroform. 1 μL of sample was injected into the Agilent 7890/5975 GC-MS system (Agilent Technologies) in Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University). The HP-INNOWax column (30m×0.25mm×0.25μm) (Agilent Technologies) was used for GC-MS analysis. The GC-oven temperature was programmed from 60°C (2min) to 240°C (2min) at 10°C/min and the flow rate of carrier gas was 1mL/min. 250°C of interface temperature, 230°C ion-source temperature, an electron-impact ionization (EI) of-70 eV with a full scan ranging from 10 to 350m/z, and a solvent delay of 5 min. Peak identification of target compounds was based on the retention times and full scan spectra of the standards.
Dehydrogenase activity was determined with the use of 2,3,5-triphenyltetrazoliumchloride (TTC) [16]. The coenzyme F420 was measured by a spectrophotometer [17].
DNA extraction, polymerase chain reaction (PCR) amplification and pyrosequencing
The eight samples were collected at the end of the experimental groups H0, H1, H2 , H3, H4, H5, H6 and H7 (15 days in total), respectively. And then these samples were frozen at -20°C for further DNA extraction.
The total DNA was extracted by PowerSoil DNA Isolation Kit (MO-BIO, USA), and then the highthroughput 16S rRNA sequencing technology was adopted to analyze the changes of microbial community structure in reactors under different OLR. During the PCR amplification process, the archaeal 16S rRNA sequences were amplified by using the primer pair of forward primer Arch 338F (5’-ACTCCTACGGGAGGCAGCA-3’) [18] and reverse primer 806R (5’-GGACTACHVGGGTWTCTAAT- 3’). After appropriate treatment, the supernatants were used for DNA extraction, PCR amplification, and pyrosequencing. The Illumina MiSeq platform (PE300, CA, USA) was applied for sequencing of the complete genome of collected samples following the manufacturer’s instructions.
Results
Biogas production under different F/I
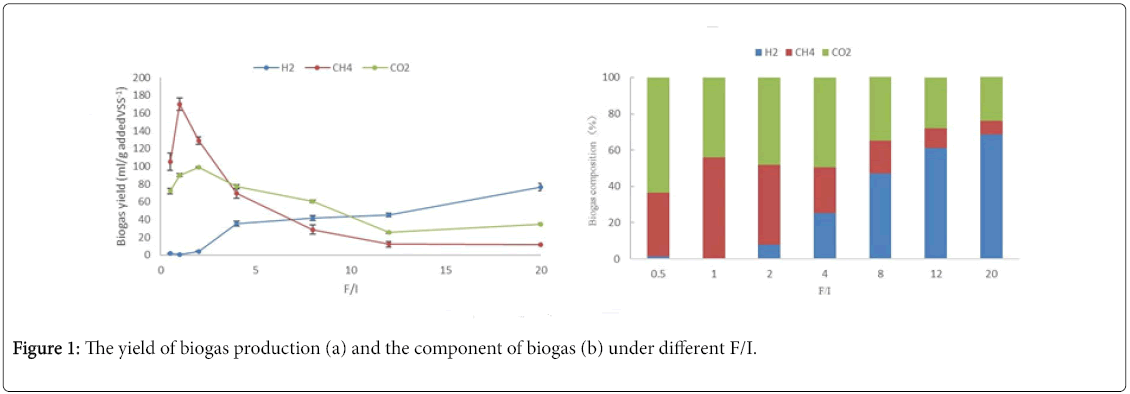
The cumulative methane production potential at different F/I was illustrated in Figure 1, which indicated that the change of F/I not only affected the methane production, but also impacted the composition of the produced biogas. Figure 1a shown the cumulative methane production under different F/I; Figure 1b clearly indicated the component of biogas at the end reaction under difference F/I. Since methane was not produced from the blank, the values of the blank were not shown in Figure 1. Frist, the cumulative methane production increased as the F/I ratio, and then decreased with the further increasing of F/I. The methane yield at 96th h with F/I of 0.5, 1, 2, 4, 8,12 and 20 was 105.3, 170.3, 128.8, 69.7, 28.6, 12.3 and 11.5ml/g addedVS-1, respectively. So, the maximum cumulative methane production (170.3/g addedVS-1) was obtained under F/I of 1, which was about 13.3 times higher than that under F/I of 20. Besides, another obvious difference was the time to reach the stable phase under difference F/I condition. The time to reach the stable phase with F/I of 0.5, 1, 2, 4, 8,12 and 20 needed about 7, 7, 11, 5, 3, 3 and 3days, respectively.
As shown in the Figure 2, the components of biogas were hydrogen, methane and carbon dioxide for every F/I, but the proportion of each component was significant difference under different F/I. The proportion of methane increased from F/I of 0 to 1, and then started to sharply drop. It seemed that F/I of 1.0, used in group H2 , exhibited the maximum methanogenic activity. The proportion of dioxide carbon had a similar trend: increased as the increase of F/I at low organic load, and reached a maximum at F/I of 1, and then deceased. At low F/I, the proportion of hydrogen was very low, but when F/I was beyond 2.0, it increased with the increase of organic load.
The accumulation of VFAs under different F/I
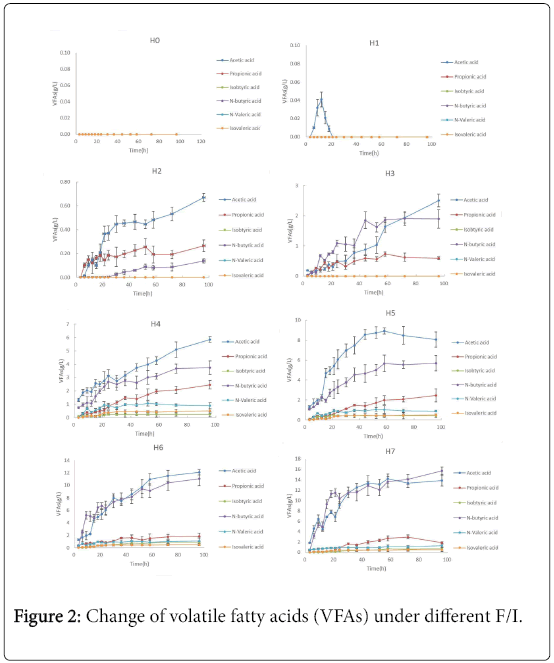
VFAs are the main intermediate products that convert to methane [19,20]. Figure 2 showed the variation of total VFAs and each constituent under different F/I condition during AD. In the control group, there was no any VFAs produced in the whole AD process. In the group with F/I of 0.5, there was only a small amount acetic acid produced. Notably, the concentration of acetic acid in the group H1 firstly increased, then decreased, at last there was no acetic acid. However, the total of VFAs produced in the H2 , H3, H4, H5, H6 and H7 showed an upward trend during AD process. And the concentration of VFAs sharply increased at the 1st day especially in the high F/I (H5, H6 and H7). For example, in the group H7, the total VFAs was 21.99g/L at 24h, which was about 0.647-fold of that at 96h. Besides, the total VFAs produced at the same time among difference groups increased as the increasing F/I. For example, the total VFAs in the H0, H1, H2 , H3, H4, H5, H6 and H7 at 96h was 0, 0, 1.07, 4.99, 13.71, 18.02, 27.44 and 33.97g/L, respectively. It was also found that the main components of VFAs during AD process were mainly acetic acid, propionic acid and N-butyric acid; and isobutyric acid; n-valeric acid and iso-valeric acid were not or only found in trace amounts in this experiment. The percentage of N-butyric acid increased with the increasing the F/I, and accounted for about 67.2% of total VFAs at the highest F/I of 20. The percentage of acetic acid and propionic acid increased as the increasing of F/I, and then both declined. However, although the concentration of acetic acid and propionic acid had a drop in the high F/I group, their concentrations were still high. Such as in the group F/I of 20, the concentration of acetic acid, propionic acid and N-butyric acid were 3.41g/L, 2.79 g/L and 13.24 g/L, respectively.
Characterization of coenzyme F420 and dehydrogenase Activity in acetic acid inhibition
For anaerobic digestion, the process stability and high treatment efficiency mainly depend on the good match of the hydrolysis/acidogenesis process and methanogenesis process. There are two main methanogenesis for the methanogenesis process: the hydrogenotrophic methanogenesis and aceticlastic methanogenesis [21]. The coenzyme F420 as H+ carriers only existing in hydrogenotrophic methanogenesis process is crucial to the whole of the AD process [21]. Hence, the concentration of coenzyme F420 can reflect the methanogenic activity.
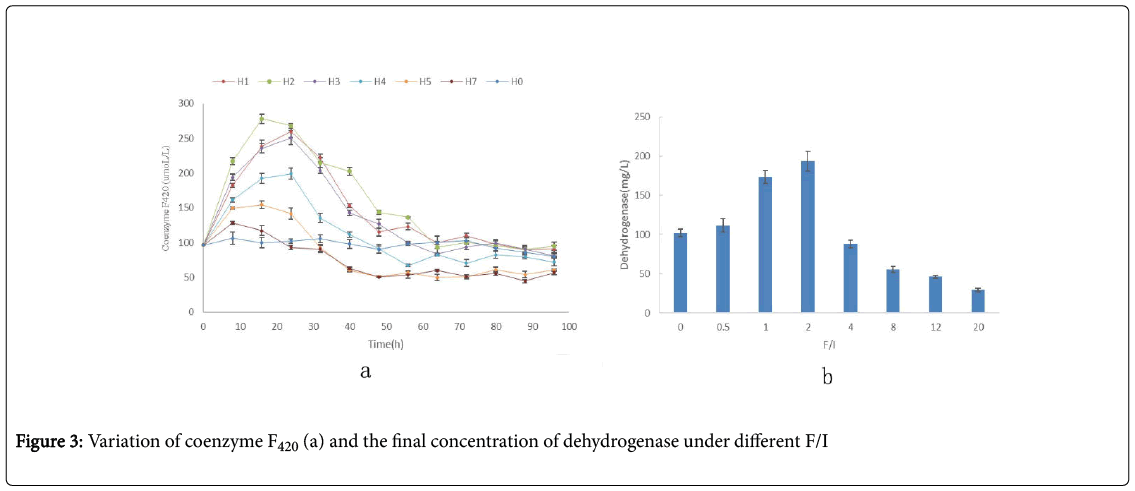
As shown in the Figure 3a, the maximum concentrations of coenzyme F420 in group H1, H2 , H3 H4, H5, H6 and H7 was 0.009, 0.017, 0.015, 0.014, 0.011 and 0.012mmol/L-1, respectively. In the group H2, the maximum concentrations of coenzyme F420 was the highest in all groups. As the increasing of F/I, the maximum concentrations of coenzyme F420 decreased. Under the high F/I, the concentrations of coenzyme F420 just increased a little at the initial 8h, then decreased. And at the end, the concentrations of coenzyme F420 was very low for the high F/I. Besides, the time need to reach the maximum concentration of coenzyme F420 became long as the increasing of F/I, and then became shorter and shorter with the further increasing of F/I. The time obtained maximum concentrations of coenzyme F420 in group H1, H2 , H3 H4, H5, H6 and H7 was the 12thh, 18thh, 30thh, 12thh, 12thh and 6thh, respectively.
Dehydrogenases is one of the most important enzymes, and is used as an indicator of overall microbial activity, which occur intracellular in all living microbial cells [22,23]. To study the effect of VFAs on the activity of microbial community, the samples were token at the end of reaction for every group, and the result was shown in the Figure 4. The concentration of dehydrogenase in every group was very different. Firstly, its concentration increased with the increasing of F/I, and reached the maximum at F/I of 2.0; then it sharply decreased.
Effect of VFAs on the methanogenic community under different F/I
During anaerobic digestion process, high F/I easily lead to VFAs inhibition, which usually effects the variation of methanogenic community structure in the bioreactor [24]. Therefore, the effects of intermediate VFAs produced by the increasing of F/I on the methanogenic community structure were analyzed at the end of each stage used the 16S rRNA high throughput sequencing technique. The results showed in Figure 4.
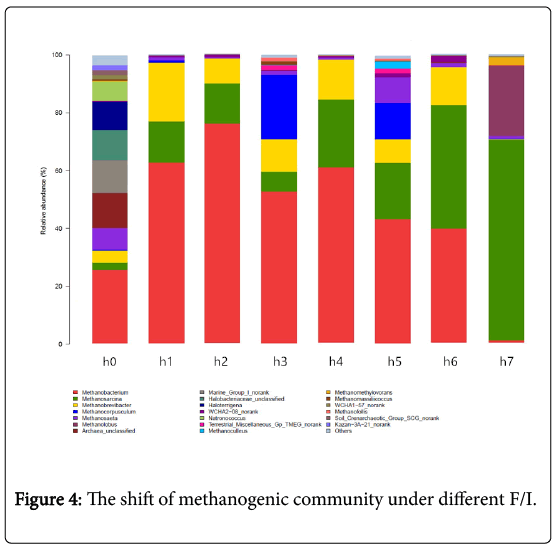
The effective gene sequences of individual sample were assigned to different taxa levels (from species to genus). In this study, the major groups (genera and phyla) were referred to those with a relative abundance no less than 1%. At the genus level (Figure 4), most of archaea were affiliated to the five-major genus: Methanobacterium, Methanosarcina, Methanobrevibacter, Methanocorpusculum and Methanosaeta, which in total accounted for 40.04% (H0),99.00% (H1), 98.80% (H2), 94.51% (H3), 98.97% (H4), 92.47% (H5), 97.00% (H6) and 71.73% (H7) of the archaeal community. As shown in the Figure 4, the dominant archaeal genera in the reactors at the low F/I (H0, H1, H2 , H3 and H4) are mainly Methanobacterium, Methanosarcina and Methanocorpusculum. While the Methanosarcina became more and more dominant in the high F/I. Besides, the most obvious shift of archaeal community structure under difference F/I was the change in relative abundance of Methanobacterium and Methanobrevibacter, especially Methanobacterium. When the F/I increased from 0 to 4.0, the relative abundance of Methanobacterium firstly increased from 25.43% (H0) to 75.89% (H2), and the decreased from 52.64% (H3) to 6.91%(H7). The relative abundance of Methanosarcina in H0, H1, H2 , H3, H4, H5, H6 and H7 was about 2.5%, 14.27%, 13.89%, 6.84%, 23.49%, 19.55%, 42.88%, and 69.81%, respectively. These data indicated that in general the Methanosarcinapresented an upward trend as the increasing of F/I. The relative abundance of Methanosaeta (acetoclastic methanogens) [25] and Methanocorpusculum also had some obvious change. The relative abundance of Methanosaeta and Methanocorpusculum was 8.82% and 22.46% in H5 with F/I of 8, and they both would decrease in lower or higher F/I. Among all the archaeal at the genus level, the change of the relative abundance of Methanobacterium was the strongest.
Discussion
The relationship of methane yield, coenzyme F420 and dehydrogenase with VFAs
VFA concentration is an important indicator in an anaerobic reactor, specifically for the activities of acetogenic bacteria and methanogens. Changes in VFAs could indicate the stability of the anaerobic reactor. Besides, the changing of VFAs concentration was the major influencing factor of pH during AD. The desired range for methanogens is generally between 6.6 and 7.6, which mean that an appropriate VFA concentration was very crucial to anaerobic digestion system. At the beginning, the biological activity for all microorganism was high in every reactor. So, the generation of VFAs was immediate, and massive VFAs were produced. Under the low F/I, the VFAs produced by acetogens was few, and could be quickly metabolized by methanogens, so there was almost no accumulation of VFAs. In comparison, high amount of VFAs would be quickly produced under high F/I by the acetogens and lead to a sharp drop of pH, which inhibited, or even killed methanogens. Hence, the VFAs couldn’t be timely converted to methane. Meanwhile, due to its ability to endure low pH, acetogens continued to produce VFAs. At last, the VFAs inhibition occurred, which caused an imbalance of the methanogenic process due to the considerable increase of VFAs [26]. Methanogen is very sensitive to the change of environment [27]. So, VFAs accumulation and pH decline could suppress the methanogens activity and digestion efficiency [28]. The methane yield decreased with the increasing of total VFAs concentration. Especially at the high VFAs concentration, the decrease of methane yield was very remarkable. Besides, propionic acid is considered to be more toxic than acetate to methanogens, the high concentration of propionic acid may be one of the reasons for lower methane production at high F/I.
As shown in the Figure 2, the accumulation of VFAs would quickly happened at the high F/I, which mean the methanogenic process was inhibited rapidly, and the inhibition became more and more with the increasing of total VFAs concertation, so the concentrations of coenzyme F420 was very low during the AD process with the high F/I; on the contrary, initially there were enough food and appropriate concertation of VFAs, so the methanogenic community were very active at the low F/I. However, as the food ran out with the AD process going on, there was not enough food for the growth and metabolism of methanogens the hydrogenotrophic methanogenesis, so the concentrations of coenzyme F420 firstly increased and then decreased at low F/I/. Because the microorganism, especially methanogenic community, are very sensitive with the change of pH, and even die at low pH. Hence, the dehydrogenase was very low at high F/I.
Archaeal community dynamics
The result of high throughput sequencing shown that Methanobacterium, Methanosarcina, Methanobrevibacter, Methanocorpusculum and Methanosaeta were the main bacteria at the genus level. It is well known that only these two methanogens – Methanosarcina and Methanosaeta – can use acetate as a substrate for methane production and that they compete for acetate. However, Methanosarcina had both hydrogenotrophic and acetoclastic methanogenesis pathways, this is, it not only could use acetate, but also H2 plus CO2 , and methyl compounds for methane production; while Methanosaeta could use only acetate for methane production. Hence, Methanosarcina are expected to be favored with high acetate concentrations under high F/I concentrations; whereas Methanosaeta, with higher affinity for acetate, are expected to be disadvantageous for use at high acetate concentrations. As shown in the Figure 4, the relative abundance of Methanosarcina was high at the high F/I. Methanobacterium and Methanobrevibacter were the hydrogenotrophic methanogen, which could use H2 plus CO2 and formate for methane production [25]. Therefore, Methanosarcina and Methanobrevibacter/Methanobacterium would remain as mainly contributors of the methane production from acetate and H2 plus CO2 under the high F/I conditions. The inhibition of the activity of hydrogenotrophic methanogens including Methanobrevibacter and Methanobacterium might be related to the propionate accumulation in the high F/I [29]. These archaeal community dynamics suggested that hydrogenotrophic methanogens were more significant during the early stages of the AD process perhaps because acetoclastic methanogens were easily inhibited by the accumulation of volatile fatty acids such as, propionate.
Conclusion
The results of this study shown that the methane yield had a very close linked with the variation of intermediate VFAs. High F/I leaded to produce excessive VFAs, and acetic acid and n-butyric acid were the main inhibitory VFAs, especially the acetic acid, to which aceticlastic methanogenesis is more sensitive than hydrogenotrophic methanogenesis. Therefore, compared with aceticlastic methanogenesis only using acetic acid, the microbial species using acetic acid, CO2 /H2 or methyl compounds might have a potential to adapt well to excessive F/I compared, which suggested that the imbalance between the two methane metabolic pathways would lead to low methane production. Besides, the results of the high-throughput sequencing also indicated that the methanogencic community composition was similar under difference F/I, however, the structure was different. And the high-throughput sequencing further indicated that the accumulation of intermediate VFAs mainly leaded to the dominant archaea shifted from Methanobacterium to Methanosarcina.
Acknowledgements
This study was supported by the National Science and Technology Pillar Program (2014BAL04B04), Natural Science Foundation of Hubei Province, China (NO. 2013CFB289; 2013CFB308) and Major Science and Technology Program for Water Pollution Control and Treatment (NO. 2009ZX07317-008-003).
References
- Xiao X, Huang Z, Ruan W, Yan L, Miao H, et al. (2015) Evaluation and characterization during the anaerobic digestion of high-strength kitchen waste slurry via a pilot-scale anaerobic membrane bioreactor. BioresourceTechnol 193: 234-242.
- Zhang DQ, Tan SK, Gersberg RM (2010) Municipal solid waste management in China: Status, problems and challenges. J Environ Manage 91: 1623-1633.
- Li Y, Jin Y (2015) Effects of thermal pretreatment on acidification phase during two-phase batch anaerobic digestion of kitchen waste. Renew Energ 77: 550-557.
- Hecht C, Griehl C (2009) Investigation of the accumulation of aromatic compounds during biogas production from kitchen waste. BioresourceTechnol 100: 654-658.
- De Vrieze J, Plovie K, Verstraete W, Boon N (2015) Co-digestion of molasses or kitchen waste with high-rate activated sludge results in a diverse microbial community with stable methane production. J Environ Manage 152:75-82.
- Vintiloiu A, Boxriker M, Lemmer A, Oechsner H, Jungbluth T, et al. (2013) Effect of ethylene diamine tetra acetic acid (EDTA) on the bioavailability of trace elements during anaerobic digestion. ChemEng J 223: 436-441.
- U Kun Kiran E, Trzcinski AP, Ng WJ, Liu Y (2014) Bioconversion of food waste to energy: A review. Fuel 134: 389-399.
- Raposo F, De la Rubia MA, Fernández-Cegrà V, Borja R (2012) Anaerobic digestion of solid organic substrates in batch mode: An overview relating to methane yields and experimental procedures. Renewable and Sustainable Energy Reviews 16: 861-877.
- Misi SN, Forster CF (2001) Batch co-digestion of multi-component agro-wastes. BioresourceTechnol 80: 19-28.
- Zhang C, Su H, Baeyens J, Tan T (2014) Reviewing the anaerobic digestion of food waste for biogas production. Renewable and Sustainable Energy Reviews 38: 383-392.
- Zhai N, Zhang T, Yin D, Yang G, Wang X, et al. (2015) Effect of initial pH on anaerobic co-digestion of kitchen waste and cow manure. Waste Manage 38: 126-131.
- Yang Z, Xu R, Zheng Y, Chen T, Zhao L et al (2016) Characterization of extracellular polymeric substances and microbial diversity in anaerobic co-digestion reactor treated sewage sludge with fat, oil, grease. BioresourceTechnol 212: 164-173.
- Liu C, Li H, Zhang Y, Si D, Chen Q (2016) Evolution of microbial community along with increasing solid concentration during high-solids anaerobic digestion of sewage sludge. BioresourceTechnol 216: 87-94.
- Liu Z, Gao Y, Chen J, Imanaka T, Bao J, et al. (2013) Analysis of metabolic fluxes for better understanding of mechanisms related to lipid accumulation in oleaginous yeast Trichosporoncutaneum. BioresourTechnol 130: 144-151.
- Li SW, Wang J, Yang Y, Liu ZJ, Cheng L, et al. (2016) Polymorphisms in FADS1 and FADS2 alter plasma fatty acids and desaturase levels in type 2 diabetic patients with coronary artery disease. J Transl Med 14: 79.
- Gabbita KV, Huang JYC (1984) A modified method for determination of dehydrogenases activity of activated sludge. Toxicological and Environmental Chemistry
- Grochowski LL, White RH (2010) Biosynthesis of the Methanogenic coenzymes. Comprehensive natural products II: chemistry and biology, Elsevier Science, Amsterdam, Netherlands pp: 711-748.
- Takai K, Horikoshi K (2000) Rapid detection and quantification of members of the archaeal community by quantitative PCR using fluorogenic probes. Appl Environ Microbiol 66: 5066-5072.
- Tian H, Duan N, Lin C, Li X, Zhong M (2015) Anaerobic co-digestion of kitchen waste and pig manure with different mixing ratios. J BiosciBioeng 120: 51-57.
- Velmurugan B, Arathy EC, Hemalatha R, Philip JE, Alwar RR (2010) Anaerobic co-digestion of fruit and vegetable wastes and primary sewage sludge. J Environ Sci Eng 52: 19-22.
- Deppenmeier U (2002) The unique biochemistry of methanogenesis. Progress in nucleic acid research and molecular biology 71: 223-283.
- Salazar S, Sánchez LE, Alvarez J, Valverde A, Galindo P, et al. (2011) Correlation among soil enzyme activities under different forest system management practices. EcolEng 37:1123-1131.
- Bing-Cheng YU, Dong-Xia YU (2012) Soil microbial and enzymatic activities across a chronosequence of Chinese pine plantation development on the loess plateau of China. Pedosphere 22: 1-12.
- Ali M, Chai L, Wang H, Tang C, Min X et al (2016) Enhanced short-cut nitrification in an airlift reactor by CaCO3 attachment on biomass under high bicarbonate condition. Biodegradation 27: 131-144.
- Xu Z, Zhao M, Miao H, Huang Z, Gao S, et al. (2014) In situ volatile fatty acids influence biogas generation from kitchen wastes by anaerobic digestion. BioresourTechnol 163: 186-192.
- Raposo F, Borja R, MartÃn MA, MartÃn A, de la Rubia MA, et al. (2009) Influence of inoculum–substrate ratio on the anaerobic digestion of sunflower oil cake in batch mode: Process stability and kinetic evaluation. ChemEng J 149: 70-77.
- Xiao X, Huang Z, Ruan W, Yan L, Miao H, et al. (2015) Evaluation and characterization during the anaerobic digestion of high-strength kitchen waste slurry via a pilot-scale anaerobic membrane bioreactor. BioresourTechnol 193: 234-242.
- Climenhaga MA, Banks CJ (2008) Anaerobic digestion of catering wastes: effect of micronutrients and retention time. Water Sci Technol 57: 687-692.
- Barredo MS, Evison LM (1991) Effect of propionate toxicity on methanogen-enriched sludge, Methanobrevibactersmithii, and Methanospirillumhungatii at different pH values. Appl Environ Microbiol 57: 1764-1769.
Citation: Zhang J, Liu M, Zhang J, He Q, Yang K, et al. (2017) The Response of Biogas Production and Methanogenic Community to the Variation of Intermediate VFAs Produced during the Anaerobic Digestion of Food Waste. J Bioremediat Biodegrad 7: 411 DOI: 10.4172/2155-6199.1000411
Copyright: ©2017 Zhang J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5686
- [From(publication date): 0-2017 - Dec 20, 2024]
- Breakdown by view type
- HTML page views: 4930
- PDF downloads: 756