The Propolis and Its Usability for the Mitigation of Methane Production in Ruminants
Received: 20-Sep-2022 / Manuscript No. ijrdpl-22-73125 / Editor assigned: 22-Sep-2022 / PreQC No. ijrdpl-22-73125 / Reviewed: 06-Oct-2022 / QC No. ijrdpl-22-73125 / Revised: 10-Oct-2022 / Manuscript No. ijrdpl-22-73125 / Accepted Date: 16-Oct-2022 / Published Date: 17-Oct-2022 QI No. / ijrdpl-22-73125
Abstract
In this review propolis was studied for mitigation of methane emission based on its origin. Propolis is a natural resinous substance collected by honeybees from different plant parts such as buds, branches, leaves, and exudates.It is a multifunctional material used by bees in the construction and maintenance of their hives. Use of propolis by humans has a long history, predated only by the discovery of honey. Use of products containing propolis has resulted in extensive dermal contact and it is now increasingly being used a dietary supplement. Propolis is a known source of polyphenols and the flavonoids which contributes to rumen fermentations. Propolis stimulates the rumen microorganism for the consumption of hydrogen by changing in total VFA. Recently, studies on the effects of propolis on animal husbandry and animal health have increased due to many issues, such as organic animal breeding, feeding or treatment methods, reducing the use of antibiotics. One of these areas is the use of propolis to improve the growth performance and productivity of the livestock. Propolis has been determined to have antibacterial and antiviral effects in humans and animals.
Introduction
Ruminant’s (cattle, buffalo, sheep, and goats) are considered as the largest contributor in the emission of greenhouse gases which results in global warming. Six gases have been identified as constituting the greenhouse gases; mainly carbon dioxide (CO2) and methane (CH4) with trace amounts of other gases are responsible for this climate change. Because animals consume plants that uses CO2, so it has not been considered as a net contributor to this climate change but the role of CH4 and N2O have contribute a lot to this climate change and has 21-times more global warming potential then CO2 (Haque [1]). Methane emission is not only related to this climate change but also associated with the energy loss, reductions in their retention and use of energy. Microbial activity in the rumen plays (Table 1) a vital role in the conversion of gross energy (GE) in feed into CH4. There is a need of sustainable and immediate mitigation strategies from ruminants through dietary manipulation, which is simplistic and pragmatic approach to alter the pathway of fermentation for the reduction of CH4 emission up to 40% depending upon the degree of change and nature of intervention (Haque, 2018 [1]). Several studies have been done for the discovery of alternative natural feed additives and propolis is one of them which has been considered as an alternative feed additive to antibiotics to improve the feed efficiency and body weight gain (Soltan et al. 2016 [2]; Morsy et al. 2015[3]). Propolis is generally known as the “bee glue” collected by honeybees from exudates and buds of various plants species, mixed with wax and pollen, and modified by the enzymatic activity of honeybees (Soltan and Patra, 2020[4]). It is used by honeybees as a defense material for the protection of hive, to fill the cavities of the beehive, for maintenance of internal temperature (350C) during cold days and protection from invasion by predators. For decades it has been the main interest of researchers to investigate its chemical composition and biological properties. Propolis chemical composition depends upon plants species and phyto-geographic conditions which generates problem for the exact standardization and quality control, but many studies have been made to overcome this and give results. It is noteworthy here that every source of propolis has its own significant biological activity.
| Microbial Types | Important Genera And Species | % Of Microbial Mass |
|---|---|---|
| Bacteria | 40–50 | |
| Acetogens | Acetitomaculum ruminis, Eubacterium limosum | |
| Acid utilizers | Megasphaera elsdeni, Wolinella succinogenes, Veillonella gazogene, Micrococcus lactolytica, Oxalobacter formigenes, Desulfovibrio desulfuricans, Desulfotomaculum ruminis, Succiniclasticum ruminis | |
| Cellulolytic | Fibrobacter succinogenes, Butyrivibrio fi brisolvens, Ruminococcus fl avefaciens, Ruminococcus albus, Clostridium cellobioparum, Clostridium longisporum, Clostridium lochheadii, Eubacterium cellulosolvens | |
| Hemicellulolytic | Prevotella ruminicola, Eubacterium xylanophilum, Eubacterium uniformis | |
| Lipolytic | Anaerovibrio lipolytica | |
| Pectinolytic | Treponema saccharophilum, Lachnospira multiparus | |
| Proteolytic | Prevotella ruminicola, Ruminobacter amylophilus, Clostridium bifermentans | |
| Amylolytic | Streptococcus bovis, Ruminobacter amylophilus, Prevotella ruminicola | |
| Saccharolytic | Succinivibrio dextrinosolvens, Succnivibrio amylolytica, Selenomonas ruminantium, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus fermentum, Lactobacillus plantarum, Lactobacillus brevis, Lactobacillus helveticus, Bifi dobacterium globosum, Bifi dobacterium longum, Bifi dobacterium thermophilum, Bifi dobacterium ruminale, Bifi dobacterium ruminantium | |
| Protozoa | Entodinium bovis, Entodinium bubalum, Entodinium bursa, Entodinium caudatum, Entodinium chatterjeei, Entodinium parvum, Entodinium longinucleatum, Entodinium dubardi, Entodinium exiguum, Epidinium caudatum, Isotricha prostoma, Isotricha intestinalis, Dasytricha, ruminantium, Diplodinium dendatum, Diplodinium indicum, Oligoisotricha bubali, Polyplastron multivesiculatum, Eremoplastron asiaticus, Eremoplastron bubalus | 40–50 |
| Fungi | Piromyces communis, Piromyces mae, Piromyces minutus, Piromyces dumbonicus, Piromyces rhizinfl atus, Piromyces spiralis, Piromyces citronii, Piromyces polycephalus, Anaeromyces, mucronatus , Anaeromyces elegans, Caecomyces | 3–4 |
| communis, Caecomyces equi, Caecomyces sympodialis, Cyllamyces aberensis, Cyllamyces icaris, Neocallimastix frontalis, Neocallimastix patriciarum, Neocallimastix hurleyensis, Neocallimastix variabilis, Orpinomyces joynii, Orpinomyces intercalaris | ||
| Methanogens | Methanobacterium formicicum, Methanobacterium bryantii, Methanobrevibacter ruminantium, Methanobrevibacter smithii, Methanomicrobium mobile, Methanosarcina barkeri, Methanoculleus olentangyi | 2–3 |
| Bacteriophages | Methanobacterium phage Ψ M1, Methanobacterium phage Ψ M10, Methanobacterium phage Ψ M100, Methanothermobacter phage Ψ M100, Methanobacterium phage ΨM2 | <0.1 |
Table1: Rumen Microbiota.
Biologically it has many antioxidant, antibacterial, antifungal, antiviral, anti-inflammatory, antiparasitic, anti-methanogenic, immune-modulatory, and anticancer properties. Due to higher antimicrobial activity of propolis against gram positive than gram negative bacteria it has been observed that it plays a key role in the modification of ruminal fermentation to reduce the loss of energy as methane (Soltan & Patra, 2020[4]). The objective of this review is to demonstrate the efficiency of propolis for the mitigation of methane emission from ruminants as a natural feed additive.
Method
Search for previous studies on the subject was made by using peer-reviewed journal articles published in English. Databases of Scopus, Science Direct, Google scholar, Academia, Research- Gate and Wiley online were used for the literature search between 2014 and 2021. The initial keywords search was Propolis Phytochemicals, ruminal microorganism, mitigation strategies. For more focused search, we looked at titles and abstracts containing predefined keywords or terms such as phytochemicals, (Table 2)
Plant Species |
Phytochemicals | Animal Species | Study | Roughage To Concentrate Ratio | Microbial | References |
|---|---|---|---|---|---|---|
| Design | Population | |||||
| Saponaria officinalis | Triterpenoid, Saponin | Holstein–Friesian dairy cows | In Vitro | 0·01, 0·25, 0·5, 1·0, | ↓Microbial | (Cieslak et al. 2014[24]) |
| 2·5, 5·0 mg/ml | Population | |||||
| Nephelium lappaceum | Saponins | Fistulated dairy bulls | In vitro | 0, 2, 4, 6% | ↓ Protozoa | (Ampapon & Wanapat, -2019[25]) |
| Licorice | Flavonoids, Terpenoids, | Kordish rams | In Vitro | 60:40:00 | ↓Protozoa | (Tedeschi et al. 2021[22]) |
| Alkaloids | 40:60 | ↓Entodinium | ||||
| Stevia rebaudiana | Saponins, Oligosaccharides | Holstein-Frisian cows | In vitro | 3.79 kg | ↓Protozoa | (Ramos-Morales et al. 2017[26]) |
| Strawberry | Tannins, | Holstein-Friesian cows | In Vitro | 21 kg DM | No Effect | (Bryszak et al. 2019[27]) |
| Flavonoids, | In Vivo | |||||
| Black pepper | Tannins | Holstein cows | In Vitro | 40 g/d | No Effect | (Rodriguez, 2018[28]) |
| In Vivo | ||||||
| Humulus lupulus L. | Acetate Propionate | Fistulated steers | In vivo | 0·1, 0·2, 0·5, 1·0, 2·5, | ≠Gram- | (Flythe & Harlow, 2019[29]) |
| 5·0 mg/ml | positive bacteria | |||||
| Eucalyptus viminalis | Flavinoids | Bull-calves | In Vitro In Vivo | 100 ml / goal | ≠ Proteobacteria | (Yausheva et al. 2019[30]) |
| ≠Fibrobacteres | ||||||
| Maize silage | Pyrogallol | Simmental cattle | In Vivo | 5.57 kg DM/d | ↑Firmicutes | (Zhou et al. 2019[31]) |
| Gallic acid | ↑Bacteroidetes | |||||
| Chestnut | Tannins | Holstein steers | In Vivo In Vitro | 2 g/kg | ↑Firmicutes | (Díaz Carrasco et al. 2017[32]) |
| ↑Bacteroidetes | ||||||
| Azadirachta indica | Acetate, Butyrate | West African Dwarf | In Vitro | 40 g/day | No Effect | (Adelusi et al. 2016[33]) |
| Goats | ||||||
| Acacia mearnsii | Tannins | Jersey steers | In Vitro | 0, 5, 10, 15, and 20 g/kg | ↑Entodinium | (Avila et al. 2020[34]) |
| Eucalyptus | Tannins | Fistulated buffaloes | In Vitro | 0, 40, 80, and 120 | ↓Protozoa | (Thao et al. 2015[35]) |
| camaldulensis | g/hd/d | ↓proteolytic bacteria | ||||
| Rambutan | Propionate, Acetate, butyrate | Crossbreed beef | In vitro | 0, 4, 8, 12, 16 and 20 | ↑Microbiota | (Gunun et al. 2018[36]) |
| fruits | mg/0.5 g |
Table 2: Effects of Phytochemicals on Microbial Ecosystem.
essential oil, phytogenic, plant bioactive compounds, rumen fermentation, rumen microbiota adaptation, rumen micro-organisms, feed intake, ruminant performance, growth performance, goat, sheep, cattle, buffalo, beef, dairy, lactating, digestibility, and composition, CH4 production and mitigation. Eligible phytogenic additives were chosen by screening arrays of different propolis extract recommended for modulating ruminal fermentation (Table 3) and microbiota, nutrient digestibility.Plant Species |
Plant Material | Animal Species | Study Design | Diet | Effect on Rumen Fermentation | Reference |
|---|---|---|---|---|---|---|
| Corn silage | Essential Oils Herbs, Spices, | Lactating | In Vivo In Vitro | 3 g/cow/d | 3g-↑ | (Kholif et al. 2020[37]) |
| Friesian | 6 g/cow/d | 6g-↓ | ||||
| cows | ||||||
| Mint, Rosemary, Clove | Herbs, Spices, essential oils | Non-lactating Holstein-Frisian cows | In vitro | 15 g per cow per day | ↑ | (Neubauer et al. 2018[38]) |
| Trifolium alexandrinum | Herbs | Damascus goats | In vitro | 10 g daily | ↑ | (Kholif et al. 2017[39]) |
| Eucalyptus Celery Monensin | Herbs | Holstein dairy calves | In vitro | EUC (23 g/kg) | No effects | (Akbarian-Tefaghi et al. 2018[40]) |
| CEL (23 g/kg) | ||||||
| MON (30 mg/kg) | ||||||
| Trifolium alexandrinum | Straw | Early lactation Friesian cow | In vitro | 14 g/cow daily | No effect | (Matloup et al. 2017[41]) |
| Moringa oleifera | Crushed leaves | Holstein steers | In vitro | 1 g | ↑ | (Parra-Garcia et al. 2019[42]) |
| Eucalyptus globulus | Essential oils | Bubalus bubalis | In vitro | 0, 20, 40, 80, 120 µL | ↑ | (Singh et al. 2020[43]) |
| Autocarpus integrifolis, Azardirachta indica Ficus bengalensis | Cereals and oil | Holstein Friesian crossbred bulls | In vitro | 0, 2·5, 5·0, 10·0, 15·0, | No Effect | (Bhatta et al. 2015[44]) |
| 20·0, 25·0 and 30·0% | ||||||
| Panicum maximum | Fresh turmeric rhizomes | West African dwarf goats | In vitro | 0, 10, 20, 30 mg/g | ↓↑ | (Aderinboye & Olanipekun, 2021[45]) |
| Different roughage | Maringa oil | Buffalo | In vitro | 30–50% | ↑ | (Ebeid et al. 2020[46]) |
Table 3: Effect of different Plant extracts on Rumen Fermentation process.
Consequently, we sorted articles on phyto-constituents in in vitro and in vivo (ruminants) assays separately. We mapped out the content of the selected literatures by extracting information based on the following questions (i) what was the composition of phytochemicals? (ii) Did the propolis affect ruminal microbiota? (iii) Did it affect ruminal fermentation? We, therefore, ensured that the methodologies used in considered articles met the selected systematic methods for reducing bias, improving the reliability of the review findings, and drawing reasonable conclusions.
Phyto-chemistry of Propolis
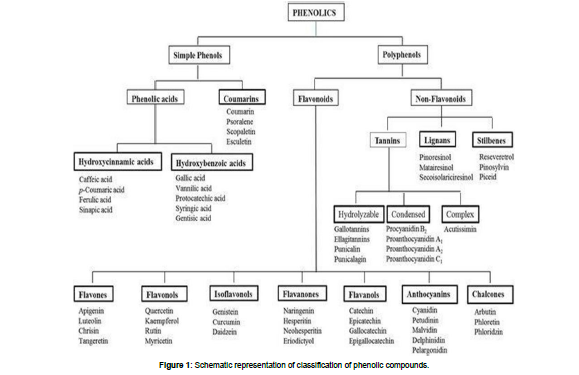
The chemical composition of propolis depends upon plant species; phytogeography, climatic factors, collection season and every source have its own significant applications. So, the researchers have a keen interest in the investigation of detailed chemical composition from every source of propolis. To date 300 compounds have been identified in propolis and Alkaloid, Flavonoid, Phenolics, (Figure 1) Terpenoid, Tannin, Glycoside and Anthraquinone, have been identified as the basic groups among these compounds (Soltan et al. 2016[2]).
Phytogeography
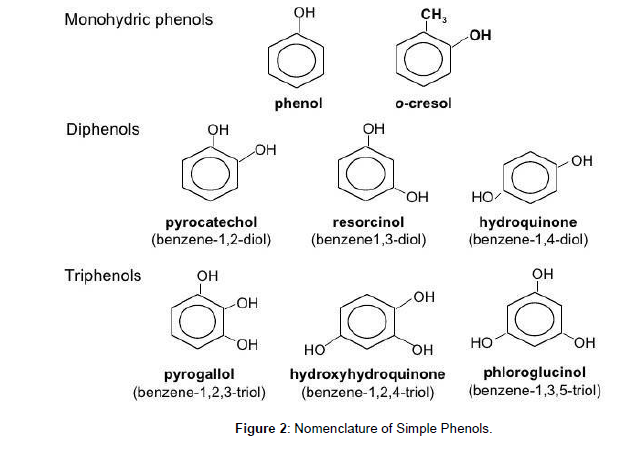
To understand the chemical composition of propolis of one region in connection with the other, researchers give a detailed description of propolis production. Generally, honeybees collect lipophilic plant substance from buds, leaves, lattices, mucilage, branches, and barks, usually within a radius of 1-2 km from the hive. Mainly in the temperate zone, honeybees collect lipophilic materials from May to November, but most frequently in the late summer (Ristivojevića et al. 2015 [5]). When propolis extracts were obtained by supercritical extraction (SCO2) and ethanolic extraction (EtOH), in eight samples of different types of propolis (red, green, and brown) from different regions in Brazil, the concentration of Artepillin C and pcoumaric acid was very high in the extracts from SCO2 (Machado et al. 2016 [6]). African propolis (Congo and Cameroon) showed twenty-one secondary metabolites belonging to four different chemical groups, three triterpenes and two diprenyl-flavonoids were identified from Congo propolis, while thirteen triterpenes, three diprenyl-flavonoids, two monoterpenic alcohols and one fatty acid ester have been identified from Cameroon propolis samples. (Papachroni et al. 2015 [7]). High-field nuclear magnetic resonance spectral profiling of propolis samples from Kangaroo Island, South Australia of sedge plant Lepidosperma sp. Montebello (Cyperaceae) showed have high proportion of prenylated hydroxystilbene and C- and O-prenylated tetrahydroxystilbenes (pTHOS) while prenylated p-coumarate concentrations were in very small amount. The isolation of five pTHOS which was not previously reported showed: (E)-4-(3- methyl-2-buten-1-yl)-3,40,5trihydroxy-30 -methoxystilbene, (E)-2,4- bis(3-methyl-2- buten-1-yl)-3,30,40,5tetrahydroxystilbene, (E)-2- (3-methyl-2-buten-1-yl)-3-(3- methyl-2-butenyloxy)- 30,40,5trihydroxystilbene, (E)-2,6-bis(3-methyl-2-buten-1-yl)-3,30,5,50 - tetrahydroxystilbene and (E)2,6- bis(3-methyl-2-buten-1-yl)-3,40,5-trihydroxy- 30 –methoxystilbene. A chemical study of propolis from the Baha region of Saudi Arabia identified 61 chemical compounds with distinct chemical structures from the following classes: 19 phenol/alcohol/ aldehydes, 10 aromatic acids, 8 esters, 6 aliphatic acids, 4 flavonoids, 4 ketones, 4 fatty acid esters, 3 terpenes 2 sugars, and 1 steroid. Turkish propolis has high total phenolic (314.36 ± 3.65 mg GAE/g propolis) and total flavonoid contents (522.71 ± 11.45 mg QE/g propolis) (Ozdal et al. 2018 [8]). The chemical composition of propolis samples collected from different geographical regions showed that flavonoid and phenolic (Figure 2) compounds are frequent in all propolis samples and determine its characteristic properties.
Seasonal effect
A limited number of studies have been conducted to evaluate the seasonal effect of propolis, however when polish propolis collected throughout three seasons of the year. The number of flavonoids and phenolic acids were highest, when harvested during the spring (125.14 mg/g) and the lowest amount in the fall (110.09 mg/g) (Wozniak et al. 2019 [9]). Artepillin C was found high in the propolis samples of southern Brazil collected during summer and autumn (Tomazzoli et al. 2020 [10]). The bioactivity of Caborca propolis (CP) collected from an arid zone of the Sonoran Desert showed that spring and autumn collection have higher amounts of bioactive compounds in comparison to the rainy seasons of summer and winter (Mendez- Pfeiffer et al. 2020 [11]). According to a comparative study conducted between March and June of 2013 and March of 2015 for the chemical composition of Africanized bees’ propolis and the author concluded that similarity of chemical profiles of collected samples were same. The main difference was in the content of phenolic acids, propolis extract produced in 2015 showed a higher content of phenolic acids caffeic, ferulic and p-coumaric and ferulic acid and this change in phenolic acid concentration was due to the difference in temperature because temperature can influence the production of secondary metabolites in plants. These environmental variabilities affect the resins, flower buds and resinous exudates which are sources of material to produce propolis and a variation in the chemical composition of these materials means changes in the composition of propolis (Calegari et al. 2017[11]). Additionally, previous reports have shown that seasonality does not significant change the chemical composition of propolis, but it can influence the quantitative chemical profile of propolis (Valencia et al. 2012 [12])
Plant Origin
In the recent year researchers focused on the chemical composition of plant origin. Phenolic profile of propolis exudates from plant Zuccagnia punctata showed eleven compounds. Among them, two uncommon dihydrochalcones, i.e., 40 -hydroxy-20 -methoxydihydrochalcone and 20 ,40 -dihydroxydihy drochalcone, were described for the first time as major constituents of Z. punctata,(Solorzano et al. 2017 [13]). A phytochemical screening of the propolis of plant Dalbergia ecastophyllum showed that it has variety of phenolic compounds including flavonoids (catechins, chalcones, aurones, flavones and flavanols), phlobaphene tannins, xanthones and pentacyclic triterpenoids (de Mendonça et al. 2015 [14]). Profiling of Trigona Apicalis propolis extract has higher concentration of flavonoid compounds as compared to phenolic (Rosli et al. 2016 [15]).
Propolis on Ruminal Microbial Fermentation and Digestion
Rumen microbiota
Rumen has a diversity of microbial ecosystem including bacteria, ciliate protozoa, anaerobic fungi, and archaea, which are involved in the degradation of feedstuffs and the production of volatile fatty acids (VFAs), lactate, amino acids, lipids, and hydrogen, which are crucial to the maintenance, growth, and production performance of ruminants (Kruger Ben Shabat et al. 2016 [16]). The host also uses microbial (Table 4) biomass and some unfermented feed components once these exit the rumen to the remainder of the digestive tract (Henderson et al. 2015 [17]).
Propolis |
Diet | Study Design | Animals | Microbil Population | Rumen Fermentation | Ammonia-Nitrogen Concentration | Methane Emission | Reference |
|---|---|---|---|---|---|---|---|---|
| Iran | 25, 50 and | In vitro | Dairy cows | 25g ↑ | ↑ | ↓ | ↓ | S. Ehtesham |
| 75 g | et al. 2018[19]) | |||||||
| Brazil | 30% | In vitro In vivo | Male sheep | ↓ | No Effect | ↓ | ↓ | (Da Silva et al. 2015[47]) |
| Brazil | 100 g | In vitro | Girolando rumen-fistulated cows | ↓ | ↑ | No Effect | ↓ | (Nascimento |
| et al. 2020[48]) | ||||||||
| Brazil | 50:50:00 | In vivo | ↓ | ↑ | Not Investigated | ↓ | (Morsy et al. 2021[23]) | |
| Brazil | 1.2 g | In vitro | Cannulated Holstein cows | ↓ | No Effect | ↓ | ↓ | (Yoshimura et al. 2018[18]) |
| Turkey | 2 g | In vitro In Vivo | Rusitec fermenters | ↓ | No Effect | ↓ | ↓ | (Ozturk et al. 2010[24]) |
| 6 g | ||||||||
| China | 3g | In vitro | Holstein heifers | ↓ | No Effect | ↓ | ↓ | (Zhang et al. 2017[49]) |
| Brazil | 35 g | In vitro | Fstulated cows | ↓ | ↑ | ↓ | ↓ | (Gomes et al. 2017[20]) |
| Egypt | 200 mg | In vitro | Females Abuduleik | ↓gram-positive | ↑ | ↓ | ↓ | (Badawy, 2021[50]) |
| sheep | ↑gram-negative |
Table 4: Effect of Propolis on Rumen Microbial Population, Rumen Fermentation, and methane Emission.
Effects of phytochemicals on ruminal microbiota
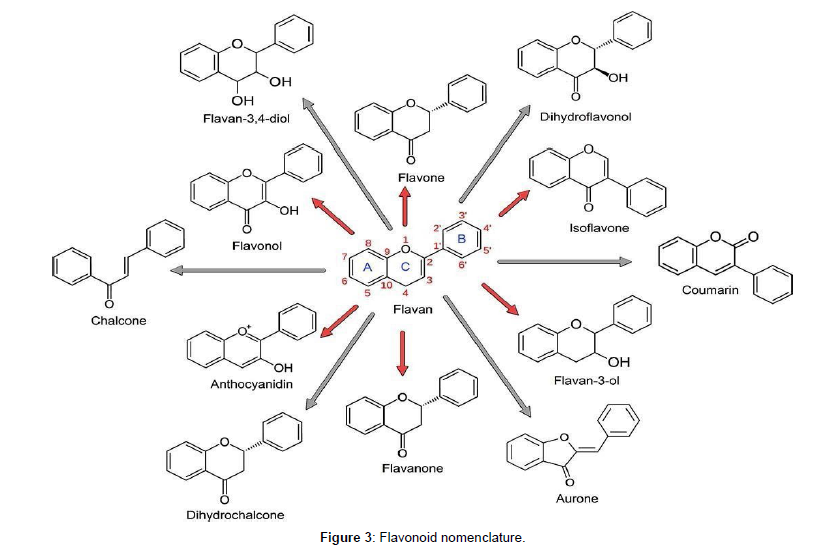
Changes in dietary supplementation strongly affects the composition, efficiency, and the fermentation of the diet-dependent ruminal microbiota (Yoshimura et al. 2018 [18]). Different bioactive compounds have different antimicrobial effect, propolis causes modulation of the ruminal fermentation (Soltan & Patra, 2020 [4]). Propolis has bacteriostatic activity against Gram-positive and some Gram-negative bacteria (S. Ehtesham et al. 2018 [19]). Cell wall of gram-negative bacteria are less rigid as compared to gram positive bacteria, so they have higher resistance to propolis due to higher complexity of these structures, with liposaccharides and high lipid content. The flavonoid (Figure 3) compounds in the propolis extract act against microorganisms through inhibition of cell membrane function, bacterial activity, or synthesis of nucleic acid, which explains the higher degradability and cumulative gas production of diets when added with propolis extract in relation to the negative control, which had complete bactericidal action and suggests that presence of flavonoids in the extract is probably capable of affecting fermentation in rumen fluid, acting through bacteria selection. It was recommended that 100% propolis extract supplementation may improve the degradation and fermentation of ruminant diet (Gomes et al. 2017 [20]). Presence of phenolic compounds in the propolis increases the microbial protein synthesis and total VFA concentration in the rumen so it has been studied that improvement in the ruminal fermentation depends upon the phenolic compounds present in propolis extract especially when high forage and low N diets are fed (de Paula et al. 2016 [21]). Three Brazilian propolis extracts (containing naringenin, caffeic acid, p-coumaric acid chrysin and artepillin C) was studied against different ruminal bacteria in vitro. It was observed that propolis extracts inhibited the growth of Ruminococcus flavefaciens, Ruminococcus albus 7, Fibro-bacter succinogenes, Butyrivibrio fibrisolvens, Prevotella albensis and Streptococcus bovis, but R. albus 20, Prevotella bryantii and Ruminobacter amylophilus were resistant to all the extracts. Propolis was effective against the hyper ammonia producing bacteria Clostridium aminophilum and Peptostreptococcus sp. (Soltan & Patra, 2020 [4]). It was assumed that phenolic compounds in the Brazilian propolis have a great antimicrobial activity (Machado et al. 2016 [6]).
Effect of Propolis on Rumen Fermentation and methane Emission
Rumen Fermentation
Anaerobic fermentation in the rumen depends upon the supply of substrate (i.e., quantity and frequency), preservation of a favorable condition for microbial growth (e.g., temperature, pH, substrate mixing), and constant removal of undesirable substances (e.g., bacterial toxins, hydrogen), so that the profile and amount of volatile fatty acids (VFA) produced and microbial protein leaving the rumen meets the ruminant's daily requirements for energy and protein without having deleterious impacts in the rumen health and functionality (Tedeschi et al. 2021 [22]). For decades different types of antibiotics are used to meet this criterion of rumen fermentation, but now the consumers denied the use of antibiotics due to some deleterious effects on ruminant health and researchers trying to implement the use of ecologically relevant ionophores because these ionophores modulates the rumen fermentation process and provides health benefits for ruminants. It has been described in the Table-3 how different plant extracts effects on rumen fermentation.
Methane mitigation
Methane emission can be done by changing rumen fermentation pattern by desirable dietary products. Increased dietary level of concentrate reduces CH4 production (Haque, 2018 [1]). However,a comprehensive exploration for a sustainable methane mitigation approach is still lacking. In the recent years it was reported that ciliates are the main source of hydrogen supply to the endosymbiotic and episymbiotic methanogens and inhibition of protozoa reduces the methane emission from ruminants (Soltan et al. 2016 [2]). Based on phyto-chemistry results, the propolis could be used as a natural alternatives product to obtain the desired rumen fermentation. Red propolis extract (RPE) supplementation in late pregnant ewes enhanced the apparent digestibility and microbial protein syntheses, and decreased CH4 emission (Morsy et al. 2021 [23]). When the propolis was examined at different doses studies showed that it did not improve the production rate and the profile of ruminal short-chain fatty acid (SCFA), while it is able to inhibit ruminal NH3-N concentration (Ozturk et al. 2010 [24]).
Conclusion
Propolis stimulates the rumen microorganism for the consumption of hydrogen by changing in total VFA and it was suggested that there is a need to study the effect of propolis for the mitigation of methane based on phytogeography, botanical origin, climatic condition, and collection methods for the further effective applications of propolis in the mitigation of methane in vitro/in vivo (Morsy [23-57]). A little work has been done on the effects of propolis from 2015 to 2021 for the mitigation of methane and rumen fermentation process as indicated in the Table-4. Further studies on the basis phytochemical constituent’s reaction to rumen microbiota can be performed to understand the effects of these propolis flavonoid and phenolic reaction with on the cell of bacteria and by using different phytochemical additives we can explore the chemical effects of these ionophores on bacterial cell wall for the investigation of methane emission process from ruminants.
References
- Haque N (2018) Dietary manipulation : a sustainable way to mitigate methane emissions from ruminants. 1–10.
- Soltan Y, Morsy A, Sallam S, Hashem N, Abdalla A, et al. (2016) Propolis As a Natural Feed Additive in Ruminant Diets; Can Propolis Affect the Ruminants Performance?: A Review.
- Morsy AS, Soltan YA, Sallam SMA, Kreuzer M, Alencar SM, et al. (2015) Comparison of the in vitro efficiency of supplementary bee propolis extracts of different origin in enhancing the ruminal degradability of organic matter and mitigating the formation of methane. Animal Feed Science and Technology 199: 51–60.
- Soltan YA, Patra AK (2020) Bee propolis as a natural feed additive: Bioactive compounds and effects on ruminal fermentation pattern as well as productivity of ruminants. Indian Journalof Animal Health 59: 50–61.
- Ristivojevića P, Trifkovićb J, Andrić FB, Milojković-Opsenicab D (2015) Poplartype Propolis: Chemical Composition, Botanical Origin and Biological Activity. Natural Product Communications 10:1869–1876.
- Machado BAS, Silva RPD, Barreto GDA, Costa SS, Da Silva DF, et al. (2016) Chemical composition and biological activity of extracts obtained by supercritical extraction and ethanolic extraction of brown, green and red propolis derived from different geographic regions in Brazil. PLoS ONE 11: 1–26.
- Papachroni D, Graikou K, Kosalec I, Damianakos H, Ingram V, et al. (2015) Phytochemical analysis and biological evaluation of selected african propolis samples from Cameroon and congo. Natural Product Communications 10: 67–70.
- Ozdal T, Sari-kaplan G, Mutlu-altundag E, Boyacioglu D (2018) Evaluation of Turkish propolis for its chemical composition , antioxidant capacity , anti- proliferative effect on several human breast cancer cell lines and proliferative effect on fibroblasts and mouse mesenchymal stem cell line. Journal of Apicultural Research 0: 1–12.
- Wozniak M, Mrówczynska L, Agnieszka W, Tomasz R, Izabela R, et al. (2019) The role of seasonality on the chemical composition , antioxidant activity and cytotoxicity of Polish propolis in human erythrocytes. 29: 301–308.
- Tomazzoli MM, Zeggio ARS, Neto RDP, Specht L, Costa C, et al. (2020) Botanical source investigation and evaluation of the effect of seasonality on brazilian propolis from apis mellifera L. Scientia Agricola 77: 1-10.
- Mendez-Pfeiffer P, Alday E, Carreño AL, Hernández-Tánori J, Montaño-Leyva B, et al. (2020) Seasonality modulates the cellular antioxidant activity and antiproliferative effect of sonoran desert propolis. Antioxidants 9: 1–15.
- Valencia D, Alday E, Robles-Zepeda R, Garibay-Escobar A, Galvez-Ruiz JC, et al. (2012) Seasonal effect on chemical composition and biological activities of Sonoran propolis. Food chemistry 131:645-651.
- Solorzano ER, Bortolini C, Bogialli S, Di Gangi IM, Favaro G, et al. (2017) Use of a LC-DAD-QTOF system for the characterization of the phenolic profile of the argentinean plant Zuccagnia punctata and of the related propolis: New biomarkers. Journal of Functional Foods 33: 425–435.
- Mendonça De, Porto ICG, de M ICC, do Nascimento TG, de Souza NS, et al. (2015) Brazilian red propolis: Phytochemical screening, antioxidant activity and effect against cancer cells. BMC Complementary and Alternative Medicine 15: 1–12.
- Rosli NL, Roslan H, Omar EA, Mokhtar N, Hapit NHA, et al. (2016) Phytochemical analysis and antioxidant activities of Trigona Apicalis propolis extract. AIP Conference Proceedings.
- Kruger Ben Shabat S, Sasson G, Doron-Faigenboim A, Durman T, Yaacoby S, et al. (2016) Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME Journal 10: 2958–2972.
- Henderson G, Cox F, Ganesh S, Jonker A, Young W, et al. (2015) Rumen microbial community composition varies with diet and host , but a core microbiome is found across a wide geographical range.
- Yoshimura EH, Santos NW, Machado E, Agustinho BC, Pereira LM, et al. (2018) Effects of dairy cow diets supplied with flaxseed oil and propolis extract, with or without vitamin E, on the ruminal microbiota, biohydrogenation, and digestion. Animal Feed Science and Technology 241: 163–172.
- Ehtesham S, Vakili AR, Danesh Mesgaran M, Bankova V (2018) The effects of phenolic compounds in iranian propolis extracts on in vitro rumen fermentation, methane production and microbial population. Iranian Journal of Applied Animal Science 8: 33–41.
- Gomes Mde FF, Itavo CCBF, Itavo LCV, Leal CRB, da Silva JA, et al. (2017) In vitro fermentation characteristics of ruminant diets using ethanol extract of brown propolis as a nutritional additive. Revista Brasileira de Zootecnia 46: 599–605.
- De Paula EM, Samensari RB, Machado E, Pereira LM, Maia FJ, et al. (2016) Effects of phenolic compounds on ruminal protozoa population, ruminal fermentation, and digestion in water buffaloes. Livestock Science 185: 136–141.
- Tedeschi LO, Muir JP, Naumann HD, Norris AB, Ramírez-Restrepo CA, et al. (2021) Nutritional Aspects of Ecologically Relevant Phytochemicals in Ruminant Production. Frontiers in Veterinary Science 8:1–24.
- Morsy AS, Soltan YA, El-Zaiat HM, Alencar SM, Abdalla AL, et al. (2021) Bee propolis extract as a phytogenic feed additive to enhance diet digestibility, rumen microbial biosynthesis, mitigating methane formation and health status of late pregnant ewes. Animal Feed Science and Technology 273: 114834.
- Cieslak A, Zmora P, Stochmal A, Pecio L, Oleszek W, et al. (2014) Rumen antimethanogenic effect of Saponaria officinalis L. phytochemicals in vitro. Journal of Agricultural Science 152: 981–993.
- Ampapon T, Wanapat M (2019) Rambutan fruit peel powder and dietary protein level influencing on fermentation characteristics, nutrient digestibility, ruminal microorganisms and gas production using in vitro fermentation techniques. Tropical Animal Health and Production 51:1489–1496.
- Ramos-Morales E, De La Fuente G, Nash RJ, Braganca R, Duval S, et al. (2017) Improving the antiprotozoal effect of saponins in the rumen by combination with glycosidase inhibiting iminosugars or by modification of their chemical structure. PLoS ONE 12:1–14.
- Bryszak M, Szumacher-Strabel M, El-Sherbiny M, Stochmal A, Oleszek W, et al. (2019) Effects of berry seed residues on ruminal fermentation, methane concentration, milk production, and fatty acid proportions in the rumen and milk of dairy cows. Journal of Dairy Science 102:1257–1273.
- Rodriguez S (2018) Evaluation of Dietary Phytochemicals as Rumen Modifiers in LactatingDairy Cows. ProQuest Dissertations and Theses 85.
- Flythe MD, Harlow BE (2019) Effects of Hops (<i>Humulus lupulus</i> L.)Beta-Acids on Short Chain Fatty Acid Production from Complex Carbohydrates by Rumen Microbiota. Advances in Microbiology 09:983–992.
- Yausheva EV, Duskaev GK, Levakhin GI, Nurzhanov BS, Yuldashbaev YA, et al. (2019) Evaluation of the effects of plant extracts on cattle rumen mi-crobiome. IOP Conference Series: Earth and Environmental Science 341.
- Zhou K, Bao Y, Zhao G (2019) Effects of dietary crude protein and tannic acid on rumen fermentation, rumen microbiota and nutrient digestion in beef cattle. Archives of Animal Nutrition 73: 30–43.
- Díaz Carrasco JM, Cabral C, Redondo LM, Pin Viso ND, Colombatto D (2017) Impact of Chestnut and Quebracho Tannins on Rumen Microbiota of Bovines. BioMed Research International.
- Adelusi O, Isah O, Afolabi R, Aderinboye R, Idowu3 O, et al. (2016) Effect of Tree Leaves on Rumen Fermentation, Microbial Count and Blood Urea Nitrogen of West African Dwarf Goats. Malaysian Journal of Animal Science 19: 19–30.
- Avila A, Zambom MA, Faccenda A, Fischer ML, Anschau FA, et al. (2020) Effects of black wattle (Acacia mearnsii) condensed tannins on intake, protozoa population, ruminal fermentation, and nutrient digestibility in jersey steers. Animals 10: 1–12.
- Thao NT, Wanapat M, Kang S, Cherdthong A (2015) Effects of supplementation of eucalyptus (E. Camaldulensis) leaf meal on feed intake and rumen fermentation efficiency in swamp buffaloes. Asian-Australasian Journal of Animal Sciences 28: 951–957.
- Gunun P, Gunun N, Cherdthong A, Wanapat M, Polyorach S, et al. (2018) In vitro rumen fermentation and methane production as affected by rambutan pee powder. Journal of Applied Animal Research 46:626–631.
- Kholif AE, Hassan AA, El Ashry GM, Bakr MH, El-Zaiat HM, et al. (2020) Phytogenic feed additives mixture enhances the lactational performance, feed utilization and ruminal fermentation of Friesian cows. Animal Biotechnology 1–11.
- Neubauer V, Petri R, Humer E, Kröger I, Mann E, et al. (2018) High-grain diets supplemented with phytogenic compounds or autolyzed yeast modulate ruminal bacterial community and fermentation in dry cows. Journal of Dairy Science 101: 2335–2349.
- Kholif AE, Matloup OH, Morsy TA, Abdo MM, Abu Elella AA, et al. (2017) Rosemary and lemongrass herbs as phytogenic feed additives to improve efficient feed utilization, manipulate rumen fermentation and elevate milk production of Damascus goats. Livestock Science 204: 39–46.
- Akbarian-Tefaghi M, Ghasemi E, Khorvash M (2018) Performance, rumen fermentation and blood metabolites of dairy calves fed starter mixtures supplemented with herbal plants, essential oils or monensin. Journal of Animal Physiology and Animal Nutrition 102: 630–638.
- Matloup OH, Abd El Tawab AM, Hassan AA, Hadhoud FI, Khattab MSA, et al. (2017) Performance of lactating Friesian cows fed a diet supplemented with coriander oil: Feed intake, nutrient digestibility, ruminal fermentation, blood chemistry, and milk production. Animal Feed Science and Technology 226: 88–97.
- Parra-Garcia A, Elghandour MMMY, Greiner R, Barbabosa-Pliego A, Camacho-Diaz LM, et al. (2019) Effects of Moringa oleifera leaf extract on ruminal methane and carbon dioxide production and fermentation kinetics in a steer model. Environmental.Science and Pollution Research 26: 15333-15344.
- Singh RK, Dey A, Paul SS, Singh M, Dahiya SS, et al. (2020) Associative effects of plant secondary metabolites in modulating in vitro methanogenesis, volatile fatty acids production and fermentation of feed in buffalo (Bubalus bubalis). Agroforestry Systems 94: 1555–1566.
- Bhatta R, Saravanan M, Baruah L, Prasad CS (2015) Effects of graded levels of tannincontaining tropical tree leaves on in vitro rumen fermentation, total protozoa and methaneProduction. Journal of Applied Microbiology 118: 557–564.
- Aderinboye RY, Olanipekun AO (2021) An in-vitro evaluation of the potentials of turmeric as phytogenic feed additive for rumen modification. Nigerian Journal of Animal Production 48 : 193– 203.
- Ebeid HM, Mengwei L, Kholif AE, Hassan Ful, Lijuan P, et al. (2020) Moringa Oleifera Oil Modulates Rumen Microflora to Mediate In Vitro Fermentation Kinetics and Methanogenesis in Total Mix Rations. Current Microbiology 77: 1271– 1282.
- Da Silva FGB, Yamamoto SM, da Silva EMS, Queiroz MAÁ, Gordiano LA, et al. (2015) Extrato de própolis e monensina sódica sobre os parâmetros de fermentação ruminal e hematológicos de ovinos. Acta Scientiarum - Animal Sciences 37: 273–280.
- Nascimento RJT, Teixeira RMA, Tomich TR, Pereira LGR, do Carmo TDJ, et al. (2020) Residue of propolis extract in bovine diets with increasing levels of protein on rumen fermentation. Pesquisa Agropecuaria Brasileira 55: 1-10.
- Zhang J, Shi H, Wang Y, Li S, Cao Z, et al. (2017) Effect of Dietary Forage to Concentrate Ratios on Dynamic Profile Changes and Interactions of Ruminal Microbiota and Metabolites in Holstein Heifers 8: 1–18.
- Badawy H (2021) Effect of Propolis as a Feed Additive on Nutritional and Productive Performance of Pregnant Ewes and their Lambs under Halaib-Shalateen Pastures Condition.Journal of Animal and Poultry Production 12: 19–26.
- Calegari MA, Prasniewski A,Silva CDA (2017) Propolis from Southwest of Parana produced by selected bees : Influence of seasonality and food supplementation on antioxidant activity and phenolic profile. 89: 45–55.
- Ozturk H, Pekcan M, Sireli M, Fidanci UR (2010) Effects of propolis on in vitro rumen microbial fermentation. Ankara Universitesi Veteriner Fakultesi Dergisi 57: 217–221.
- Ehtesham, Shahab, Vakili A, Mesgaran MD (2016) Effect of Brown Iranian Propolis Extracts on in vitro Rumen Gas Production. 75: 119-125.
- Bakdash A, Almohammadi1 OH, Taha NA, Kumar AARS (2018) Chemical Composition of Propolis from the Baha Region in Saudi Arabia. 1–10.
- Duke CC, Tran VH, Duke RK, Abu-Mellal A, Plunkett GT, et al. (2017) A sedge plant as the source of Kangaroo Island propolis rich in prenylated p-coumarate ester and stilbenes. Phytochemistry 134: 87– 97.
- Montoya-Flores MD, Molina-Botero IC, Arango J, Romano-Muñoz JL, Solorio-Sánchez FJ, et al. (2020) Effect of dried leaves of leucaena leucocephala on rumen fermentation rumen microbial population and enteric methane production in crossbred heifers. Animals 10: 1–17.
- Puniya AK, Singh R, Kamra DN (2015) Rumen Microbiology: From Evolution to Revolution. In Rumen Microbiology: From Evolution to Revolution. Egyptian Journal of Nutrition and Feed.19: 73–79.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Jabbar M, Hira H, Saeed MS, Bibi M, Nawaz H (2022) The Propolis and Its Usability for the Mitigation of Methane Production in Ruminants. Int J Res Dev Pharm L Sci, 8: 136.
Copyright: © 2022 Jabbar M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1886
- [From(publication date): 0-2022 - Mar 14, 2025]
- Breakdown by view type
- HTML page views: 1592
- PDF downloads: 294