Review Article Open Access
The Prevention of Alzheimer's Disease and Parkinson's Disease by Monascus purpureus NTU 568-Fermented Compounds
Chun Lin Lee1 and Tzu Ming Pan2,3*1Department of Life Science, National Taitung University, Taitung, Taiwan, ROC
2Department of Biochemical Science and Technology, National Taiwan University, Taipei 106, Taiwan, ROC
3Department of Research and Development, SunWay Biotechnology Company, Taipei 114, Taiwan, ROC
- Corresponding Author:
- Tzu Ming Pan
Department of Biochemical Science and Technology
College of Life Science, National Taiwan University
No. 1, Sec. 4, Roosevelt Road, Taipei
10617 Taiwan, ROC
Tel: 886-233664519
E-mail: tmpan@ntu.edu.tw
Received date: June 09, 2017; Accepted date: June 28, 2017; Published date: July 05, 2017
Citation: Lee CL, Pan TM (2017) The Prevention of Alzheimer's Disease and Parkinson’s Disease by Monascus purpureus NTU 568-Fermented Compounds. J Alzheimers Dis Parkinsonism 7:342. doi:10.4172/2161-0460.1000342
Copyright: © 2017 Lee CL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Alzheimer's disease (AD) and Parkinson’s disease (PD), which are senile neurodegenerative diseases, have become more common in recent year. An important cause for AD is currently the deposition of amyloid β (Aβ) in the brain, leading to severe oxidative stress and inflammation, as well as neuronal death and memory damage in AD patients. PD is a chronic degeneration of the central nervous system that mainly affects the motor system. Its symptoms usually appear gradually, and are most obviously tremors, body stiffness, slowed movement, and gait abnormality during early stage; though the mechanism for the death of dopaminergic neurons is not yet completely understood, oxidative stress has been shown to be a very important factor in triggering the development of PD in many human and animal studies. As has been revealed in past research, fermented products of Monascus and their extracts can effectively improve the course of AD and PD, possessing significant preventative and alleviating effects. The main effectors are confirmed to be monascin and ankaflavin for AD treatment, and dimerumic acid and deferricoprogen for PD treatment. This review, therefore, focuses on the analysis and comprehensive discussion of these four active components, for an overall understanding of their role in the AD and PD improvement function of Monascus fermented products.
Keywords
Alzheimer’s disease; Parkinson’s disease; Monascus
Introduction
As the aging population grows each year, Alzheimer's disease (AD) and Parkinson’s disease (PD), which are senile neurodegenerative diseases, have become more common in recent year. An important cause for AD is currently the deposition of amyloid β (Aβ) in the brain, leading to severe oxidative stress and inflammation, as well as neuronal death and memory damage in patients with AD [1]. In a number of in vitro and in vivo studies, it has been found that the use of antioxidants can effectively prevent the memory disability due to nerve impairment, caused by Aβ [1-3]. Epidemiology data also indicate the reduction in occurrence rate of AD by non-steroidal anti-inflammatory drugs [4]. On the other hand, a high cholesterol and high caloric diet increases the lipid content of the brain and causes lipid oxidation [5,6]. As such, a high caloric diet and Aβ deposition heighten the oxidation stress of the brain. Studies have pointed out that red mold rice also inhibits the increase in brain cholesterol caused by a high caloric diet, and lowers the expression of the risk factor apolipoprotein E (ApoE) and activity of β-secretase, ultimately reducing the accumulation of Aβ40 [7].
PD is a chronic degeneration of the central nervous system that mainly affects the motor system [8]. Its symptoms usually appear gradually and are most commonly tremors, body stiffness, slowed movement and gait abnormality during early stage [9]; these symptoms may be accompanied by cognitive and behavioral problems. Though the mechanism for the death of dopaminergic neurons is not yet completely understood, oxidative stress has been shown to be a very important factor in triggering the development of PD in many human and animal studies [10]. Antioxidants such as vitamins E and D are thought to protect brain cells from PD. Relevant research however, has not reached consistent conclusions, with some studies indicating that fats and fatty acids also have protective effect on neurons, and can reduce the risk of disease. Furthermore, studies have tentatively shown that estrogen and non-steroidal anti-inflammatory drugs may also have protective functions [11-13]. Based on the above, inhibition of oxidative stress may be an important way of alleviating and preventing PD.
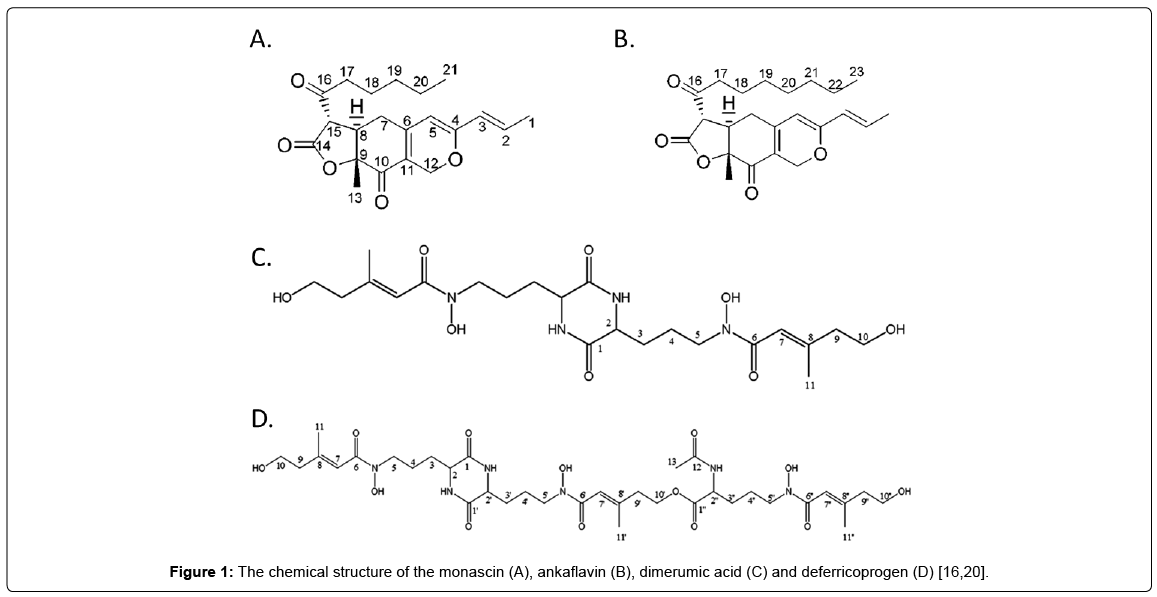
Monascus fermented product is a traditional natural food coloring used in many food products. The secondary metabolite of Monascus – monacolin K (MK) have been proven to have cholesterol lowering, blood pressure regulating, and anti-oxidative effects. As functional foods are receiving increasing attention, Monascus fermented products, with their many physiological benefits are becoming important. As has been revealed in past research, fermentation products of Monascus and their extracts can effectively improve the course of AD and PD, possessing significant preventative and alleviating effects [7,14-19]. The functional compounds are confirmed to be the novel yellow pigment monascin (MS) and ankaflavin (AK) for AD treatment, and novel antioxidative agent dimerumic acid (DMA) and deferricoprogen (DFC) for PD treatment. Their structures are shown in Figure 1 [16,20]. This review, therefore, focuses on the analysis and comprehensive discussion of these four active components, for an overall understanding of their role in the AD and PD improvement function of Monascus fermented products.
The Pathogenesis of AD and PD.
The pathogenesis of AD
As the aging population increases year by year, there is also an increase in patients with dementia. Dementia can be divided into multiple types, with Alzheimer's disease being the most common. AD is a degenerative disease of the cranial nerves, with common clinical symptoms of memory impairment, spatial operation disorder, language disorder, dysgraphia, loss of self-cognition, damaged judgment ability, attention dispersion, and others. The senile plaques accumulated around nerve cells are mainly composed of a peptide with 39-43 amino acids [21], called the Aβ, which is hard to dissolve. Amino acid sequence analysis of the amyloid separated from senile plaques shows that it is of the same type as the β-protein from the cerebrovascular plaques of patients with AD. Aβ is expressed in the neurons and glia of the central nervous system, and other body tissues [22,23], it is a peptide of 39-43 amino acids formed by the cleavage of amyloid precursor protein (APP) by the enzymes β-secretase and gamma-secretase. Aβ could be produced in large excess due to genetic mutation or other oxidative stresses. Its deposition in the brain causes nerve cells to lose their functions and die [24]. The main structural component of paired helical filament (PHF) in tangle cells is the tau protein, which cannot combine with microtubules as normal due to its high level of phosphorylation, thus forming PHF. Microtubules also lose their physiological functions due to the lack of tau protein binding [25]. The accumulation of these two abnormal proteins causes death of nerve cells and glial proliferation [26]. These pathological changes could be the results of genetic defects, aging, brain cell injury, local inflammation and Aβ toxicity. A production of Aβ in large quantities or a reduction in its scavenging rate leads to its aggregation. This is followed by the polymerization and deposition of Aβ to form diffuse plaques. Aβ oligomers act on synapses and activate neuroglial cells and astrocytes, causing progressive synapse and nerve damage. This changes the ionic balance of the neurons and brings about oxidative damage and alteration in kinase phosphatase activity, leading to PHF, and shortage of neurotransmitters, followed by extensive loss of neuronal function and nerve cell death and eventually AD [27].
The pathogenesis of PD
Parkinson's disease is a chronic degeneration of the central nervous system that mainly affects the motor system [8]. Its symptoms usually appear gradually with time, which are most obviously tremors, body stiffness, slowed movement and gait abnormality during early stage [9] and may be accompanied by cognitive and behavioral problems.
Although the mechanism of dopaminergic neuronal death remains unclear, it has been shown through human trials and animal studies that oxidative stress is an important factor for triggering the development of PD [10]. Vitamins E and D are thought to protect brain cells from the disease. Relevant research, however, has not reached consistent conclusions, with some indicating that fats and fatty acids also have protective effect on neurons, and can reduce the risk of disease. Furthermore, studies have tentatively shown that estrogen and non-steroidal anti-inflammatory drugs may also have protective functions [11-13]. Based on the above, inhibition of oxidative stress may be an important way of alleviating and preventing PD. Numerous studies have developed a number of PD animal models to be used as a testing platform for screening neuroprotective drugs. Many research-related neurotoxins, such as methyl-4-phenyl- 1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA) are used in these animal models. 6-OHDA is commonly used to induce PD in both in vitro and in vivo models through oxidative stress [28], which is shown to enhance the generation of ROS, a key player in 6-OHDA induced PD models [29]. In addition, mitogen-activated protein kinases (MAPKs) are usually associated with neural cell death and apoptosis [30] and the Ak mouse strain thymoma (Akt) pathway is, thus, believed to be a necessity in protecting the neurons from cell death and safeguarding cell survival [30,31]. Various studies have revealed the regulation of 6-OHDA induced neuronal cell damage by MAPK pathway activation and Akt inhibition [32].
Past Researches on the Pathogenesis of AD and the Improvement by Monascus Fermented Product
Neuroprotective effects of Monascus fermented product and its relation to AD improvement
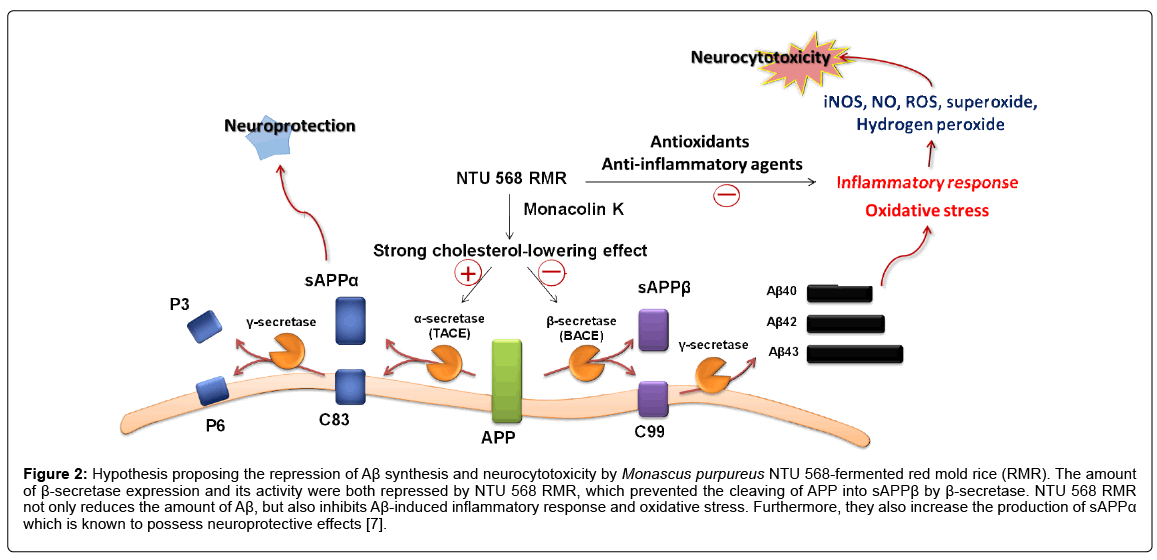
Inhibition of Aβ toxicity in neurons: Research has shown that increase in cholesterol mevalonate concentrations in IMR-32 and PC- 12 cells leads to rise in risk factor levels such as APPβ and Aβ, and the decrease in the production of neurotrophic factor sAPPα [18]. The ethanol extract of red mold rice fermented by M. purpureus NTU 568 could significantly inhibit the expression of the inflammation factor iNOS induced by Aβ40, and could reduce NO and ROS production, as well as oxidative stress. From this review, we conclude that the ethanol extract inhibits the cytotoxicity mechanism of Aβ40 (Figure 2) [7].
Figure 2: Hypothesis proposing the repression of Aβ synthesis and neurocytotoxicity by Monascus purpureus NTU 568-fermented red mold rice (RMR). The amount of β-secretase expression and its activity were both repressed by NTU 568 RMR, which prevented the cleaving of APP into sAPPβ by β-secretase. NTU 568 RMR not only reduces the amount of Aβ, but also inhibits Aβ-induced inflammatory response and oxidative stress. Furthermore, they also increase the production of sAPPα which is known to possess neuroprotective effects [7].
Inhibition of Aβ deposition via anti-oxidation and antiinflammation: Introduction of Aβ40 raises the level of AChE activity in the brain, as well as levels of active oxygen atoms and lipid peroxidation, while reducing the total anti-oxidation power and superoxide dismutase activity. Oxidative stress and inflammatory response lead to the formation of more Aβ fibers and greater severity in the pathogenesis of AD, but these damages are reduced prominently by dietary supplementation of red mold rice (RMR). This is mainly due to the anti-oxidation and anti-inflammation abilities of Monascus fermented products and the protection offered by RMR against the formation of fibrous Aβ and its deposition in the hippocampus, thereby improving memory learning ability [17]. As such, metabolites from Monascus fermentation should be effective in preventing the advancement of Aβ-induced memory impairment into AD.
Suppression of Aβ formation and promotion of sAPPα formation: The AD risk factor Aβ is predominantly formed by the cholesterol catalyzed β-secretase lysis of the precursor protein APP in the brain, forming the toxic Aβ. As indicated by previous studies, a high caloric diet with high cholesterol increases the chance of AD and worsens the condition of the disease, via elevated formation of apoE and β-secretase activity, which increase the deposition of Aβ in the brain, worsening the damage in memory learning ability. Research shows that AD and formation of Aβ are related to lipid metabolism in cells [33-35]. The ethanol extracts of NTU 568 RMR reduce the amount of Aβ and sAPPβ synthesized [36], and increase the production of the neuroprotective sAPPα. The expression and activity of β-secretase are both inhibited, avoiding its cleavage of APP to form APPβ. The inhibition of Aβ synthesis by the alcohol extracts of RMR is mainly through the reduction in cholesterol formation in nerve cells (Figure 2) [7], which could prevent the secretion of β-secretase induced by cholesterol and block the paths for Aβ and APPβ generation, leading to enhanced α-secretase activity and the level of neuroprotective sAPPα. RMR improves the memory learning ability in the water maze task, significantly. The simultaneous introduction of Aβ40 and high caloric diet causes brain damage, including rise in acetylcholine and more intense oxidative and inflammatory responses. RMR could also inhibit the increase in cholesterol concentration in the brain caused by a high caloric diet, and lower the expression of the risk factor apoE and activity of β-secretase, ultimately reducing the accumulation of Aβ40 in hippocampal tissue, and raising the production of the neuroprotective factor sAPPα [18]. In addition, RMR has also been shown to suppress the increase in cholesterol concentration caused by a high caloric diet, minimizing the expression of risk factor apoE and β-secretase activity.
Past studies on PD improvement by Monascus fermented products
PD improvement by Monascus-fermented products: Past research has indicated that M. purpureus NTU 568-fermented rice extracts could improve neural toxicity in SH-SY5Y cells and PD in rat models, both induced by 6-OHDA [15]. This research confirms the attenuation of PD by M. purpureus NTU 568-fermented rice extracts using the in vitro and in vivo neural toxicity models of 6-OHDA induced PD. The basic mechanism of these processes includes the suppression of oxidative stress and damage by the down-regulation of NOX expression and reduction of inflammatory factors activated by 6-OHDA. This result is of importance for the development of novel PD therapy, and shows that the neural protective function of the ethanol extract of M. purpureus NTU 568-fermented rice could come from the anti-oxidative and anti-inflammatory response abilities of its bioactive components. Thus, M. purpureus NTU 568-fermented rice extracts can be used as a dietary constituent in the prevention and treatment of PD. This is the first confirmation that Monascus-fermented products exhibit neural protective effects in PD cells and animal models [15].
Anti-inflammatory and anti-oxidative metabolites produced from Monascus purpureus NTU 568
Anti-inflammatory yellow pigment–MS and AK: MS and AK are the yellow pigments in Monascus fermented product. As shown in Table 1, they have been identified as anti-inflammation components and their health effects have been applied to the prevention and improvement of various conditions as follows:
| Functional ingredients | Beneficial properties | References |
|---|---|---|
| Monascin | Anti-inflammation | [37] |
| Anti-skin cancer | [49] | |
| Hypolipidemic | [50] | |
| Anti-obesity | [51] | |
| Anti-diabetic | [52] | |
| Blood pressure regulation | [53] | |
| Liver protective | [54] | |
| Anti-allergic | [55] | |
| Ankaflavin | Anti-inflammation | [38] |
| Anti-liver cancer | [56] | |
| Hypolipidemic | [50] | |
| Anti-obesity | [51] | |
| Anti-diabetic | [41] | |
| Blood pressure regulation | [53] | |
| Liver protective | [54] | |
| Anti-allergic | [55] | |
| Dimerumic acid | Anti-oxidation | [42] |
| Anti-diabetic | [57] | |
| Liver protective | [44] | |
| Anti-cancer metastasis | [58] | |
| Deferricoprogen | Kidney protective | [46] |
Table 1: The application of Monascus fermented metabolite in the preventive medicine.
In an experiment studying the TPA-induced inflammation of mouse, MS was found to have effective anti-inflammation results [37]. For the inflammation triggered by lipopolysaccharide (LPS) induction of murine RAW 264.7 macrophages, AK could achieve anti-inflammation through the reduction of nitrite content, and inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX- 2) expressions [38]. MS could suppress the inflammation caused by ovalbumin (OVA) induction of human THP-1 monocytes. MS also effectively attenuates the expression of TNF-α, interleukin-6 (IL-6) protein and mRNA. For the OVA-induced inflammation, MS could downregulate the phosphorylation of mitogen-activated protein kinase (MAPK) of c-Jun NH2-terminal kinase (JNK), but not that of ERK or p-38 MAPK. In addition, the assay of PPAR-γ antagonist GW9662 shows that MS suppresses JNK phosphorylation by increasing PPAR-γ expression. Therefore, it can be used as a functional component for anti-inflammation [39].
MS has been confirmed by previous research to possess inhibitory effects on the receptor for advanced glycation end products (RAGE) of hepatic stellate cells (HSCs). MS could increase PPAR-gamma activity to reduce the expression of α-smooth muscle actin (α-SMA) in HSCs and production of ROS; thus, delaying or inhibiting the development of fibrosis through activation of PPAR-gamma [40]. Past research has also shown the regulation on the expression of anti-oxidation enzyme Nrf- 2 by AK, which upregulates the signal transduction pathway of Nrf-2 in a methylglyoxal-induced diabetes rat model to enhance the activity of anti-oxidation enzymes, and promotes the activity of PPAR-α, improving insulin sensitivity [41].
Anti-oxidative agents-dimerumic acid (DMA) and deferricoprogen (DFC): DMA and DFC are the anti-oxidative agents in M. purpureus NTU 568 fermented products. As shown in Table 1, they have been identified as anti-oxidative agents and their health effects have been applied to the prevention and improvement of various conditions as follows:
Aniya et al. discovered the anti-oxidation effect of Monascus fermented product. Upon further purification, it is known that this anti-oxidation effect comes from DMA, which is capable of removing α,α-diphenyl-β-picrylhydrazyl (DPPH) free radical, even at low concentration, as well as reducing the level of ROS [42]. Taira et al. proposed a mechanism for the anti-oxidative ability of DMA in 2002, which states that DMA could inhibit the peroxidation of NADPH and Fe2+-dependent lipid in rat liver microsomes at a concentration of 20- 200 μM. Through clearing OH,·O2 -, ferryl-Mb and peroxyl radicals, and inhibiting lipid peroxidation (LPO), it donates an electron to the oxidizing agent to be oxidized to nitroxide radical, which is removed to achieve the anti-oxidation effect [43].
Yamashiro et al. in 2008 explored the effects of DMA on rat liver microsomes and hepatocyte oxidative stress, i.e., cytotoxicity, induced by salicylic acid (SA) and tert-butylhydroperoxide (t-BHP) and found that DMA could prevent the damage of hepatocytes by inhibiting oxidative stress [44]. As research indicates, the large quantity of carboxymethyllysine (CML) present in patients with diabetes is the cause for hepatic fibrosis. As such, the effects of DMA on RAGE signal transduction and HSCs activation were discussed by the authors, which lead to the finding that DMA could suppress collagen production in HSCs, and mRNA expression for α-SMA, platelet derived growth factor-β receptor (PDGF-βR) and procollagen 1a1 (proCol-1a1). These results show that DMA achieves anti-fibrosis in HSCs by increasing glutamate-cysteine ligase activity and suppressing oxidative stress [45].
Past research has uncovered the high anti-oxidative activity of DFC. Hsu et al. found that DFC could reduce the oxidative damage caused to the human embryonic kidney cell line HEK-293 by citrinin, effectively preventing renal cell apoptosis [46]. Upon further investigation of the antagonistic mechanism of DFC against renal cell toxicity induced by citrinin, the authors found that while citrinin increases the number of renal cells detained in the sub-G1 phase, causing their death, the introduction of DFC could effectively reduce this phenomenon. In terms of the proteins involved in apoptosis, citrinin enhances the activity of caspase-3 and caspase-9 in renal cells, which is significantly diminished by the action of DFC, showing that DFC safeguards kidney cells from damage by suppressing the mitochondrial apoptosis pathways [46].
Past researches on the improvement of AD and PD by Monascus fermented functional compounds
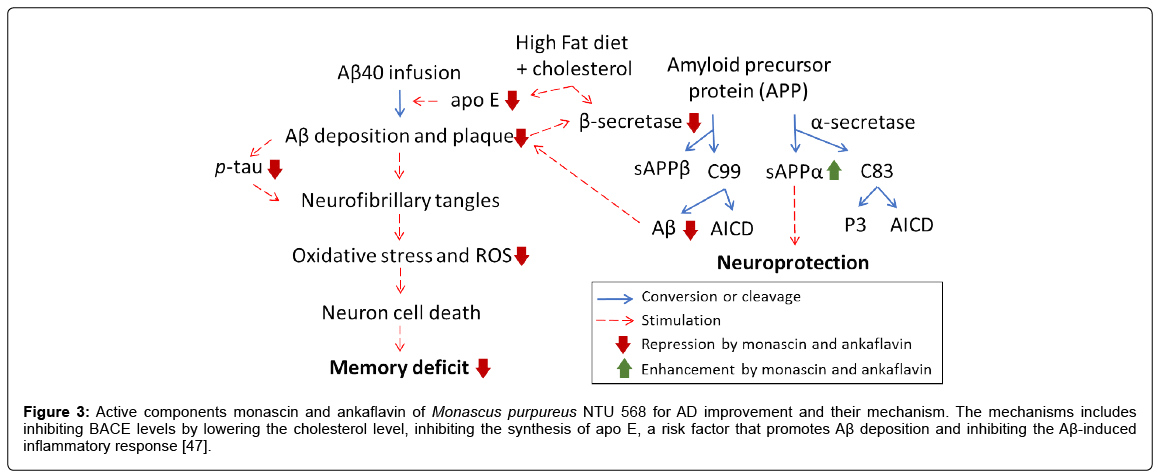
Active components of Monascus fermented product for AD improvement and their mechanism: In the cortex cerebra and hippocampus, the performance of the donepezil group is not as efficient as MS and AK in inhibiting β-secretase, p-tau and apo E. Similar trends are observed in the water maze test as well as MDA and ROS levels in the cortex cerebra and hippocampus of rats introduced with Aβ40 (Figure 3) [47].
Figure 3: Active components monascin and ankaflavin of Monascus purpureus NTU 568 for AD improvement and their mechanism. The mechanisms includes inhibiting BACE levels by lowering the cholesterol level, inhibiting the synthesis of apo E, a risk factor that promotes Aβ deposition and inhibiting the Aβ-induced inflammatory response [47].
The influence of MS and AK on the deposition of the protein Aβ40 in the cortex cerebra and hippocampus: From these results and previous studies, it can be seen that the Aβ40 administered could stimulate oxidation stress and inflammatory response in the brain, resulting in deposition of Aβ40 [23]. As deposition continues with time, oxidative stress and inflammation of greater severity are triggered. This vicious cycle repeats and leads to continued worsening of brain damage. MS and AK lower Aβ40 accumulation in the cortex cerebra by their suppression of oxidation stress and inflammation, so that the Aβ40 introduced into the brain will not be stimulated by oxidants or inflammatory factors to deposit or cause damage to the brain; thus, effectively improving memory learning competence. The inhibition in Aβ40 deposition by oral feeding of MS and AK leads to reduced expression of MDA, ROS and AD related proteins, as well as inflammation-related proteins in the cortex cerebra and hippocampus (Figure 3) [47].
The influence of MS and AK on the deposition of the protein sAPPα in the cortex cerebra and hippocampus: sAPPα is a protective factor for brain nerve cells, capable of promoting synapse growth and protecting nerve cells. In the previous study, the animals in the vehicle group are provided with a series of nerve cell protections to lower the deposition tendency of the Aβ40 introduced, and to inhibit Aβ formation caused by oxidation stress and inflammatory response [23]. The deposition of the protein sAPPα in the donepezil group is not as significant as in the MS and AK groups, which confirms the expression enhancement of the nerve cell protective factor sAPPα by MS and AK feeding. The synthesis route of sAPPα is opposite to that of Aβ. Studies have shown that cholesterol could elevate β-secretase activity and increase Aβ formation, which, together with oxidative stress, will cause the production of more Aβ and inhibit the synthesis of sAPPα. MS and AK could reduce the occurrence of risk factors such as oxidation stress in the brain and the deposition of Aβ40; thus, effectively promoting the expression of sAPPα. As such, the improvement in memory learning ability could be attributed to the elevation of sAPPα expression by MS and AK (Figure 3) [47].
Dimerumic acid and deferricoprogen as the active ingredients in Monascus-fermented products for PD improvement
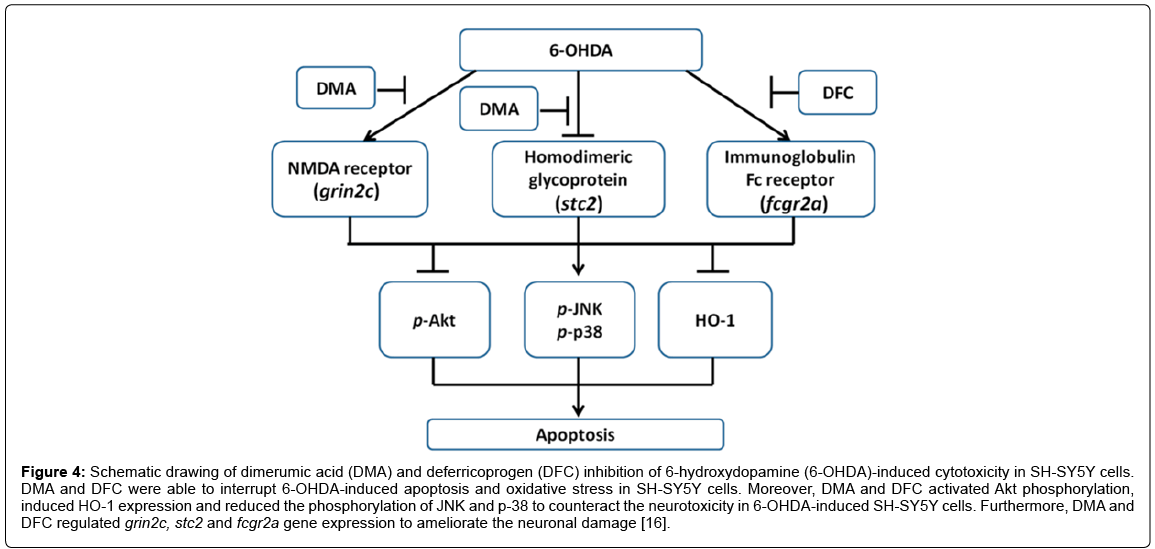
As proven in another ongoing study on red mold rice, the active ingredients DMA and DFC can prevent 6-OHDA induced apoptosis and oxidative stress in SH-SY5Y cells [16]. In addition, DMA and DFC activated Akt phosphorylation could stimulate the expression of HO-1 and reduce the phosphorylation of JNK and p-38 to offset the neurotoxicity in SH-SY5Y cells induced by 6-OHDA. In addition, DMA and DFC can regulate the gene expression of grin2c, stc2 and fcgr2a to improve neuronal damage (Figure 4). These results of mechanism-based therapies are critical for progress in PD prevention and treatment. In addition, we deduce that the DMA and DFC from M. purpureus fermentation can be used as functional components in preventing and treating PD [16].
Figure 4: Schematic drawing of dimerumic acid (DMA) and deferricoprogen (DFC) inhibition of 6-hydroxydopamine (6-OHDA)-induced cytotoxicity in SH-SY5Y cells. DMA and DFC were able to interrupt 6-OHDA-induced apoptosis and oxidative stress in SH-SY5Y cells. Moreover, DMA and DFC activated Akt phosphorylation, induced HO-1 expression and reduced the phosphorylation of JNK and p-38 to counteract the neurotoxicity in 6-OHDA-induced SH-SY5Y cells. Furthermore, DMA and DFC regulated grin2c, stc2 and fcgr2a gene expression to ameliorate the neuronal damage [16].
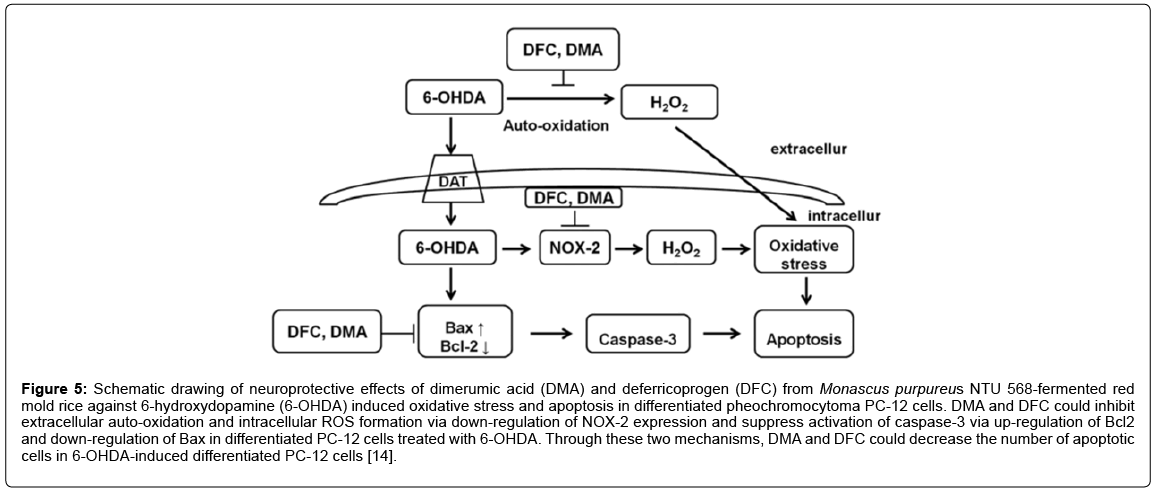
The previous finding that 6-OHDA triggers cell oxidative stress has been widely used in the generation of PD cells and animal models [28]. Past research has suggested that the ROS involved initiate auto-oxidation [28], and that NADPH oxidase (NOX)-derived ROS plays a vital role in the 6-OHDA induced cell death [48] by mediating the mitochondrial caspase cascade to activate the caspase for 6-OHDA-induced apoptosis [48]. However, the details on the molecular mechanism of cytotoxicity induced by 6-OHDA are not fully understood yet. Research results show that in differentiated pheochromocytoma PC-12 cells treated with 6-OHDA, DMA, and DFC could suppress extracellular auto-oxidation and the formation of intracellular ROS through the downregulation of NOX-2 expression, and suppress the activation of caspase-3 by increasing Bcl2 expression and reducing Bax expression. Through these two mechanisms, DMA and DFC lower the number of apoptotic cells in 6-OHDA-induced differentiated PC-12 cells (Figure 5) [14]. These results are significant for the development of anti-oxidation therapy for PD. As such, metabolites from M. purpureus NTU 568 fermentation can be used as functional foods in the prevention or treatment of PD.
Figure 5: Schematic drawing of neuroprotective effects of dimerumic acid (DMA) and deferricoprogen (DFC) from Monascus purpureus NTU 568-fermented red mold rice against 6-hydroxydopamine (6-OHDA) induced oxidative stress and apoptosis in differentiated pheochromocytoma PC-12 cells. DMA and DFC could inhibit extracellular auto-oxidation and intracellular ROS formation via down-regulation of NOX-2 expression and suppress activation of caspase-3 via up-regulation of Bcl2 and down-regulation of Bax in differentiated PC-12 cells treated with 6-OHDA. Through these two mechanisms, DMA and DFC could decrease the number of apoptotic cells in 6-OHDA-induced differentiated PC-12 cells [14].
Prospects of Monascus Fermented Constituents for AD and PD
According to the results of the presented studies, M. purpureus NTU 568-fermented products contain anti-oxidative and antiinflammatory substances. The anti-oxidative components DMA and DFC could improve PD pathogenesis by combating oxidative stress and reducing apoptosis. The anti-inflammatory agents MS and AK can inhibit the brain inflammatory response induced by Aβ40 and dampen the occurrence of related risk factors. In terms of future development, the amount of active components present will affect the treatment of AD and PD. Relevant studies have indicated that to be effective for AD and PD improvement, the content of active ingredients needs to reach a certain level, thus requiring advanced extraction and enrichment techniques for their production in high concentration, while still maintaining effectiveness. Though Monascus is a healthbenefiting genus of fungus commonly seen in recent years, not all its species are able to produce DFC, DMA, MS and AK. The M. purpureus NTU 568 often used in past research are able to produce these active compounds, with speed accelerated by solid-state fermentation, and in large quantities. Therefore, the fermentation products obtained from this strain can possibly become functional products for the prevention and improvement of PD and AD in the future.
References
- Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G (2000) Oxidative stress in Alzheimer's disease. Biochim Biophys Acta 1502: 139-144.
- Garcia T, Esparza JL, Nogues MR, Romeu M, Domingo JL, et al. (2010) Oxidative stress status and RNA expression in hippocampus of an animal model of Alzheimer's disease after chronic exposure to aluminum. Hippocampus 20: 218-225.
- Yamada K, Ren X, Nabeshima T (1999) Perspectives of pharmacotherapy in Alzheimer's disease. Jpn J Pharmacol 80: 9-14.
- Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE (2000) Inflammatory mechanisms in Alzheimer's disease: Inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J Neurosci 20: 558-567.
- Ransmayr G (2003) Cholesterol and statins in Alzheimer disease. Wien Med Wochenschr 153: 258-259.
- Sabbagh MN, Thind K, Sparks DL (2009) On cholesterol levels and statins in cognitive decline and Alzheimer disease: Progress and setbacks. Alzheimer Dis Assoc Disord 23: 303-305.
- Lee CL, Pan TM (2011) Red mold fermented products and Alzheimer's disease: A review. Appl Microbiol Biotechnol 91: 461-469.
- Duvoisin RC (1977) Problems in the treatment of Parkinsonism. Adv Exp Med Biol 90: 131-155.
- Cheng FK (2016) The use of acupuncture in patients with Parkinson's disease. Geriatr Nurs.
- Sankhla CS1 (2017) Oxidative stress and Parkinson's disease. Neurol India 65: 269-270.
- Taghizadeh M, Tamtaji OR, Dadgostar E, Kakhaki DR, Bahmani F, et al. (2017) The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson's disease: A randomized, double-blind, placebo-controlled trial. Neurochem Int.
- Calvello R, Cianciulli A, Nicolardi G, De Nuccio F (2017) Vitamin D treatment attenuates neuroinflammation and dopaminergic neurodegeneration in an animal model of Parkinson's disease, shifting M1 to M2 microglia responses. J Neuroimmune Pharmacol 12: 327-339.
- Rimmelzwaan LM, van Schoor NM, Lips P, Berendse HW, Eekhoff EM (2016) Systematic review of the relationship between vitamin D and Parkinson's disease. J Parkinsons Dis 6: 29-37.
- Tseng WT, Hsu YW, Pan TM (2016) Neuroprotective effects of dimerumic acid and deferricoprogen from Monascus purpureus NTU 568-fermented rice against 6-hydroxydopamine-induced oxidative stress and apoptosis in differentiated pheochromocytoma PC-12 cells. Pharm Biol 54: 1434-1444.
- Tseng WT, Hsu YW, Pan TM (2016) The ameliorative effect of Monascus purpureus NTU 568-fermented rice extracts on 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells and the rat model of Parkinson's disease. Food Funct 7: 752-762.
- Tseng WT, Hsu YW, Pan TM (2016) Dimerumic Acid and deferricoprogen activate Ak mouse strain thymoma/heme oxygenase-1 pathways and prevent apoptotic cell death in 6-hydroxydopamine-induced SH-SY5Y cells. J Agric Food Chem 64: 5995-6002.
- Lee CL, Kuo TF, Wang JJ, Pan TM (2007) Red mold rice ameliorates impairment of memory and learning ability in intracerebroventricular amyloid beta-infused rat by repressing amyloid beta accumulation. J Neurosci Res 85: 3171-3182.
- Lee CL, Kuo TF, Wu CL, Wang JJ, Pan TM (2010) Red mold rice promotes neuroprotective sAPPalpha secretion instead of Alzheimer's risk factors and amyloid beta expression in hyperlipidemic Abeta40-infused rats. J Agric Food Chem 58: 2230-2238.
- Lee CL, Wang JJ, Pan TM (2008) Red mold rice extract represses amyloid beta peptide-induced neurotoxicity via potent synergism of anti-inflammatory and anti-oxidative effect. Appl Microbiol Biotechnol 79: 829-841.
- Lee CL, Pan TM (2012) Development of Monascus fermentation technology for high hypolipidemic effect. Appl Microbiol Biotechnol 94: 1449-1459.
- Frederikse PH, Garland D, Zigler JS, Piatigorsky J (1996) Oxidative stress increases production of beta-amyloid precursor protein and beta-amyloid (Abeta) in mammalian lenses, and Abeta has toxic effects on lens epithelial cells. J Biol Chem 271: 10169-10174.
- Netland EE, Newton JL, Majocha RE, Tate BA (1998) Indomethacin reverses the microglial response to amyloid beta-protein. Neurobiol Aging 19: 201-204.
- Weldon DT, Rogers SD, Ghilardi JR, Finke MP, Cleary JP, et al. (1998) Fibrillar beta-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase and loss of a select population of neurons in the rat CNS in vivo. J Neurosci 18: 2161-2173.
- Esler WP, Wolfe MS (2001) A portrait of Alzheimer secretases--new features and familiar faces. Science 293: 1449-1454.
- Hasegawa M, Smith MJ, Goedert M (1998) Tau proteins with FTDP-17 mutations has a reduced ability to promote microtubule assembly. FEBS Lett 437: 207-210.
- Casanova MF, Troncoso JC, Price DL (1986) Hematogenous origin of brain macrophages: A case report. Neurology 36: 844-847.
- Citron M1 (2004) Beta-secretase inhibition for the treatment of Alzheimer's disease--promise and challenge. Trends Pharmacol Sci 25: 92-97.
- Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, et al. (2001) Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson's disease. Prog Neurobiol 65: 135-172.
- Hanrott K, Gudmunsen L, O'Neill MJ, Wonnacott S (2006) 6-Hydroxydopamine-induced apoptosis is mediated via extracellular auto-oxidation and caspase 3-dependent activation of protein kinase Cdelta. J Biol Chem 281: 5373-5382.
- Rodriguez-Blanco J, Martin V, Herrera F, Garcia-Santos G, Antolín I, et al. (2008) Intracellular signaling pathways involved in post-mitotic dopaminergic PC12 cell death induced by 6-hydroxydopamine. J Neurochem 107: 127-140.
- Chong ZZ, Li F, Maiese K (2005) Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol 75: 207-246.
- Kim MK, Kim SC, Kang JI, Hyun JH, Boo HJ, et al. (2011) 6-Hydroxydopamine-induced PC12 cell death is mediated by MEF2D down-regulation. Neurochem Res 36: 223-231.
- Frears ER, Stephens DJ, Walters CE, Davies H, Austen BM (1999) The role of cholesterol in the biosynthesis of beta-amyloid. Neuroreport 10: 1699-1705.
- Kuo YM, Emmerling MR, Bisgaier CL, Essenburg AD, Lampert HC, et al. (1998) Elevated low-density lipoprotein in Alzheimer's disease correlates with brain abeta 1-42 levels. Biochem Biophys Res Commun 252: 711-715.
- Roher AE, Kuo YM, Kokjohn KM, Emmerling MR, Gracon S (1999) Amyloid and lipids in the pathology of Alzheimer disease. Amyloid 6: 136-145.
- Roher AE, Kuo YM (1999) Isolation of amyloid deposits from brain. Methods Enzymol 309: 58-67.
- Akihisa T, Tokuda H, Yasukawa K, Ukiya M, Kiyota A, et al. (2005) Azaphilones, furanoisophthalides and amino acids from the extracts of Monascus pilosus-fermented rice (red-mold rice) and their chemopreventive effects. J Agric Food Chem 53: 562-565.
- Hsu LC, Hsu YW, Liang YH, Pan TM (2011) Anti-tumor and anti-inflammatory properties of ankaflavin and monaphilone A from Monascus purpureus NTU 568. J Agric Food Chem 59: 1124-1130.
- Hsu WH, Lee BH, Liao TH, Hsu YW, Pan TM (2012) Monascus-fermented metabolite monascin suppresses inflammation via PPAR-gamma regulation and JNK inactivation in THP-1 monocytes. Food Chem Toxicol 50: 1178-1186.
- Hsu WH, Lee BH, Hsu YW, Pan TM (2013) Peroxisome proliferator-activated receptor-gamma activators monascin and rosiglitazone attenuate carboxymethyllysine-induced fibrosis in hepatic stellate cells through regulating the oxidative stress pathway but independent of the receptor for advanced glycation end products signaling. J Agric Food Chem 61: 6873-6879.
- Lee BH, Hsu WH, Chang YY, Kuo HF, Hsu YW, et al. (2012) Ankaflavin: A natural novel PPARgamma agonist upregulates Nrf2 to attenuate methyl glyoxal-induced diabetes in vivo. Free Radic Biol Med 53: 2008-2016.
- Aniya Y, Ohtani II, Higa T, Miyagi C, Gibo H, et al. (2000) Dimerumic acid as an antioxidant of the mold, Monascus anka. Free Radic Biol Med 28: 999-1004.
- Taira J, Miyagi C, Aniya Y (2002) Dimerumic acid as an antioxidant from the mold, Monascus anka: The inhibition mechanisms against lipid peroxidation and hemeprotein-mediated oxidation. Biochem Pharmacol 63: 1019-1026.
- Yamashiro J, Shiraishi S, Fuwa T, Horie T (2008) Dimerumic acid protected oxidative stress-induced cytotoxicity in isolated rat hepatocytes. Cell Biol Toxicol 24: 283-290.
- Lee BH, Hsu WH, Hsu YW, Pan TM (2013) Suppression of dimerumic acid on hepatic fibrosis caused from carboxymethyl-lysine (CML) by attenuating oxidative stress depends on Nrf2 activation in hepatic stellate cells (HSCs). Food Chem Toxicol 62: 413-419.
- Hsu LC, Hsu YW, Liang YH, Lin ZH, Kuo YH, et al. (2012) Protective effect of deferricoprogen isolated from Monascus purpureus NTU 568 on citrinin-induced apoptosis in HEK-293 cells. J Agric Food Chem 60: 7880-7885.
- Lee CL, Lin PY, Hsu YW, Pan TM (2015) Monascus-fermented monascin and ankaflavin improve the memory and learning ability in amyloid ß-protein intracerebroventricular-infused rat via the suppression of Alzheimer’s disease risk factors. J Func Food 18: 387-399.
- Choi DH, Cristóvão AC, Guhathakurta S, Lee J, Joh TH, et al. (2012) NADPH oxidase 1-mediated oxidative stress leads to dopamine neuron death in Parkinson's disease. Antioxid Redox Signal 16: 1033-1045.
- Akihisa T, Tokuda H, Ukiya M, Kiyota A, Yasukawa K, et al. (2005) Anti-tumor-initiating effects of monascin, an azaphilonoid pigment from the extract of Monascus pilosus fermented rice (red-mold rice). Chem Biodivers 2: 1305-1309.
- Lee CL, Kung YH, Wu CL, Hsu YW, et al. (2010) Monascin and ankaflavin act as novel hypolipidemic and high-density lipoprotein cholesterol-raising agents in red mold dioscorea. J Agric Food Chem 58: 9013-9019.
- Lee CL, Wen JY, Hsu YW, Pan TM (2013) Monascus-fermented yellow pigments monascin and ankaflavin showed anti-obesity effect via the suppression of differentiation and lipogenesis in obese rats fed a high-fat diet. J Agric Food Chem 61: 1493-1500.
- Lee BH, Hsu WH, Huang T, Chang YY, Hsu YW, et al. (2013) Effects of monascin on anti-inflammation mediated by Nrf2 activation in advanced glycation end product-treated THP-1 monocytes and methylglyoxal-treated wistar rats. J Agric Food Chem 61: 1288-1298.
- Hsu WH, Lee BH, Lu IJ, Pan TM (2012) Ankaflavin and monascin regulate endothelial adhesion molecules and endothelial NO synthase (eNOS) expression induced by tumor necrosis factor-alpha (TNF-alpha) in human umbilical vein endothelial cells (HUVECs). J Agric Food Chem 60: 1666-1672.
- Hsu WH, Chen TH, Lee BH, Hsu YW, Pan TM (2014) Monascin and ankaflavin act as natural AMPK activators with PPARα agonist activity to down-regulate non-alcoholic steatohepatitis in high-fat diet-fed C57BL/6 mice. Food Chem Toxicol 64: 94-103.
- Chang YY, Hsu WH, Pan TM (2015) Monascus secondary metabolites monascin and ankaflavin inhibit activation of RBL-2H3 cells. J Agric Food Chem 63: 192-199.
- Su NW, Lin YL, Lee MH, Ho CY (2005) Ankaflavin from Monascus-fermented red rice exhibits selective cytotoxic effect and induces cell death on Hep G2 cells. J Agric Food Chem 53: 1949-1954.
- Lee BH, Hsu WH, Hsu YW, Pan TM (2013) Dimerumic acid attenuates receptor for advanced glycation end products signal to inhibit inflammation and diabetes mediated by Nrf2 activation and promotes methylglyoxal metabolism into d-lactic acid. Free Radic Biol Med 60: 7-16.
- Ho BY, Wu YM, Chang KJ, Pan TM (2011) Dimerumic acid inhibits SW620 cell invasion by attenuating Hâ??Oâ??-mediated MMP-7 expression via JNK/C-Jun and ERK/C-Fos activation in an AP-1-dependent manner. Int J Biol Sci 7: 869-880.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 4524
- [From(publication date):
August-2017 - Jul 06, 2025] - Breakdown by view type
- HTML page views : 3593
- PDF downloads : 931