Research Article Open Access
The Prevalence of Anemia In Pregnancy In A Developed Country – How Well Understood Is It?
| Hayley Messenger and Boon Lim* | |
| Department of Obstetrics and Gynecology, Royal Hobart Hospital, Liverpool St, Hobart, Tasmania 7000 Australia | |
| *Corresponding Author : | Boon Lim Department of Obstetrics and Gynecology Centenary Hospital for Women and Children Canberra, ACT 2605, Australia Tel: +61431689293 E-mail: boon.lim@act.gov.au |
| Received date: Feb 19, 2016; Accepted date: Mar 18, 2016; Published date: Mar 21, 2016 | |
| Citation: Messenger H, Lim B (2016) The Prevalence of Anemia in Pregnancy in A Developed Country – How Well Understood is it?. J Preg Child Health 3:231. doi:10.4172/2376-127X.1000231 | |
| Copyright: © 2016 Messenger H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Background: Anemia is known to be common in pregnancy, yet prevalence has not been comprehensively assessed by gestational age and nutritional cause in a large cohort from developed countries, with consideration of socio-demographic influence. Aims: To quantify the prevalence of anemia (hemoglobin less than 11g/dL) during each trimester of pregnancy and determine possible causative nutritional deficiencies in a developed country with a homogenous population relative to mainland Australia. Materials and Methods: This retrospective cohort study was undertaken in Tasmania, an Australian state with 6608 registered births in 2011. Results for the majority of pregnant women in 2011 were obtained from databases of major laboratories in the state, encompassing 5696 pregnancy episodes, to determine anemia prevalence in each trimester and around term. Results: A significant decrease in hemoglobin levels during pregnancy was observed from 13 ± 0.96 g/dL (0-13 weeks gestation, 1.9%, p=0.522) to 11.9 ± 1.04 g/dL (14-25 weeks, 13.4%, p=0.01), 11.8 ± 1.01 g/dL (26-36 weeks, 17.7%, p=<0.001) and 11.9 ± 1.56 g/dL (37-43 weeks, 21.9%, p=<0.001). Anemia was more prevalent in Hobart (difference=0.7% (n.s.), 4.5% (p=0.01), 6.2%, 11.2%). Conclusions: Prevalence of anemia in pregnancy was higher than previously estimated in Australia, being more common in the tertiary public hospital setting, and with advancing gestation. Limited testing of vitamin B12, iron and folate status made determining aetiology impossible. Assuming anemia in pregnancy is due to iron deficiency without considering other possibilities may limit opportunities to treat these causes.
| Keywords |
| Pregnancy; Anemia; Iron; Deficiency; Hemoglobin; Developed countries |
| Introduction |
| Anemia in pregnancy, defined by the World Health Organization (WHO) as hemoglobin level of less than 11 g/dL, is a global health problem affecting 41.8% of women worldwide and up to 75% in developing countries [1]. Maternal anemia has correlates with poor fetal outcomes [2] and increased morbidity and mortality for mother and child [1]. Surveys of anemia in pregnancy undertaken in malaria endemic developing countries describe anemia rates over 90% [3]. In developed countries, socio-demographic background represents the most important risk factor for anemia and disordered iron stores in pregnancy [4]. Reducing rates of peripartum anemia reduces the need for blood transfusion [5]. |
| In 1969, Fleming et al demonstrated a prevalence of anemia in Australian women of 10.6% [6]. Subsequent cohort studies of anemia in pregnancy showed a prevalence of 18% [7], higher in adolescents [8] and up to 40% in indigenous populations [9]. |
| Iron deficiency anemia rates have been shown to increase more than five-fold from “booking” for antenatal care to delivery [10] demonstrating the importance of assessing prevalence of anemia by gestation. Tasmania, an Australian state with a population of 495,354 [11] recorded 6608 registered births in 2011 [12]. Three laboratories support 70% of births in 4 public hospitals and 30% in private units [13]. The objective of this retrospective analysis is to quantify the prevalence of anemia by gestational age, helping to understand nutritional causes of anemia in pregnancy. |
| Materials and Method |
| Ethics approval (reference number H001248) was granted by the University of Tasmania Health and Human Research Ethics Committee. |
| Reference ranges from the RHH Laboratory were used due to their applicability to the local population and similarity to those of the Royal College of Pathologists of Australasia [14] (Table 1). |
| Descriptive statistics are reported for continuously scaled variables (median, minimum, maximum). In case of irrelevant deviation from the normal distribution the mean, standard deviation (SD) and categorically scaled variables (absolute and relative frequencies) are reported. Distributions of numbers of samples as well as proportions of distinct samples were evaluated by kernel density and LOESS regression, respectively. |
| Data collection |
| In Tasmania, pregnant women routinely undergo full blood count (FBC) at booking for antenatal care, generally in the first trimester. Repeat FBC in conjunction with routine gestational diabetes screening is performed at 24-28 weeks gestation with further testing if indicated. Data was retrieved from the two main laboratory service providers. A private laboratory (Sonic [BS]) services women cared for in the public and private sector in two of three geographical regions (Burnie and Hobart), and women cared for privately in the region surrounding Launceston. Women in the Hobart region under public care have investigations performed by the Royal Hobart Hospital (RHH) public laboratory. |
| Pregnancies were identified in the two laboratory databases by clinical notes and cross-referencing to the performance of gestational diabetes screening (RHH) and by clinical notation of pregnancy status (BS). Datasets of test results included full blood count (FBC), iron studies, vitamin B12 and folate levels; date of investigation and information about gestational age, such as estimated date of delivery, gestational age at the time of testing or date of last menstrual period. Results were de-identified, but results for each individual woman remained linked. All results for women identified as pregnant between 27th August 2010 and 22nd April 2012 were included in the original data retrieval to ensure inclusion of women in any stage of viable pregnancy (from 24 weeks’ gestation) during 2011. |
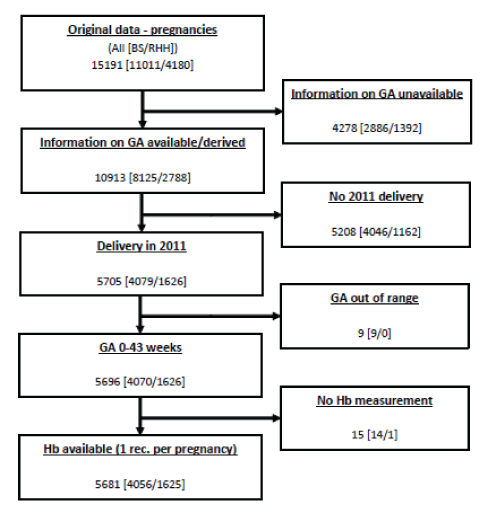
| A total of 30294 “records”, or pathology tests (BS: 18958, RHH: 11336) from 15191 (BS: 11011, RHH: 4180) were available. The process for record capture is outlined in Figure 1. Information about hemoglobin was available for 96.7% of these records (13541 [BS: 8204, RHH: 5337]). |
| Gestational age allocation |
| Gestational age was derived by examining laboratory provided records and clinical remarks provided on the pathology request form. Remarks were checked for flagged terms providing information that may be related to gestational age to allow further classification. Records were subdivided to gestational age periods 0-13, 14-25, 26-36 and 37-43 weeks to reflect the trimesters of pregnancy, and grouped investigations that may have been performed postnatally separately to avoid bias. Variations from one to 29 records per period were observed, with 76.5% (BS: 82.7%, RHH: 62.9%) of women having one record. A further 15.3% (BS: 13.3%, RHH: 20.4%) had two records per gestational age period and 8.0% (BS: 4.0%, RHH: 16.7%) had 3 or more records per period. After random selection of one record per woman subject per period 9867 (BS: 6776, RHH: 3091) records remained, and hemoglobin results were available for 9610 records (BS: 6670, RHH: 2935). Women were assessed in one or more gestational age periods (2458: 1 period, 2393: 2 periods, 757: 3 periods, 88: 4 periods, total: 5696 different women) (Figure 1). In each gestational age period (0-13, 14-25, 26-36 and 37-43 weeks) the following numbers of women, were analysed respectively: 2657, 1845, 3449 and 1669 (Table 2). |
| Discrepancy resolution |
| Discrepancies were clarified and resolved by manually considering all available patient information in available records for each individual woman. |
| Queries regarding inconsistencies were resolved using predefined steps within data setup and in meetings with clinicians and statisticians to ensure inconsistencies were analysed systematically and reliably. |
| If several records per gestational age period existed for a woman, one record was randomly selected in order to avoid varying the impact of different subjects due to clustered data. Other selection methods were applied (first, last record, mean), and the influence of selection method was investigated by comparing results in a sensitivity analysis. |
| A discrepancy between two sets of results for the same woman occurred when a woman had at least two informative records (usually from physician comments on pathology request forms) and based on provided or derived gestational age information contradictory birth dates were obtained (differing by 4 weeks or more). Differences of more than 4 weeks within a record occurred in 1295 out of 30294 individual records (BS data: 1284 of 18958; RHH data: 11 of 11336). |
| Discrepancies between several records for the same woman may occur where there is uncertainty of gestational age. For example, the clinical comments may note a gestation that later investigation reveals to be incorrect. A data collection issue is that unique identifiers refer to patients rather than pregnancies within original datasets. |
| This resulted in discrepancies particularly when serial miscarriages occurred in the studied timeframe. Six women with three pregnancies and 268 women with two were detected. In case of multiple serial pregnancies in the timeframe studied, a pregnancy related identification was introduced ensuring reference to separate pregnancies and to each woman. |
| Results |
| Analysis undertaken for 9610 records pertained to 5696 individually identified pregnant women in all parameters of FBC, iron, B12 and folate status that were available. |
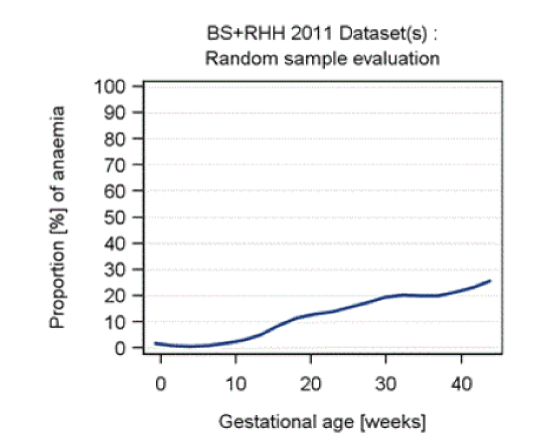
| The proportion of anaemic samples related to all samples is shown in Figure 3 indicating increasing anemia with gestation. The peak at 26 weeks gestation reflects local policy for timing of gestational diabetes screening and antenatal visits. |
| Statistically significant differences in anemia rates in each gestational age period were observed between the two laboratory datasets. The mean and standard deviation for hemoglobin, incidence of anemia and number of records are presented by geographical site (Table 3). The quotient of both curves, as yielded by LOESS regression, showed similar increases in the proportion of anemic samples with gestational week. |
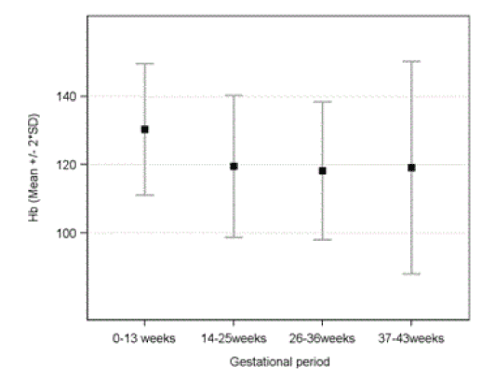
| A significant decrease in hemoglobin levels (mean ± SD) and rise in anemia rates were noted, from 13 ± 0.96 g/dL (0-13 weeks: anemia 1.9%, p = 0.522) to 11.9 ± 1.04 g/dL (14-25 weeks: anemia 13.4%, p = 0.01), 11.8 ± 1.01 g/dL (26-36 weeks: anemia 17.7%, p < 0.001) and 11.9 ± 1.56 g/dL (37-42 weeks: anemia 21.9%, p < 0.001) Figure 2 and Table 3. Review of all records showed that 22.3% (1269 of 5696) women were anemic in at least one test during pregnancy. Descriptive statistics for laboratory values among anaemic patients well as proportion of patients with values outside reference interval are shown in Table 3. |
| Nutritional causes of anemia |
| Normal MCV was present in 97.6% of records with normal hemoglobin and in 87% of records with anemia. Macrocytic anemia (elevated MCV) was noted in 5 records and microcytic anemia (reduced MCV) in 302; with 32 and 456 records with normal hemoglobin levels. |
| Nutritional deficiencies were rarely tested for and some infrequently tested parameters are not included in table 2. Serum folate and red cell folate were tested and normal in 0.1% and 1% of records respectively. Vitamin B12 status was not tested in the vast majority of women, even in the setting of normocytic anemia. Overall, only 184 women had vitamin B12 levels quantified during pregnancy (1.9%). Low Vitamin B12 was shown in 27 women, and of this group 6 were anemic. There is a need for confirmation with B12 metabolite levels to determine significance during pregnancy [15]. |
| Macrocytic anemia was noted in 0.3% of the population, and microcytic anemia in 11.2% (Table 3). The majority of available records revealed normal MCV in the presence of anemia (87%) or normal hemoglobin levels (97.6%). Ferritin, an important marker of iron stores [14], was low in the presence of anemia in 38.9% of women whose ferritin levels were tested. Transferrin saturation was reduced in 438 records and in 75 (17.1%) there was concurrent anemia, implicating iron deficiency anemia. |
| Both MCH and MCHC tend to be reduced in iron deficiency anemia [14]. Among 1268 women with anemia, 33.8% and 1% had low MCH and MCHC respectively. The majority of records for these parameters were normal, implying poor sensitivity for indicating iron deficiency or coexisting nutritional deficiencies. |
| Discussion |
| In this study, we observed gestation related prevalence of anemia during pregnancy up to 21.9%. Women accessing public antenatal services at the state tertiary referral centre were more likely to be anemic than women accessing private services. Determining nutritional deficiency causes of anemia in the majority of women was impossible due to limited vitamin B12 and folate testing. Full blood count and iron status investigations simply confirmed the recognised pattern in iron deficiency. |
| Because we analysed routinely measured data, considerable effort was made to ensure data integrity and consistency of results. We were unable to obtain access to information for all Tasmanian women, as we did not receive permission to do so from one laboratory. We were not able to access quality assurance information for all laboratories to ensure data consistency. This was relevant in view of the difference in anemia prevalence between laboratories. Nevertheless, due to the sample size, we were confident that the findings reflected the general population of the state. Reliance on clinical notes and laboratory records for access to data and allocation to trimester of pregnancy was a significant limitation, and raises the possibility that some pregnant women were missed in data collection. In addition, data collection could not collate identifying information from each laboratory in order to ensure women who may have used both public and private laboratories were not duplicated. This may account for the large population studied. The methodology above described how this was overcome. De-identified data gives incomplete knowledge of the woman’s medical history, demographic and ethnic background that may affect her anemia status. |
| Strengths of this analysis include its large cohort, completing survey of the entire state by geographical areas. The previous north-eastern Tasmanian study demonstrated rates of anemia amongst pregnant women higher than in previous Australian estimates [8]. This research adds that by term, Tasmanian women have a greater prevalence of anemia than previously recognised. There is some difficulty in comparison as this is the first Australian study to survey anemia in pregnancy by gestational age, and would likely have been affected to some extent by postnatal hemoglobin values. |
| Tasmania is ethnically less diverse, (88% Caucasian and <1% indigenous population) and more socioeconomically disadvantaged [16] earning Australia’s lowest wages [17]. Socioeconomic disadvantage and rurality are known to worsen health outcomes [18]. |
| The burden of anemia among indigenous populations has been shown to be higher than in the general population [19], yet despite high anemia prevalence, Tasmania’s indigenous population is small relative to other Australian states [20]. Undertaking this study in a developed country removes further confounding factors such as major nutritional deficiencies (poor food security and poverty) and endemic disease such as malaria. |
| Although increasing prevalence of anemia with gestation was noted, hemodilutional effects, which peak at around 24 to 32 weeks gestation [21], would not solely account for this observation. Nutritional status or contamination of data with postpartum anemia values relating to early delivery due to obstetric complications may account for some of these differences. |
| The number of visits per period is considerably larger for RHH. Within a subpopulation having only one record per period, the site effects are less dominant (Table 3). A similar result is found if reviewing records at 26 weeks. Investigations at this time are part of routine screening for gestational diabetes. |
| These findings may be related to a discrepancy in socioeconomic status between the two geographical regions studied. Burnie residents, in rural northwest Tasmania, are more likely to be more socially disadvantaged; while the metropolitan Hobart region has larger migrant populations, smaller proportions of single parent families, higher employment rates and average wages [20]. Nutritional status of the two demographic groups is likely to be quite different. Women accessing private antenatal care throughout Tasmania utilize the same pathology provider as public patients in Burnie, but are from a different demographic and socioeconomic background. The significantly higher proportion of anemia in the RHH group may reflect lower socioeconomic status, as well as relative complexity of the obstetric patient cared for, being Tasmania’s tertiary referral centre. Heterogeneity within the BS dataset makes it difficult to draw conclusions from comparison between the two sets of data, however the cohort as a whole is relatively homogenous compared to pregnant populations on mainland Australia. |
| In a population where anemia is commonly due to iron deficiency, the most cost effective method to prove this assumption while excluding other treatable causes must be considered. In this study, it is apparent that no other treatable nutritional causes were investigated in the vast majority of women, despite potential impact of vitamin B12 and folate deficiency on pregnancy outcomes [22]. Iron deficiency, with or without anemia, is known to be associated with poorer obstetric outcome and associated with a higher blood transfusion rate in the postnatal period [21]. This study demonstrates that this is not routinely investigated, even though it is the most common cause of anemia, based on the indices of the blood picture. |
| One reasonable approach is to test for ferritin, iron, vitamin B12 and folate status for situations where parameters such as MCV, MCH and MCHC are abnormal. An agreed algorithm for investigation of anemia in pregnancy is Australia is lacking. Understanding the prevalence of anemia throughout pregnancy assists in developing pathways for appropriately timed investigation and treatment of various causes of anemia. There may be a role for iron supplementation during pregnancy to reduce the risk of preterm delivery or low birth-weight babies [23]. This area requires further research. Availability of socio-demographic and ethnic characteristics may assist in providing reliable and relevant results. The anemia rates quantified in this study exceed Australian data previously quoted [1,6-8] despite the state’s ethnic homogeneity [11]. This may indicate a more accurate reflection of the Tasmanian population and their particular socioeconomic situation, revealing Tasmanian women suffer anemia prevalence during pregnancy at rates exceeded only by remote indigenous women [9]. |
| This study has shown that the prevalence of anemia in pregnancy, even in a developed country is higher than common perception. Anemia can be associated with maternal and fetal morbidity and mortality. Whilst it is assumed that iron deficiency is the most common cause of anemia, the causes are not always well investigated. Clear guidelines need to be available for the management of this important problem. |
| Acknowledgment |
| Vifor Pharma Pty. Ltd. provided unrestricted funding for statistical support by Stephan Weber and Thomas Keller at ACOMED statistik (Leipzig, Germany). Dr Lindsay Edwards and Dr Anna Johnston were involved in study conception and design. Leigh Murfett and Daniel Owens at Sonic Healthcare, and Andrew Hudspeth at Royal Hobart Hospital Pathology Services were instrumental in accessing and retrieving data. We are grateful for the assistance of Dr Stephen Raymond, Dr Lindsay Edwards and Dr Anna Johnstone in the editing process. |
References
- World Health Organization (2008). Worldwide prevalence of anemia 1993-2005: WHO global database on anemia. Geneva: WHO Press.
- Akhter S, Momen MA, Rahman MM, Parveen T, Karim RK (2010) Effect of maternal anemia on fetal outcome.Mymensingh Med J 19: 391-398.
- Baig-Ansari N, Badruddin SH, Karmaliani R, Harris H, Jehan I, et al. (2008) Anemia prevalence and risk factors in pregnant women in an urban area of Pakistan.Food Nutr Bull 29: 132-139.
- Bencaiova G, Burkhardt T, Breymann C (2012) Anemia--prevalence and risk factors in pregnancy.Eur J Intern Med 23: 529-533.
- Rouse DJ, MacPherson C, Landon M, Varner MW, Leveno KJ, et al. (2006) Blood transfusion and cesarean delivery.ObstetGynecol108: 891-897.
- Fleming AF, Stenhouse NS (1969) Anemia in pregnancy in Western Australia.Med J Aust 2: 673-677.
- Khalafallah A, Dennis A, Bates J, Bates G, Robertson IK, et al. (2010) A prospective randomized, controlled trial of intravenous versus oral iron for moderate iron deficiency anemia of pregnancy.J Intern Med 268: 286-295.
- Lewis LN, Hickey M, Doherty DA, Skinner SR (2009) How do pregnancy outcomes differ in teenage mothers? A Western Australian study.Med J Aust 190: 537-541.
- Steenkamp M, Rumbold AR, Kildea S(2012) Measuring what matters in delivering services to remote-dwelling Indigenous mothers and infants in the Northern Territory, Australia. Australian Journal of Rural Health 20: 228-237.
- Turner S, Seybold D, Celestine C, Williams D (2012) Incidence of anemia among obstetric patients in an Appalachian teaching clinic.Mil Med 177: 1212-1216.
- Australian Bureau of Statistics (2011) Census QuickStats: All people, usual residents, Tasmania.
- Australian Bureau of Statistics (2012) 3301.0- Births, Australia.
- Council of Obstetric and Paediatric and Perinatal Mortality (2011). Department of Health and Human Services. Tasmania: DHHS Annual report.
- Royal College of Pathologists of Australasia (2009). RCPA Manual [monograph online]. Sydney, NSW: RCPA.
- Metz J, McGrath K, Bennett M, Hyland K, Bottiglieri T (1995) Biochemical indices of vitamin B12 nutrition in pregnant patients with subnormal serum vitamin B12 levels. Am J Hematol 48: 251-255.
- Australian Bureau of Statistics (2011) Population distribution and structure census.
- Australian Bureau of Statistics (2013) 6302.0 Average weekly earnings, Australia : state and territory earnings.
- Australian Bureau of Statistics (2011) Australian social trends: health and socioeconomic disadvantage.
- Khambalia AZ, Aimone AM, Zlotkin SH (2011) Burden of anemia among indigenous populations.Nutr Rev 69: 693-719.
- Australian Bureau of Statistics (2011). National regional profiles 2007-2011: Hobart, West and Northwest.
- Milman N (2006) Iron and pregnancy--a delicate balance.Ann Hematol 85: 559-565.
- Molloy AM, Kirke PN, Brody LC, Scott JM, Mills JL (2008) Effects of folate and vitamin B12 deficiencies during pregnancy on fetal, infant, and child development.Food Nutr Bull 29: S101-111.
- Scholl TO, Reilly T (2000) Anemia, iron and pregnancy outcome.J Nutr 130: 443S-447S.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 13688
- [From(publication date):
April-2016 - Apr 07, 2025] - Breakdown by view type
- HTML page views : 12309
- PDF downloads : 1379
