The Predictive Role of Intra-Tumoral CD8+T Cells with Stem Cell-Like Properties in Response to and Efficacy of Anti-PD-1/L1 Treatment
Received: 19-Jan-2022 / Manuscript No. AOT-22-50790 / Editor assigned: 21-Jan-2022 / PreQC No. AOT-22-50790(PQ) / Reviewed: 04-Feb-2022 / QC No. AOT-22-50790 / Revised: 08-Feb-2022 / Manuscript No. AOT-22-50790 (R) / Published Date: 15-Feb-2022 DOI: 10.4172/aot.7.s1.1000002
Abstract
A Checkpoint Inhibitor-Based Immunotherapy (ICIs) has demonstrated outstanding efficacy in many solid tumors. However, some treated patients with positive expression of PD-L1 do not respond, and even develop hyper progression, and some patients with negative expression of PD-L1 can also benefit from immunotherapy combined with chemotherapy. There is growing need to identify biomarkers that will improve the selection of patients who will best respond to therapy. Intra-tumoral effective T cells are critical for anti-tumor effect, and some subpopulation of it have been verified as potential biomarkers for ICIs. In our previous study, a subset of intra-tumoral CD8+ T cells with stem-like properties has been identified as sensitive biomarker for predicting the response to and efficacy of PD-1/L1 blockade treatment with or without chemotherapy. In the current comment, the role and challenges of this cell subpopulation in anti-PD-1/L1 immunotherapy will be discussed.
Keywords: Non-small cell lung cancer; PD-1/L1 immunotherapy; CD8+ T cells; Biomarker
About the Study
Nowadays, PD-1/L1 blockades, a Checkpoint Inhibitor-Based Treatment (ICIs), have been widely used in various solid tumors and demonstrated favorable therapeutic effect [1-3]. Some ICIs, such as pembrolizumab and nivolumab, have been adopted to treat advanced Non-Small Cell Lung Cancer (NSCLC) as first-line treatment depends on the expression of PD-L1 [4].
However, a large body of clinical evidence showed that many ICIs combined with chemotherapy significantly improve survival time of patients with NSCLC regardless of PD-L1 expression [5,6]. These results indicate that PD-L1 may not absolutely reflect the patients’ response to immunotherapy. There is a growing need to identify biomarkers that will improve the selection of patients who will best respond to therapy.
Tumor infiltrating lymphocytes as potentially sensitive markers
It is well known that PD-1/L1 immunotherapy perform the antitumor effect due to its preventive influence against CD8+ T cell exhaustion. Therefore, the function of T lymphocytes may critical for PD-1/L1 blockade. Lately, some researches demonstrated that a few subsets of Tumor-Infiltrating Lymphocytes (TILs) served as potential biomarkers for response to PD-1/L1 immunotherapy in patients with NSCLC. CD103+CD8+ TILs have been identified to be associated with the efficacy of PD-1/L1 blockade [7]. PD-L1+CD4 +CD25+Tregs served as a diagnostic biomarker for response to ICIs [8].
Furthermore, the higher density of intra-tumoral CD3+CD45RO+CD8+T cells in melanoma was associated with durable beneficial in anti- PD-1 therapy [9]. Therefore, tumor with an active immune microenvironment, which exemplified by infiltration of activated, effector T cells, will be better primed to respond to PD-1/L1 immunotherapy.
Intra-tumoral CD8+ T cells with stem cell-like properties mediate tumor control and response to ICIs
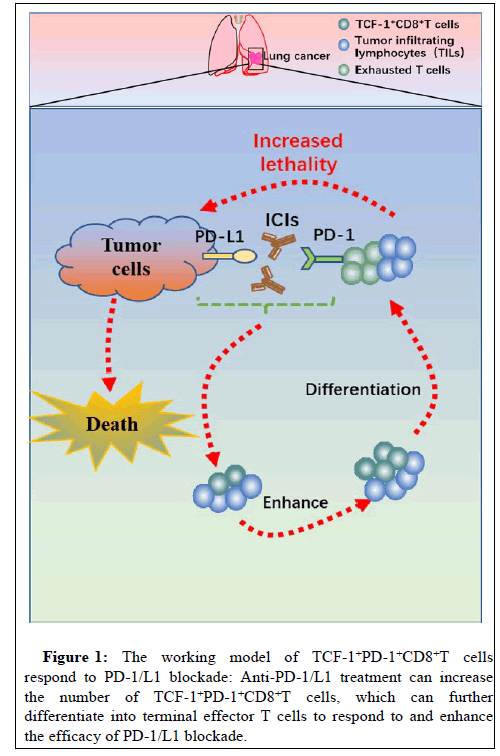
As TILs are critical for anti-tumor and response to immunotherapy, the evolution of TILs, especially for CD8+ T cells are studied extensively. Recently, a subpopulation of CD8+ T cells were identified to possess stem cell-like properties that express the T cell Transcription Factor 1 (TCF-1) and differentiate into ‘terminally exhausted’ TILs, namely “progenitor exhausted cells” (TCF-1+PD-1+CD8+ T cells) [10].
This subset was first identified during inflammatory infection; it was needed for PD-1+CD8+ T cells expansion in response to PD-1 blockade [11]. Subsequently, few researches confirmed the presence of TCF-1+PD-1+CD8+T cells in human melanoma using multiplex staining, and this subpopulation expanded and/or persisted preferentially in response to PD-1 blockade [12]. The importance of TCF-1+PD-1+CD8+T cells in immunotherapy-driven tumor control was further confirmed in preclinical mouse models using adoptive cell transfers [12]. Collectively, these results indicated that TCF-1+PD-1+CD8+T cells are potential target cells of PD-1 blockade; it may represent a biomarker for therapy outcome prediction (Figure 1).
TCF-1+PD-1+CD8+T cells are associated with the response to and benefit of PD-1/L1 immunotherapy in NSCLC
As the progenitor exhaust T cells are potential biomarker for PD-1 blockade in patients with melanoma, the predictive role of TCF-1+PD-1+CD8+T cells in response to and prognosis of anti- PD-1/L1 treatment in NSCLC has been evaluated in our previous study [13]. Our results demonstrated that patients with high frequency of TCF-1+PD-1+CD8+T cells have a longer PFS and OS after PD-1/L1 immunotherapy with or without chemotherapy. Importantly, a significant association was established between increased frequency of this subset and response to anti-PD-1/L1 therapies in patients with NSCLC, which was not observed in patients with melanoma as aforementioned study. Furthermore, this subpopulation was almost undetected in hyper progressive patients after anti-PD-1/L1 treatment, indicating it may also sensitive biomarker for predicting the hyper progression of PD-1/L1 blockade in NSCLC. Collectively, our research is not only confirming the positive correlation between the expression of TCF-1+PD-1+CD8+T cells and efficacy of anti-PD1/L1 treatment with or without chemotherapy, but also uncover that this cell subset may be a favorable biomarker for selecting the patients with clinical benefit or hyper progressive disease after immunotherapy.
Conclusion
Although PD-L1 expression in tumor cells enriches the responders to ICIs in many solid tumors, it is not an absolute predictor of therapeutic response since the mechanism of ICIs is to inhibit the exhaustion of effective T cells. Therefore, it is necessary to consider the status of T cells in addition to the expression of PD-L1 in tumor cells. Currently, some subpopulations of intra-tumoral T cells have been identified as potentially sensitive biomarkers for ICIs. In our previous study, we found that subsets of intra-tumoral CD8+ T cells, which possess the stem cell-like properties, are tightly correlated with the response to and efficacy of anti-PD-1/L1 immunotherapy with or without chemotherapy in NSCLC. Thus, we need to comprehensively consider the function of intra-tumoral T cells and the expression of PD-L1 when selecting patients who will be benefit from PD-1/L1 blockade treatment. However, there are also some challenges for exploring the intra-tumoral T cells as potential biomarkers. Firstly, the specific mechanism of the response of TCF-1+PD-1+CD8+T cells to anti-PD-1/L1 treatment remains largely unknown. Secondly, the predictive role of this cell subset needs to be verified by larger samples and in multiple types of solid tumors. Finally, it is necessary to establish a standard process for detecting this subpopulation in order to be transformed easily into clinical application in the further.
References
- Kammerer-Jacquet SF, Deleuze A, Saout J, Mathieu R, Laguerre B, et al. (2019) Targeting the PD-1/PD-L1 pathway in renal cell carcinoma. Int J Mol Sci 20: 1692.
[CrossReff], [Google Scholar], [PubMed]
- Velho PI, Antonarakis ES (2018) PD-1/PD-L1 pathway inhibitors in advanced prostate cancer. Expert Rev Clin Pharmacol 11: 475-486.
[CrossReff], [Google Scholar], [PubMed]
- Xia L, Liu Y, Wang Y (2019) PD-1/PD-L1 blockade therapy in advanced non-small-cell lung cancer: Current status and future directions. Oncologist 24: S31-S41.
[CrossReff], [Google Scholar], [PubMed]
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, et al. (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer: A Randomised, open-label, controlled, phase 3 trial. Lancet 393: 1819-1830.
[CrossReff], [Google Scholar], [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, et al. (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378: 2078-2092.
[CrossReff], [Google Scholar], [PubMed]
- Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, et al. (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379: 2040-2051.
[CrossReff], [Google Scholar], [PubMed]
- Wang P, Huang B, Yang J, Liang Z, Zhang N, et al. (2018) CD103+CD8+ T lymphocytes in non-small cell lung cancer are phenotypically and functionally primed to respond to PD-1 blockade. Cell Immunol 325: 48-55.
[CrossReff], [Google Scholar], [PubMed]
- Wu SP, Liao RQ, Tu HY, Wang WJ, Dong ZY, et al. (2018) Stromal PD-L1-positive regulatory T cells and PD-1-positive CD8-Positive T cells define the response of different subsets of non-small cell lung cancer to PD-1/PD-L1 blockade immunotherapy. J Thorac Oncol 13: 521-532.
[CrossReff], [Google Scholar], [PubMed]
- Balatoni T, Mohos A, Papp E, Sebestyén T, Liszkay G, et al. (2018) Tumor-infiltrating immune cells as potential biomarkers predicting response to treatment and survival in patients with metastatic melanoma receiving ipilimumab therapy. Cancer Immunol Immunother 67: 141-151.
[CrossReff], [Google Scholar], [PubMed]
- Held W, Siddiqui I, Schaeuble K, Speiser DE (2019) Intratumoral CD8+ T cells with stem cell-like properties: Implications for cancer immunotherapy. Sci Transl Med 11: e6863.
[CrossReff], [Google Scholar], [PubMed]
- Chen Z, Ji Z, Ngiow SF, Manne S, Cai Z, et al. (2019) TCF-1-centered transcriptional network drives an effector versus exhausted CD8 T cell-fate decision. Immunity 51: 840-855.
[CrossReff], [Google Scholar], [PubMed]
- Siddiqui I, Schaeuble K, Chennupati V, Marraco SAF, Calderon-Copete S, et al. (2019) Intratumoral Tcf1+PD-1+CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50: 195-211.
[CrossReff], [Google Scholar], [PubMed]
- Fang X, Wu G, Hua J, Zhao P, Shan M, et al. (2021) TCF-1+ PD-1+ CD8+T cells are associated with the response to PD-1 blockade in non-small cell lung cancer patients. J Cancer Res Clin Oncol.
[CrossReff], [Google Scholar], [PubMed]
Citation: Fang X, Tang R, Chen R, Luo Z, Xu X, et al. (2022) The Predictive Role of Intra-Tumoral CD8+T Cells with Stem Cell-Like Properties in Response to and Efficacy of Anti-PD-1/L1 Treatment. J Oncol Res Treat S1: 002. DOI: 10.4172/aot.7.s1.1000002
Copyright: © 2022 Fang X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 2405
- [From(publication date): 0-2022 - Mar 26, 2025]
- Breakdown by view type
- HTML page views: 1996
- PDF downloads: 409