Commentary Open Access
The Power of Music on Alzheimer Disease and the Need to Understand the Underlying Molecular Mechanisms
Carmela Matrone1* and Elvira Brattico2
1Institute of Biomedicine, Aarhus University, Bartholins Alle, 6, 8000 Aarhus, Denmark
2Center for Music in the Brain (MIB), Department of Clinical Medicine, Aarhus University & Royal Academy of Music Aarhus/Aalborg, Denmark
- Corresponding Author:
- Carmela Matrone
Institute of Biomedicine, Aarhus University
Bartholins Alle, 6, 8000 Aarhus, Denmark
Tel: +45-87167252
E-mail: matrone@ biomed.au.dk
Received date: September 07, 2015; Accepted date: October 13, 2015; Published date: October 20, 2015
Citation: Matrone C, Brattico E (2015) The Power of Music on Alzheimer’s Disease and the Need to Understand the Underlying Molecular Mechanisms. J Alzheimers Dis Parkinsonism 5:196. doi: 10.4172/2161-0460.1000196
Copyright: © 2015 Matrone C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Alzheimer’s disease (AD), the most common form of dementia, is a neurodegenerative disorder that leads to memory loss. The prevalence of AD varies among many different factors, including age, comorbidities, genetics, and education level. There is no way to definitively diagnose AD before performing an autopsy. There is no cure for AD, although large economical efforts are currently capitalized in promising research and development of new strategies. The purpose of this commentary is to review what we already know about the effects of music treatment on AD. Beside not curative of AD, the use of music seems to exert beneficial effects on Alzheimer’s symptoms. In turn, we briefly summarize the accumulated evidence on the effects of music on brain plasticity, discussing the necessity to further investigate the molecular mechanisms governing this plasticity, with a particular focus on the brain derived neurotrophic factor (BDNF). We believe that a further comprehension of how music influences the molecular processes in the human neuronal network might open new perspectives to enhance quality of life for both the patient and his or her caregivers.
Introduction
As our population grows older, one of the most common chronic mental health conditions is dementia. According to a recent report the number of people living with dementia worldwide was 35,6 million in 2010 and the it will nearly double in 20 years, to 65.7 million in 2030 and 115.4 million in 2050 (Alzheimer’s Disease International, 2013). Given the increasing aging population, the overwhelming incidence of dementia will likely become a public health priority and one of the greatest social and economic challenges of our time [1-4].
Alzheimer’s disease (AD) is the most prevalent form of dementia, accounting for 60–80% of cases. AD is a progressive neurodegenerative disease, characterized by an early stage in which people experience a progressive memory loss and a decrease in thinking ability such as decision-making. Later the disease evolves in the incapacity to perform daily activities or to recognize loved ones [5-7]. Such behavioural changes are frequently associated to molecular changes in the brain, such as the development of amyloid beta plaques and neurofibrillary tangles. Accordingly, a disruption of balance between production and clearance of amyloid precursor protein is considered as a major cause of amyloid-β plaques formation, consequently leading to neural dysfunction, regional brain atrophy and finally dementia [8,9].
A rarely occurring form of AD (amounting to only 2% of AD cases) has a proven genetic background, namely a mutation on the gene coding for amyloid precursor proteins or presenilin 1 and 2, and it generally starts in early age [10]. The largest AD forms, normally appearing over 65 years of age, are instead defined as being of a sporadic nature because no precise cause has been established [9]. For the common AD forms, the strongest predictor of developing AD, aside from age, is the genetic risk factor apolipoprotein (apo) E4 [11,12]. Additionally, epidemiological studies strongly support associations between limited education, depression, hypertension, diabetes, high cholesterol, smoking, head trauma and AD [13]. Furthermore, an unbalance between blood and cerebrospinal fluid in growth factor levels, such as Brain Derived Neurotrophic factor (BDNF) and Nerve Growth factor (NGF), or alterations in their downstream signalling have been largely reported in patients with AD [14-17] (Table 1). Particularly, BDNF levels change during the progression of the disease with an increase in the early stages and then a decrease in the late stages, likely indicative of a different neuronal response to the insult. Accordingly, the increase of BDNF in the early stages might reflect a compensatory neuronal repair mechanism. Afterwards, with the progression of the neuronal degeneration, BDNF levels decrease as result of the increased neuronal loss and the severity of dementia [18].
| Table1:RiskfactorsinAD. |
| Advancingage |
| Familyhistory |
| Headtrauma |
| Lackofmentalstimulation |
| Down’ssyndrome |
| Environmentaltoxins:aluminum,mercury |
| Oxidativestressduetoaccumulationoffreeradicalsand/orlowantioxidant levels |
| Abnormalproteinprocessing |
| Neurotransmitterdeficit |
| Geneticpolymorphism |
Table 1: Risk factors in AD.
As matter of fact, AD is a multifactorial disorder and each of these possible age-related causes provides little possibility in developing clinical prevention and therapies. For many years, the pharmacological treatment of AD has been symptomatic, focusing on the improvement of cognitive and behavioural symptoms in patients with moderate to severe dementia. Those symptoms (frequently occurring in cluster and varying by time, severity, and diagnosis) mainly include hyperactivity cluster (agitation, aggression, euphoria, irritability, aberrant motor activity), psychosis cluster (hallucinations and delusions), mood liability cluster (depression and anxiety), and instinctual cluster (appetite disturbance, sleep disturbance, and apathy) [19]. Hence, in AD the main drugs used have been antipsychotic and anxiolytic, along with a limited number of drugs specifically approved for dementia, such as cholinesterase inhibitors and memantine. However, treatment effects are generally limited, and they only prevent symptoms from becoming worse for a limited time and/or partially help in controlling some behavioural symptoms (Table 2).
| Symptomatic Treatments Acetylcholinesterase Inhibitors |
| NMDA-receptor Antagonists, Nicotinic-receptor Agonists |
| Disease-modifying Treatments: |
| Inhibition of amyloid formation: beta and gamma-secretase inhibitors Inhibition of Ab aggregation |
| Tau phosphorylation inhibitors |
| Other Therapies |
| Cholesterol-lowering therapies, Anti-inflammatory therapies |
| Therapies involving antioxidants: vitamin E and gingko biloba |
| Therapies involving neurotrophic factors: nerve growth factor (NGF) and estrogen |
| Alternative therapies: Diet control, Use of exercise, Stress control, Herbal remedies, Music |
| Psychotic Treatments: Antidepressants [depression], Anxiolytics [anxiety] |
| Antipsychotics [severe confusion, paranoia, and hallucinations] |
| Hormone replacement |
Table 2: Pharmacological and non-pharmacological treatments of AD.
Moreover, some of the latest pharmacological treatments targeting the toxicity of amyloids or NMDA receptor antagonists acting on the glutammatergic system (memantine) are associated with increased morbidity and mortality in patients, particularly after long period of exposure [20-22]. As consequence, after a recent systematic review of all the results obtained by pharmacologically treating AD patient, it has been even recommended to avoid any pharmacological intervention [22-24]. Although restraining patients from taking medication is a very extreme position, it is evident that no pharmacological treatment can delay or halt the progression of the disease, and in too many cases, even symptomatic drugs have only limited efficacy. Conclusively, alternative approaches have been proposed and several non-pharmacological approaches have been developing, consisting in the regularly use of various cognitively stimulating leisure activities to help the cognitive and emotional capacity of the patients [25].
One such potential leisure activity is music. Other potential nonpharmacological interventions include cognitive training/stimulation, rehabilitative care, massage/touch, physical activity, education/training for professionals, and education and psychosocial support for informal caregivers. In this commentary we aim at providing a short overview of the findings on music-derived brain plasticity over the life span and in AD. Particular focus will be given to the potential connections between music and BDNF serum levels, and how that can be of help in AD. Finally, future perspectives on the use of musical activities as nonpharmacological therapy will be discussed.
Music in the Brain
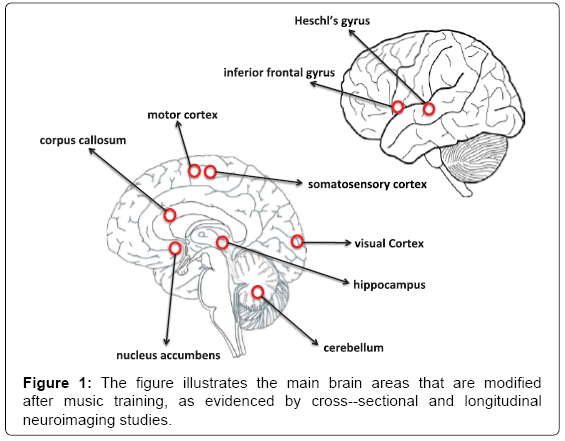
Music is a powerful stimulator of the brain. In the simplest listening conditions in solitude at home or by means of mobile devices when commuting, music consists of time-varying sound events characterized by a large number of acoustic features (in music information retrieval, more than a hundred of features can be computationally extracted from songs). Low-level features, such as the timbre of an instrument and pitch, are tracked by several regions of the brain, particularly the Heschl’s gyrus and the right anterior part of the superior temporal gyrus, in which the primary and non-primary auditory cortices are located [26], Also motor regions, like the supplementary motor area and the cerebellum are involved, if the listeners have an active musical background [27], due to the audio-motor transformations that are necessary for instrument playing.
Indeed, when music is played, score reading, fine movements of the limbs, haptic feedback, emotional arousal are all co-occurring and the related regions of the brain become activated [28,29] (Figure 1). The repetitive activation of these regions in professional musicians playing instruments for decades induces neuroplasticity that is clearly observable with modern neuroimaging techniques, e.g., magnetic resonance imaging (MRI). Increased grey matter volume of the Heschl’s gyrus [30], primary motor cortex, premotor cortex [31,32], cerebellum [33] and hippocampus [34] have been found in musicians when compared with non-musicians.
Many studies document the efficacy of music in increasing some cognitive functions such as memory, executive functions, speech, and attention in both adults and children [35-38]. Effects on verbal intelligence, positively correlated with activity in prefrontal brain regions, have been documented in preschool children even after short-term music training [39]. In adults, larger brain activity in the hippocampus and the dorsolateral prefrontal cortex, strongly related to cognitive control abilities [40]. Overall, these findings demonstrate the relevance of music for brain plasticity in the cortex and the hippocampus, making musicians a good model for non-invasively studying neuroplasticity processes in humans [41,42].
In elderly professional musicians, musical practice seems to preserve the volumes of the neuronal fibers of the arcuate fasciculus, connecting the supratemporal lobe with the inferofrontal regions of the brain, hence halting the apoptosis related to normal aging [43]. Additionally, in adults music is a reliable elicitor of memories, including autobiographical ones. Janata presented subjects to songs from the rock/pop charts of the years when subjects were between 7 and 19 years old [44]. The songs that were rated by subjects as familiar and autobiographically salient activated the dorsomedial prefrontal cortex, previously associated with self-referential processes and integration of sensory information with self-knowledge. The repetition of motifs in a musical piece also activates the prefrontal cortex and the hippocampus [45], further supporting the links between music listening and memory processes [46].
Music In Alzheimer’s Disease
In recent years, music has been introduced as a treatment modality for several central nervous system (CNS) pathologies extending from disturbed behaviour caused by senile dementia [46,47] to schizophrenic-like disorders [48] and AD [49]. In addition, recent findings have reported beneficial effects of music in Parkinson’s disease (PD) [50] cerebral ischemia [51], pain [52], autism [53], anxiety and depression [54]. Furthermore, it also has been shown that music can change the pattern of brain electrical activity in electroencephalography recordings [55,56]. Interestingly, neuroimaging studies show that rhythm perception activates basal ganglia that are compromised to varying degrees in PD [57].
Music therapy has the ability to alleviate some symptoms of dementia [58], by providing access to lost memories or enhancing affective state and communicative skills [59-62]. Although some criticisms and some methodological limitations have been pointed out [63,64], most part of the studies have showed the benefits of music therapy in AD patients. Music has been reported to improve healthy cognition in healthy adults, such as autobiographical memory [60-62], semantic memory [65], language ability and cognitive function [59], or in neuropsychiatric symptoms, such as agitation [59,66] apathy [67], depression, and anxiety [68,69]. Moreover, regular musical activities, such as singing, improve the well-being and cognitive functions in healthy older persons [37,70-74] reducing the risk of developing dementia [75]. In a recent study 10 weeks singing practice by both the patients and the caregivers positively affected not only the shortterm and working memory of patients but even the quality of life of caregivers [76].
AD causes the impairment of episodic and autobiographical memories, and difficulties in interaction between the self and the world. Music seems to mostly alleviate the negative effect of AD by affecting autobiographical memory. In a pioneering study, Foster and Valentine [77] asked AD patients to complete an autobiographical adaptation of the Mini-Mental State Examination [78] while listening to the famous Vivaldi’s Four Seasons, unfamiliar music, cafeteria noise, or in silence. They reported different autobiographical performance among the patients analysed as compared to controls, but, in general, AD patients remembered significantly better in the sound conditions than in silence, and while listening to music than in the cafeteria noise. Afterwards, these outcomes were replicated and confirmed by others [60-62,79]. This result pattern has been attributed to several different factors associated with music listening, such as arousal enhancement [77], anxiety reduction [79], involuntary recall, and verbal narration enhancement [60-62]. Overall, the effects of music on brain functions (musical memory) seem to be carried out via the neural circuits in both limbic and neocortical regions of the brain [80,81]. However, it is still unclear whether these areas are preserved in demented patients.
Although some reports have described impaired music memory in AD [82], and defects in musical episodic memory [83], most of the studies indicate that musical memory remains relatively intact even in the advanced stages of the illness [84,85]. If it is true, it means that these areas have a great potential in designing rehabilitation or therapeutic strategies and might provide promising prospects for clinical interventions. In this regard, Jacobsen and colleagues have recently provided an important contribution by demonstrating that the anterior cingulate and the ventral pre-supplementary motor areas are both responsible in translating musical memory and show minimal cortical atrophy and minimal disruption of glucose metabolism in young adult AD patients [86].
Music and BDNF
BDNF and NGF are believed to be involved in several CNS pathologies, including AD [15,87] and PD [88,89] schizophrenia and depressive disorders [90,91]. In addition the loss of BDNF levels is likely related to the degenerative processes of Huntington’s Disease [92]. These proteins also play an important role in cerebral recovery after ischemic damage [93] and are able to stimulate neurogenesis in the hippocampus [94] and influence the behavioural performance of rats [95]. In various mouse, rat, and primate models, BDNF administration has reversed synaptic damage, partially normalized genetic errors, improved cell signaling, partially rescued learning, memory deficits and cognitive decline, and reduced oxidative stress and cell death [96]. In addition, there is indication demonstrating the potential neuroprotective effect of BDNF against Aβ-induced neurotoxicity [97]. Compelling evidence supports the idea that reduced BDNF levels in serum and plasma predispose and associate to the severity of depression [98,99], a potent risk factor for dementia and neurodegenerative disease. In addition, a decrease in BDNF levels has been largely associated to stress, which plays an important role in suicidal behaviour and constitutes a major risk factor for depression [100-102]. Stress and depression can also be related to dysregulated neuroinflammation reactions [103]. In relation to this, neuroinflammation is associated to a decrease in BDNF levels in specific brain areas, such as the hippocampus and prefrontal cortex [104]. While a direct link between BDNF levels in depressed humans and music intervention has not been established yet, human and animal evidence exists that music has a beneficial effect in alleviating stress symptoms and depression [105-107], and it is capable of influencing the neuroinflammation response by reducing the number and the activity of natural killer cells and controlling cytokine production [103,108].
A major problem for the therapeutic use of BDNF is that the molecule is too large to penetrate the blood-brain barrier resulting in the failure of its action in brain and its consequent accumulation in blood [109]. When BDNF is taken by routes common for other drugs, such as orally or injections into the body, it cannot reach the brain where it is needed. Although in vitro and animal data are promising, it is unlikely that BDNF therapy will be feasible in the next coming future.
Initial experimental data on animal models indicate that exposure to music can influence brain function, probably through modulation of neurotransmitters and/or other neuronal mediators. In the mouse, music exposure differentially alters the levels of BDNF and NGF in the hypothalamus and hippocampus [110,111]. Some studies have shown the effect of Mozart’s music on hippocampal content of BDNF in prenatal/postpartum and adult exposed animals [110-114]. Moreover, it has been reported that perinatal exposure of mice to music has an influence on BDNF/TrkB level and its intracellular signaling [115]. These data have been obtained by studying post mortem the BDNF levels in mice hippocampus and hypothalamus slices. In humans, the measurement of BDNF levels can be done in vivo only via blood serum. While the accurate localization of the changes in BDNF expression in the central nervous system cannot be obtained noninvasively in humans, the systemic changes of BDNF levels could still be established before and after music intervention in future studies.
The effectiveness of Mozart’s sonatas on behaviour has been demonstrated in humans in a study performed on three groups of college students before taking an intelligence quotient (IQ) test. The first group listened to a Mozart sonata. The second group listened to a relaxation tape before their test. The third group did not listen to anything before the test. The first group had the highest score [116,117]. This finding, baptized the “Mozart effect” by the mass media, was erroneously interpreted as the power of classical music to make people smarter simply by listening to it. Subsequent behavioural studies did not replicate the effect of music listening but provided consistent proof of the impact of long-term music training on cognitive abilities, such as working memory, spatial cognition, second language phoneme acquisition, discrimination of prosody, and mathematic skills [118,119].
In the field of dementia, many studies document the efficacy of music in increasing some cognitive functions such as memory, executive functions, speech, and attention [35-38]. As mentioned above effects on verbal intelligence, positively correlated with activity in prefrontal brain regions, have been documented in preschool children even after short-term music training [39]. Larger brain activity in attention and cognitive control-related networks [120], especially in the dorsolateral prefrontal cortex, strongly related to cognitive control abilities, but also in the hippocampus, middle cingulate cortex, motor and auditory areas [40].
Whether or not BDNF production might underlie the cognitive and neural effects of music training in younger and elderly population is an intriguing aspect that should be speculated. Indeed, studies carried out in BDNF Met/Met mutated mice suggest that music exposure induces a relaxing and anxiolytic effect by increasing BDNF levels in hippocampus [121].
Hence, the increase in the BDNF concentration via music exposure and the activation of BDNF downstream signaling, if confirmed in humans, might provide a molecular explanation for the role played by music in dementia. Additionally, understanding the molecular mechanisms behind music effects on brain plasticity and AD might provide a solid ground for increasing the use of music for alleviating the symptoms and improving the life quality of demented patients.
Music and Sex Hormones
An alternative molecular mechanism that might explain the beneficial effects of music on AD can be searched from sex hormones. Aging seems to affect sex hormone levels. In particular, a reduction in sex hormones have been associated to various symptoms in the elderly, including diminished cognitive function, disturbance of memory, mood depression, and climacteric disturbance [122-125]. In AD, the aging-related reductions in sex hormones, especially estrogen, represent a critical risk factor [126,127]. In vitro studies indicate that estrogen decreases β amyloid peptide content and protects neurons from neuronal death [128]. In addition to these effects on amyloid metabolism, estrogen improves cognitive function and delays the onset of dementia by increasing cholinergic activity in the brain, stimulating axonal budding and dendrite formation and retarding cerebral arteriosclerosis [122-125].
Hormone replacement therapies (HRT) are frequently used to normalize sex hormone levels and even though the issue is largely debated, HRT is also indicated to treat some symptoms in AD patients [129-131]. Interesting recent studies indicate that music can be effective in the treatment of AD, by increasing the secretion of 17-estradiol and testosterone [132]. A link between musicality and musical creative behaviour and vasopressin (acting in synergy with testosterone) receptor gene variations has been recently made [133,134]. Hence, sex hormones might mediate the positive effects of music in AD, although the interaction with the neural processes involved is most likely separated from the processes involved in BDNF mediated effects.
Conclusions
Music is something that most people will take for granted, but it has demonstrated effects on the quality of life via emotion regulation in everyday life [135] and on cognitive and motor abilities through daily instrumental practice [136]. As mentioned above, these effects are observable in the behavior and in the brain. Furthermore, evidence from several studies suggests that music therapy effectively reduces some behavioral features of AD patients and, besides some skepticism, overall there is a large assent in considering music therapy at least as an effective supplemental non-pharmacological strategy in AD.
This commentary has not the purpose to provide a reliable ground on which to justify the use of music therapy as a treatment for dementia, but rather to encourage further research on determining the scientific reasons for music effects on AD. In conclusion, we highlight the need for a deeper comprehension of the molecular mechanism linking music benefits to brain functions in demented patients.
Supporting Grants
This study was funded by the Lundbeck Foundation (Grant Number: R151- 2013 14806 to CM) and Danish Council (DFF-4004-00330) to CM and the Danish National Research Foundation DNRF117 to EB.
References
- Minati L, Edginton T, Bruzzone MG, Giaccone G (2009) Current concepts in Alzheimer's disease: a multidisciplinary review. Am J Alzheimers Dis Other Demen 24: 95-121.
- Herrup K (2015) The case for rejecting the amyloid cascade hypothesis. Nat Neurosci 18: 794-799.
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, et al. (2005) Global prevalence of dementia: a Delphi consensus study. Lancet 366: 2112-2117.
- Comas-Herrera A (2007) Cognitive impairment in older people: future demand for long-term care services and the associated costs. Int J Geriatr Psychiatry 22:1037-1045.
- Cash DM, Ridgway GR, Liang Y, Ryan NS, Kinnunen KM, et al. (2013) The pattern of atrophy in familial Alzheimer disease: volumetric MRI results from the DIAN study. Neurology 81: 1425-1433.
- Chhatwal JP, Schultz AP, Johnson K, Benzinger TL, Jack C Jr, et al. (2013) Impaired default network functional connectivity in autosomal dominant Alzheimer disease. Neurology 81: 736-744.
- Stark SL, Roe CM, Grant EA, Hollingsworth H, Benzinger TL, et al. (2013) Preclinical Alzheimer disease and risk of falls. Neurology 81: 437-443.
- Huang Y, Chen H, Wang J, Bunjhoo H, Xiong W, et al. (2013) Relationship between CCR2-V64I polymorphism and cancer risk: a meta-analysis. Gene 524: 54-58.
- Huang Y, Mucke L (2012) Alzheimer mechanisms and therapeutic strategies. Cell 148: 1204-1222.
- Sperling R, Mormino E, Johnson K (2014) The evolution of preclinical Alzheimer's disease: implications for prevention trials. Neuron 84: 608-622.
- Liu CC, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9: 106-118.
- Bu G (2009) Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat Rev Neurosci 10: 333-344.
- Kumar A, Singh A, Ekavali (2015) A review on Alzheimer's disease pathophysiology and its management: an update. Pharmacol Rep 67: 195-203.
- O'Bryant SE, Hobson VL, Hall JR, Barber RC, Zhang S, et al. (2011) Serum brain-derived neurotrophic factor levels are specifically associated with memory performance among Alzheimer's disease cases. Dement GeriatrCognDisord 31: 31-36.
- Calissano P, Amadoro G, Matrone C, Ciafrè S, Marolda R, et al. (2010) Does the term 'trophic' actually mean anti-amyloidogenic? The case of NGF. Cell Death Differ 17: 1126-1133.
- Calissano P, Matrone C, Amadoro G (2009) Apoptosis and in vitro Alzheimer disease neuronal models. CommunIntegrBiol 2: 163-169.
- Calissano P, Matrone C, Amadoro G (2010) Nerve growth factor as a paradigm of neurotrophins related to Alzheimer's disease.DevNeurobiol 70: 372-383.
- Laske C, Stransky E, Leyhe T, Eschweiler GW, Wittorf A, et al. (2006) Stage-dependent BDNF serum concentrations in Alzheimer's disease. J Neural Transm 113: 1217-1224.
- Petrovic M, Hurt C, Collins D, Burns A, Camus V, et al. (2007) Clustering of behavioural and psychological symptoms in dementia (BPSD): a European Alzheimer's disease consortium (EADC) study. ActaClinBelg 62: 426-432.
- McShane R, AreosaSastre A, Minakaran N (2006)Memantine for dementia. Cochrane Database SystRev : CD003154.
- Hope T, Keene J, Fairburn C, McShane R, Jacoby R (1997) Behaviour changes in dementia. 2: Are there behavioural syndromes? Int J Geriatr Psychiatry 12: 1074-1078.
- Abraha I, Cruz-Jentoft A, Soiza RL, O'Mahony D, Cherubini A (2015) Evidence of and recommendations for non-pharmacological interventions for common geriatric conditions: the SENATOR-ONTOP systematic review protocol. BMJ Open 5: e007488.
- Olazarán J, Reisberg B, Clare L, Cruz I, Peña-Casanova J, et al. (2010) Nonpharmacological therapies in Alzheimer's disease: a systematic review of efficacy. Dement GeriatrCognDisord 30: 161-178.
- Sadowsky CH, Galvin JE (2012) Guidelines for the management of cognitive and behavioral problems in dementia. J Am Board Fam Med 25: 350-366.
- Hall CB, Lipton RB, Sliwinski M, Katz MJ, Derby CA, et al. (2009) Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology 73: 356-361.
- Alluri V, Toiviainen P, Lund TE, Wallentin M, Vuust P, et al. (2013) From Vivaldi to Beatles and back: predicting lateralized brain responses to music. Neuroimage 83: 627-636.
- Alluri V, Toiviainen P, Jääskeläinen IP, Glerean E, Sams M, et al. (2012) Large-scale brain networks emerge from dynamic processing of musical timbre, key and rhythm. Neuroimage 59: 3677-3689.
- Tramo MJ (2001) Biology and music. Music of the hemispheres. Science 291: 54-56.
- Zatorre RJ, Baum SR (2012) Musical melody and speech intonation: singing a different tune. PLoSBiol 10: e1001372.
- Schneider P, Scherg M, Dosch HG, Specht HJ, Gutschalk A, et al. (2002) Morphology of Heschl'sgyrus reflects enhanced activation in the auditory cortex of musicians. Nat Neurosci 5: 688-694.
- Gaser C, Schlaug G (2003) Brain structures differ between musicians and non-musicians. See comment in PubMed Commons below J Neurosci 23: 9240-9245.
- Gaser C, Schlaug G (2003) Gray matter differences between musicians and nonmusicians. See comment in PubMed Commons below Ann N Y AcadSci 999: 514-517.
- Hutchinson S, Lee LH, Gaab N, Schlaug G (2003) Cerebellar volume of musicians. Cereb Cortex 13: 943-949.
- Groussard M, Viader F, Hubert V, Landeau B, Abbas A, et al. (2010) Musical and verbal semantic memory: two distinct neural networks? Neuroimage 49: 2764-2773.
- Brotons M, Koger SM (2000) The impact of music therapy on language functioning in dementia. J Music Ther 37: 183-195.
- Ceccato E, Vigato G, Bonetto C, Bevilacqua A, Pizziolo P, et al. (2012) STAM protocol in dementia: a multicenter, single-blind, randomized, and controlled trial. Am J Alzheimers Dis Other Demen 27: 301-310.
- Bugos JA, Perlstein WM, McCrae CS, Brophy TS, Bedenbaugh PH (2007) Individualized piano instruction enhances executive functioning and working memory in older adults. Aging Ment Health 11: 464-471.
- Van de Winckel A, Feys H, De Weerdt W, Dom R (2004) Cognitive and behavioural effects of music-based exercises in patients with dementia. ClinRehabil 18: 253-260.
- Moreno S, Lee Y, Janus M, Bialystok E (2015) Short-term second language and music training induces lasting functional brain changes in early childhood.ChildDev 86: 394-406.
- Groussard M, La Joie R, Rauchs G, Landeau B, Chételat G, et al. (2010) When music and long-term memory interact: effects of musical expertise on functional and structural plasticity in the hippocampus. PLoS One 5.
- Jäncke L (2002) The case of a left-handed pianist playing a reversed keyboard: a challenge for the neuroscience of music. Neuroreport 13: 1579-1583.
- Jäncke L (2002) Does "callosal relay" explain ear advantage in dichotic monitoring? Laterality 7: 309-320.
- Wan CY, Schlaug G (2010) Neural pathways for language in autism: the potential for music-based treatments. Future Neurol 5: 797-805.
- Janata P (2009) The neural architecture of music-evoked autobiographical memories. Cereb Cortex 19: 2579-2594.
- Burunat I, Alluri V, Toiviainen P, Numminen J, Brattico E (2014) Dynamics of brain activity underlying working memory for music in a naturalistic condition. Cortex 57: 254-269.
- Sung HC, Chang AM (2005) Use of preferred music to decrease agitated behaviours in older people with dementia: a review of the literature. J ClinNurs 14: 1133-1140.
- Sung HC, Chang SM, Tsai CS (2005) Working in long-term care settings for older people with dementia: nurses' aides. J ClinNurs 14: 587-593.
- Gold C, Heldal TO, Dahle T, Wigram T (2005) Music therapy for schizophrenia or schizophrenia-like illnesses. Cochrane Database SystRev : CD004025.
- Brotons M, Marti P (2003) Music therapy with Alzheimer's patients and their family caregivers: a pilot project. J Music Ther 40: 138-150.
- Haneishi E (2001) Effects of a music therapy voice protocol on speech intelligibility, vocal acoustic measures, and mood of individuals with Parkinson's disease.J Music Ther 38: 273-290.
- Särkämö T, Tervaniemi M, Laitinen S, Forsblom A, Soinila S, et al. (2008) Music listening enhances cognitive recovery and mood after middle cerebral artery stroke. below Brain 131: 866-876.
- Siedliecki SL, Good M (2006) Effect of music on power, pain, depression and disability. J AdvNurs 54: 553-562.
- Geretsegger M, Elefant C, Mössler KA, Gold C (2014) Music therapy for people with autism spectrum disorder.Cochrane Database Syst Rev 6: CD004381.
- Burns JM, Andrews G, Szabo M (2002) Depression in young people: what causes it and can we prevent it? Med J Aust 177 Suppl: S93-96.
- Lin YP, Jung TP, Chen JH (2009) EEG dynamics during music appreciation. ConfProc IEEE Eng Med BiolSoc 2009: 5316-5319.
- Overman AA, Hoge J, Dale JA, Cross JD, Chien A (2003) EEG alpha desynchronization in musicians and nonmusicians in response to changes in melody, tempo, and key in classical music. Percept Mot Skills 97: 519-532.
- Grahn JA (2009) The role of the basal ganglia in beat perception: neuroimaging and neuropsychological investigations. Ann N Y AcadSci 1169: 35-45.
- Svansdottir HB, Snaedal J (2006) Music therapy in moderate and severe dementia of Alzheimer's type: a case-control study. IntPsychogeriatr 18: 613-621.
- Ziv N, Granot A, Hai S, Dassa A, Haimov I (2007) The effect of background stimulative music on behavior in Alzheimer's patients. J Music Ther 44: 329-343.
- El Haj M, Fasotti L, Allain P (2012) The involuntary nature of music-evoked autobiographical memories in Alzheimer's disease. Conscious Cogn 21: 238-246.
- El Haj M, Fasotti L, Allain P (2012) Source monitoring in Alzheimer's disease.BrainCogn 80: 185-191.
- El Haj M, Moroni C, Samson S, Fasotti L, Allain P (2013) Prospective and retrospective time perception are related to mental time travel: evidence from Alzheimer's disease. Brain Cogn 83: 45-51.
- Watling C, Driessen E, van der Vleuten CP, Vanstone M, Lingard L (2013) Music lessons: revealing medicine's learning culture through a comparison with that of music. Med Educ 47: 842-850.
- Vanstone AD1, Wolf M, Poon T, Cuddy LL (2015) Measuring engagement with music: development of an informant-report questionnaire. See comment in PubMed Commons below Aging MentHealth .
- Simmons-Stern NR, Budson AE, Ally BA (2010) Music as a memory enhancer in patients with Alzheimer's disease. Neuropsychologia 48: 3164-3167.
- Zare M, Ebrahimi AA, Birashk B (2010) The effects of music therapy on reducing agitation in patients with Alzheimer's disease, a pre-post study. Int J Geriatr Psychiatry 25: 1309-1310.
- Holmes C, Knights A, Dean C, Hodkinson S, Hopkins V (2006) Keep music live: music and the alleviation of apathy in dementia subjects. IntPsychogeriatr 18: 623-630.
- Guétin S, Portet F, Picot MC, Pommié C, Messaoudi M, et al. (2009) Effect of music therapy on anxiety and depression in patients with Alzheimer's type dementia: randomised, controlled study. Dement GeriatrCognDisord 28: 36-46.
- Guétin S, Soua B, Voiriot G, Picot MC, Hérisson C (2009) The effect of music therapy on mood and anxiety-depression: an observational study in institutionalised patients with traumatic brain injury. Ann PhysRehabil Med 52: 30-40.
- Hanna-Pladdy B, MacKay A (2011) The relation between instrumental musical activity and cognitive aging. Neuropsychology 25: 378-386.
- Kattenstroth JC, Kolankowska I, Kalisch T, Dinse HR (2010) Superior sensory, motor, and cognitive performance in elderly individuals with multi-year dancing activities. Front Aging Neurosci 2.
- Parbery-Clark A, Strait DL, Anderson S, Hittner E, Kraus N (2011) Musical experience and the aging auditory system: implications for cognitive abilities and hearing speech in noise. PLoS One 6: e18082.
- Zendel BR, Alain C (2012) Musicians experience less age-related decline in central auditory processing. Psychol Aging 27: 410-417.
- Cohen GD, Perlstein S, Chapline J, Kelly J, Firth KM, et al. (2006) The impact of professionally conducted cultural programs on the physical health, mental health, and social functioning of older adults. Gerontologist 46: 726-734.
- Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, et al. (2003) Leisure activities and the risk of dementia in the elderly. N Engl J Med 348: 2508-2516.
- Särkämö T, Tervaniemi M, Laitinen S, Numminen A, Kurki M, et al. (2014) Cognitive, emotional, and social benefits of regular musical activities in early dementia: randomized controlled study. Gerontologist 54: 634-650.
- Foster NA, Valentine ER (2001) The effect of auditory stimulation on autobiographical recall in dementia. Exp Aging Res 27: 215-228.
- Folstein MF, Folstein SE, McHugh PR (1975) "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189-198.
- Irish M, Cunningham CJ, Walsh JB, Coakley D, Lawlor BA, et al. (2006) Investigating the enhancing effect of music on autobiographical memory in mild Alzheimer's disease. Dement GeriatrCognDisord 22: 108-120.
- Menon V, Levitin DJ (2005) The rewards of music listening: response and physiological connectivity of the mesolimbic system. Neuroimage 28: 175-184.
- Levitin DJ, Tirovolas AK (2009) Current advances in the cognitive neuroscience of music. Ann N Y AcadSci 1156: 211-231.
- Halpern AR, O'Connor MG (2000) Implicit memory for music in Alzheimer's disease. Neuropsychology 14: 391-397.
- Baird A, Samson S (2009) Memory for music in Alzheimer's disease: unforgettable? Neuropsychol Rev 19: 85-101.
- Johnson JK, Chang CC, Brambati SM, Migliaccio R, Gorno-Tempini ML, et al. (2011) Music recognition in frontotemporal lobar degeneration and Alzheimer disease.CognBehavNeurol 24: 74-84.
- Cuddy LL, Duffin J (2005) Music, memory, and Alzheimer's disease: is music recognition spared in dementia, and how can it be assessed? Med Hypotheses 64: 229-235.
- Jacobsen JH, Stelzer J, Fritz TH, Chételat G, La Joie R, et al. (2015) Why musical memory can be preserved in advanced Alzheimer's disease. Brain 138: 2438-2450.
- Matrone C, Ciotti MT, Mercanti D, Marolda R, Calissano P (2008) NGF and BDNF signaling control amyloidogenic route and Abeta production in hippocampal neurons. ProcNatlAcadSci U S A 105: 13139-13144.
- Porritt MJ, Batchelor PE, Howells DW (2005) Inhibiting BDNF expression by antisense oligonucleotide infusion causes loss of nigral dopaminergic neurons. ExpNeurol 192: 226-234.
- Howells DW, Porritt MJ, Wong JY, Batchelor PE, Kalnins R, et al. (2000) Reduced BDNF mRNA expression in the Parkinson's disease substantianigra.ExpNeurol 166: 127-135.
- Angelucci F, Oliviero A, Pilato F, Saturno E, Dileone M, et al. (2004) Transcranial magnetic stimulation and BDNF plasma levels in amyotrophic lateral sclerosis. Neuroreport 15: 717-720.
- Angelucci F, Brenè S, Mathé AA (2005) BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry 10: 345-352.
- Ferrer I, Goutan E, Marín C, Rey MJ, Ribalta T (2000) Brain-derived neurotrophic factor in Huntington disease. Brain Res 866: 257-261.
- Ferrer I, Krupinski J, Goutan E, Martí E, Ambrosio S, et al. (2001) Brain-derived neurotrophic factor reduces cortical cell death by ischemia after middle cerebral artery occlusion in the rat. ActaNeuropathol 101: 229-238.
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, et al. (2005) Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. ExpNeurol 192: 348-356.
- Pelleymounter MA, Cullen MJ, Baker MB, Gollub M, Wellman C (1996) The effects of intrahippocampal BDNF and NGF on spatial learning in aged Long Evans rats.MolChemNeuropathol 29: 211-226.
- Nagahara AH, Tuszynski MH (2011) Potential therapeutic uses of BDNF in neurological and psychiatric disorders Nat Rev Drug Discov 10: 209-219.
- Song JH, Yu JT, Tan L (2015) Brain-Derived Neurotrophic Factor in Alzheimer's Disease: Risk, Mechanisms, and Therapy.MolNeurobiol 52: 1477-1493.
- Terracciano A, Lobina M, Piras MG, Mulas A, Cannas A, et al. (2011) Neuroticism, depressive symptoms, and serum BDNF.Psychosom Med 73: 638-642.
- Kim YK, Lee HP, Won SD, Park EY, Lee HY, et al. (2007) Low plasma BDNF is associated with suicidal behavior in major depression. ProgNeuropsychopharmacolBiol Psychiatry 31: 78-85.
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, et al. (2003) Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry 60: 804-815.
- Deveci A, Aydemir O, Taskin O, Taneli F, Esen-DanaciA (2007) Serum BDNF levels in suicide attempters related to psychosocial stressors: a comparative study with depression. Neuropsychobiology 56: 93-97.
- Deveci A (2007) Serum brain-derived neurotrophicfactorlevels in conversion disorder: Comparative study with depression. Psychiatry ClinNeurosci 61:571-573.
- Calabrese F, Rossetti AC, Racagni G, Gass P, Riva MA, et al. (2014) Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity.Front Cell Neurosci 8: 430.
- Hritcu L, Gorgan LD (2014) Intranigral lipopolysaccharide induced anxiety and depression by altered BDNF mRNA expression in rat hippocampus. ProgNeuropsychopharmacolBiol Psychiatry 51: 126-132.
- Baste VS, Gadkari JV (2014) Study of stress, self-esteem and depression in medical students and effect of music on perceived stress. Indian J PhysiolPharmacol 58: 298-301.
- CevascoAM,RKennedy,NR (2005) Generally,Comparison of movement tomusic, rhythm activities, and competitivegames on depression,stress,anxiety,and anger of females in substance abuse rehabilitation.J Music Ther42: 6480.
- Moradipanah F, Mohammadi E, Mohammadil AZ (2009) Effect of music on anxiety, stress, and depression levels in patients undergoing coronary angiography. East Mediterr Health J 15: 639-647.
- Koyama M, Wachi M, Utsuyama M, Bittman B, Hirokawa K, et al. (2009) Recreational music-making modulates immunological responses and mood states in older adults. J Med Dent Sci 56: 79-90.
- Slevin JT (2006) Unilateral intraputaminalglial cell line-derived neurotrophic factor in patients withParkinsondisease:response to 1year each of treatment and withdrawal. Neurosurg Focus 20: E1.
- Angelucci F, Fiore M, Ricci E, Padua L, Sabino A, et al. (2007) Investigating the neurobiology of music: brain-derived neurotrophic factor modulation in the hippocampus of young adult mice.BehavPharmacol 18: 491-496.
- Angelucci F(2007) Music exposure differentially alters the levels of brain-derived neurotrophic factor and nerve growthfactor in the mouse hypothalamus. NeurosciLett 429:152-155.
- Li B, Arime Y, Hall FS, Uhl GR, Sora I (2010) Impaired spatial working memory and decreased frontal cortex BDNF protein level in dopamine transporter knockout mice.Eur J Pharmacol 628: 104-107.
- Barbagallo AP, Weldon R, Tamayev R, Zhou D, Giliberto L, et al. (2010) Tyr(682) in the intracellular domain of APP regulates amyloidogenic APP processing in vivo.PLoS One 5: e15503.
- Chaudhury S, Wadhwa S (2009) Prenatal auditory stimulation alters the levels of CREB mRNA, p-CREB and BDNF expression in chick hippocampus. Int J DevNeurosci 27: 583-590.
- Chikahisa S, Sei H, Morishima M, Sano A, Kitaoka K, et al. (2006) Exposure to music in the perinatal period enhances learning performance and alters BDNF/TrkB signaling in mice as adults. Behav Brain Res 169: 312-319.
- Rauscher FH, Shaw GL, Ky KN (1995) Listening to Mozart enhances spatial-temporal reasoning: towards a neurophysiological basis. NeurosciLett 185: 44-47.
- Rauscher FH, Shaw GL (1998) Key components of the Mozart effect.Percept Mot Skills 86: 835-841.
- Miendlarzewska EA, van Elswijk G, Cannistraci CV, van Ee R (2013) Working memory load attenuates emotional enhancement in recognition memory. Front Psychol 4: 112.
- Tierney A, Kraus N (2013) Music training for the development of reading skills. Prog Brain Res 207: 209-241.
- Pallesen KJ, Brattico E, Bailey CJ, Korvenoja A, Koivisto J, et al. (2010) Cognitive control in auditory working memory is enhanced in musicians. PLoS One 5: e11120.
- Li WJ, Yu H, Yang JM, Gao J, Jiang H, et al. (2010) Anxiolytic effect of music exposure on BDNFMet/Met transgenic mice. Brain Res 1347: 71-79.
- Watson RR (1998) Aging and endocrinology. Science 279: 304-305.
- Mazur A (1998) Aging and endocrinology. Science 279: 305-306.
- Martini L, Dondi D, Limonta P, Maggi R, Messi E, et al. (1992) Endocrinology of aging. Ann N Y AcadSci 673: 214-225.
- Lamberts SW, van den Beld AW, van der Lely AJ (1997) The endocrinology of aging. Science 278: 419-424.
- Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ (2011) Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer's disease. Neurobiol Aging 32: 604-613.
- Barron AM, Pike CJ (2012) Sex hormones, aging, and Alzheimer's disease. Front Biosci (Elite Ed) 4: 976-997.
- Xu H, Gouras GK, Greenfield JP, Vincent B, Naslund J, et al. (1998) Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nat Med 4: 447-451.
- Farlow MR, Cummings JL (2007) Effective pharmacologic management of Alzheimer's disease. Am J Med 120: 388-397.
- Aisen PS (2005) Pharmacologic treatment options in Alzheimer's disease: optimizing disease management. J Am Acad Nurse PractSuppl: 5-7.
- Kumar V, Durai NB, Jobe T (1998) Pharmacologic management of Alzheimer's disease. ClinGeriatr Med 14: 129-146.
- Fukui H, Arai A, Toyoshima K (2012) Efficacy of music therapy in treatment for the patients with Alzheimer's disease.Int J Alzheimers Dis 2012: 531646.
- Ukkola LT, Onkamo P, Raijas P, Karma K, Järvelä I (2009) Musical aptitude is associated with AVPR1A-haplotypes.bMe PLoS One 4: e5534.
- Ukkola-Vuoti L, Oikkonen J, Onkamo P, Karma K, Raijas P, et al. (2011) Association of the arginine vasopressin receptor 1A (AVPR1A) haplotypes with listening to music. J Hum Genet 56: 324-329.
- Saarikallio S (2011) Music as emotional self-Ô?Éregulation throughout adulthood. Psychology of Music 39:307-327.
- Schellenberg EG (2007) Exposure to music and cognitive performance: Tests of children and adults. Psychology of Music 35:5-19.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 18223
- [From(publication date):
December-2015 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 17139
- PDF downloads : 1084