Research Article Open Access
The Potential Effect of Caffeine and Nicotine Co-administration against Aluminum-induced Alzheimer's disease in Rats
Azza A Ali1*, Hebatalla I Ahmed1, Hanan A Abd El-Samea1 and Ebtehal El-Demerdash2
1Pharmacology and Toxicology Department, Faculty of Pharmacy, Al-Azhar University, Cairo, Egypt
2Pharmacology and Toxicology Department, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt
- *Corresponding Author:
- Azza A Ali
Head of Pharmacology and Toxicology Department, Faculty of Pharmacy
Al -Azhar University, Cairo, Egypt
Tel: +20 01061905439
E-mail: azzamoro@gmail.com
Received date: May 03, 2016; Accepted date: May 09, 2016; Published date: May 16, 2016
Citation: Azza AA, Ahmed HI, El-Samea HAA, El-Demerdash E (2016) The Potential Effect of Caffeine and Nicotine Co-administration against Aluminuminduced Alzheimer’s disease in Rats. J Alzheimers Dis Parkinsonism 6:236. doi: 10.4172/2161-0460.1000236
Copyright: © 2016 Azza AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disease characterized clinically by cognitive decline and memory loss. Caffeine and nicotine are the most commonly co-used psycho stimulants. Caffeine is one of the major contributors to the dietary antioxidants which prevent oxidative damage and may reduce the risk of chronic neurodegenerative diseases. Nicotine has the ability to decrease level of reactive oxygen species (ROS) in the hippocampus and suggested to attenuate the impairment of memory associated with AD. The study aimed to evaluate the influence of caffeine and nicotine co-administration against aluminium-induced neurotoxicity that mimics AD in rats. Five groups of rats were used and received daily for five weeks: Saline for control, aluminium chloride (AlCl3) (70 mg/kg, IP) for AD mimic group, while treated groups received together with AlCl3, either Caffeine (5mg/kg, IP), Nicotine (1 mg/kg, SC) or their combination. Three behavioral experiments were performed: Forced Swimming Test (FST), Morris Water Maze (MWM) task and Conditioned-Avoidance and Learning (CAL) test. Histo pathological changes in the brain and biochemical changes in Acetyl cholinesterase (AchE) as well as oxidative parameters; malon dialdehyde (MDA), superoxide dismutase (SOD), total anti oxidane capacity (TAC) were also evaluated for all groups. Results of the behavioral tests showed that caffeine and nicotine co-administration had more pronounced protecting effect from learning and memory impairment induced by AlCl3 than each one alone. Caffeine and nicotine co-administration also prevent neuronal degeneration in the hippocampus and the eosinophilic plagues in the striatum induced by AlCl3 while nicotine alone still showed mild gliosis in striatum. The marked protection of caffeine and nicotine co-administration confirmed also by the significant increase in TAC and SOD and decrease in MDA and AchE in brain tissue. In conclusion, co-administration of caffeine and nicotine can reduce the risk of neuronal degeneration in the hippocampus and attenuate the impairment of learning and memory associated with AD.
Keywords
Alzheimer’s disease; AlCl3; Neuronal degeneration; Caffeine; Nicotine; Oxidative stress; Rats
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by degeneration of the hippocampus and cortical neurons that leads to cognitive decline and memory loss [1]. It is a complex disease that causes accumulation of β-amyloid (Aβ) plaques and neurofibrillary tangles composed of tau amyloid fibrils associated with synapse loss and neuro degeneration [2]. AD is usually classified according to its age of onset to either late-onset or early-onset disease. The majority of patients who develop the disease are aged above 65 years (late-onset AD), while few of AD cases exhibiting an earlier onset, typically in the late 40s or early 50s (early-onset AD) [3]. Three genes have been firmly implicated in the pathophysiology of the early-onset disease; AD-linked mutations in these genes can be considered diagnostic biomarkers of this disease [3]. Late-onset AD etio pathogenesis is unclear [4]; Apolipoprotein E (APOE) is the established susceptibility gene for late-onset AD [3-5]. There are three main hypotheses to explain AD using its hallmarks: the cholinergic hypothesis, the amyloid hypothesis, and the tau hypothesis [6]. Oxidative stress (OS) is involved in the pathogenesis of AD [7]. The importance of OS in AD is due to the high susceptibility of the brain to ROS. The brain has no powerful antioxidant activity; moreover it is rich in fatty acids which are liable to peroxidation, it also consumes a lot of oxygen therefore; it is strongly exposed to free-radicals accumulation [8].
Aluminum (Al) is a well-established neurotoxin and is suspected to be linked with various neurodegenerative diseases including AD and dementia [9]. It is used in cooking utensils and in pharmacological agents including antacids and antiperspirants from which the element enters the human body [10]. Aluminum crosses the blood brain barrier via specific high affinity receptors [11] and causes different neurological disturbances ultimately result in learning and memory deficits in animals and humans. Furthermore, aluminum has been reported to be found in both senile plaques and neurofibrillary tangles-bearing neurons in the brains of AD patients [12]. Moreover, it is a potent cholinotoxin and causes apoptotic neuronal loss, which is a characteristic symptom of neuro degeneration associated with AD [13]. Owing to its chemical characteristics, it can bind to the phosphate groups of DNA and RNA, affecting DNA topology and influencing the expression of various genes essential for brain functions [10]. It also causes spatial memory deficit, influences emotional reactivity, and impairs various brain functions related to learning and memory [10].
Caffeine is the most widely consumed behavioral stimulant present primarily in coffee and tea. It acts by increasing cortical activity, cerebral energy metabolism and extracellular acetylcholine concentration [14]. It is also a natural methyl xanthine and a nonselective A1 and A2A adenosine receptor antagonist, moreover it is well known as a neuromodulator with associative effect on cognitive function, motor behavior and information processing [15]. The increased state of alertness and arousal is experienced with caffeine due to its CNS stimulation activity [15]. Acetylcholine is involved in caffeine’s stimulant properties as cholinergic nerve terminals arising from forebrain cholinergic complex have been involved in arousal and attention processes [16]. Besides, caffeine represents one of the major contributors to the dietary antioxidants, which prevent neurons and other cells from free radical-induced oxidative damage and thus can reduce the risk of chronic degenerative diseases [17]. It also augments the rate of cerebral glucose utilization which contributes to enhance the cognitive functions [15].
Nicotine is a major component of tobacco and exerts many effects on the central nervous system (CNS). It activates specific receptor molecules called nicotinic acetylcholine receptors (nAChRs) which are physiologically gated by the endogenous neurotransmitter acetylcholine [18]. Nicotine interacts with a variety of pre-synaptic nAChR to facilitate the release of a number of neurotransmitters including acetylcholine, dopamine, noradrenaline, serotonin, γ-aminobutyric acid and glutamate; many of which have been implicated in mediating/ modulating a number of behavioral tasks [19]. Activation of nAchRs can lead to potentiating of cognitive functions, psychomotor stimulation, analgesia, reinforcement and synaptic potentiation [20]. It is worthy to note that progressive accumulation of Aβ in AD impairs nAchR functions [21].
In the light of what was mentioned, the study aimed to evaluate the possible protective effects of caffeine and nicotine co-administration against aluminum-induced - neurotoxicity that mimics AD using a rat model.
Materials and Methods
Animals
The study was conducted in accordance with ethical guidelines of Faculty of Pharmacy, Al-Azhar University, Egypt. Forty male Sprague Dawley rats, weighing 220-250 g were used. They were obtained from Nile Co. for Pharmaceuticals and Chemical Industries, Cairo, Egypt. Rats were housed in stainless-steel cages, four per cage, with alternatively 12 hour light and dark cycles at a temperature of 25 ± 1°C. All rats were kept under the same adequate conditions. They provided with their daily dietary requirements of standard diet pellets (El-Nasr, Abu Zaabal, Cairo, Egypt) contained not less than 20% protein, 5% fiber, 3.5% fat, 6.5% ash and vitamin mixture and water was given adlibitum.
One hour before each experiment and after food and water had been removed from the cages; rats were taken to the test situation for adaptation. All experiments were carried during fixed time (from 8 AM to 12 PM).
Drugs and chemicals
Aluminum chloride - hydrated (AlCl3.6H2O), caffeine, and nicotine were purchased from Sigma Chemical Co. (St. Louis, MO, USA). AlCl3 and caffeine were freshly dissolved in distilled water; nicotine was freshly dissolved in saline. All other chemicals and solvents were of highest grade-commercially available.
Experimental design
Five groups of rats (8/group) were used and received daily for five weeks: Saline for control group, AlCl3 (70 mg/kg, IP) for AD group [22], while treated groups received together with AlCl3, either Caffeine (5 mg/kg, IP) [23], Nicotine (1 mg/kg, SC) [24] or both Caffeine and Nicotine. All drugs were administered at a dose volume 0.5 ml/250 g body weight. Three behavioral experiments were performed: Forced swimming test, Morris water maze task and Conditioned-avoidance and Learning test. Rats were sacrificed 24 h after the last test, the brain tissues were dissected and washed with ice-cold saline. All of the brain tissues were either subjected for analysis immediately or kept frozen at – 80°C till the time of analysis. They were homogenized in saline; the homogenates were used to assess oxidative stress markers as lipid peroxides expressed as malon dialdehyde (MDA), superoxide dismutase (SOD) and total antioxidant capacity (TAC), in addition, acetylcholine esterase (AchE) activity was also estimated. On the other hand, specimens from the whole brain areas for all treated groups were taken for histo pathological examination.
Behavioral experiments
Three experiments of behavioral assessments with different degree of stressfulness were selected to formulate an integrative testing pattern. The chosen tests allowed measuring the most behavioral responses to the mentioned drugs. This pattern includes the following experiments which were performed according to certain order depending on their level of stressfulness:
Forced Swimming Test (FST)
It is a behavioral test for hopelessness and despair which used frequently to evaluate the potential efficacy of the prospective antidepressant drugs [25,26]. When the animals were forced to swim in a confined space and after an initial phase of vigorous activity, they ceased to struggle surrendering themselves. The despair behavior reflected a state of lowered mood [27]. The system consists of individual glass cylinders 40 cm tall × 20 cm in diameter containing 30 cm of water, maintained at 23-25 ºC, so the rats could not support themselves by touching the bottom with their feet [28]. The procedure for the used FST has been described previously; two swimming sessions were conducted, an initial 15-min pre-test followed 24 h later by a 5-min test [29]. Following both swimming sessions, rats were removed from cylinders, dried with towels and returned to their home cages. Test sessions were videotaped for later scoring. Water was changed in the cylinders after every other trial to avoid confounding results because of possible alarm substances from urine or faeces. The measured parameters were; immobility score, swimming score and climbing score. Immobility: The animal remained floating passively in the water and only making those movements necessary to keep its head above the water, Swimming: movements of the four extremities that allow the rat to move around or cross the cylinder, Climbing: active movements with forepaws in and out of the water, usually directed against the walls. Scores for each behavior were expressed as the total number of counts per 5-min session [28].
Morris Water Maze (MWM) Task
It is a hippocampus dependent spatial learning task as previously described [30] in which rats are required to learn to locate an escape platform in a pool of water, using visual cues surrounding the maze. Water maze was a black circular tank 150 cm in diameter and 62.5 cm in height. The tank was filled with water (20 ± 1°C) to a depth of 40 cm. around the room; numerous visual cues (e.g. bookcase and tables) were present which remained constant throughout the experiment. The maze was divided geographically into four quadrants northeast (NE), northwest (NW), southeast (SE), southwest (SW), and starting positions, north (N), south (S), east (E), west (w) that were equally spaced around the perimeter of the pool, a hidden circular platform (diameter: 13 cm) was located in the centre of the SW quadrant, 1 cm below the surface of the water. Rats were trained to find a submerged escape platform, located in a fixed position during four consecutive daily sessions. Each session consisted of four trials; four different starting positions and equally spaced around the perimeter of the pool were used in a fixed order. Each trial had a maximum duration of 60 sec began with releasing the rats in MWW, then escape latency was calculated which is the time in seconds taken to escape on to the submerged platform. Rats which couldn’t find the platform within 60 sec were placed on it. At the end of each trial, rats were allowed to remain on the platform for 20 sec in order to recognize the place well. For all training trials, escape latency was averaged per rat (four different positions), then calculated the averages of the groups. Two hours after the last training trial (the fourth trial of the fourth day), rats were subjected to a memory probe trial during which they swam for 60 sec in the absence of the training platform. All rats started from the same position, opposite to the target quadrant (i.e. the quadrant where the escape platform had been positioned). Time of probe trial was calculated (i.e. time in seconds spent in the target quadrant).
Conditioned-Avoidance and Learning (CAL) test
All rats were trained in the apparatus of the conditioned avoidance test which was previously described [31,32] and modified [33]. The use of CAL test was extended and parameters were manipulated for evaluating learning ability and memory consolidation in high stressful conditions. The apparatus consists of five interconnected chambers; four of them can be electrified using a laboratory DC power supply (Model GPR-6060 D) to deliver the foot shock (Un-conditioned stimulus; 50 volts, 25 pulse /sec) through their stainless steel grid floor. The fifth chamber represents the safety area (Glass floor). Training was conducted by pairing of auditory stimulus (Conditioned stimulus; electric bell) for 5 sec. followed by another 5 sec. of foot shock. Number of trials to avoid the electric shock and reach to safety area during 5 sec. of the conditioned stimulus was calculated for each rat at the 1st and the 2nd day of training which indicating learning ability and short term memory retention.
Biochemical Parameters
Assessment of oxidative stress markers
In all groups, MDA, TAC and SOD were measured in the brain homogenate of each rat. Lipid peroxidation was determined be estimating the level of thiobarbituric acid reactive substances (TBARS) measured as MDA [34]. The determination of TAC is performed by the reaction of antioxidants with a defined amount of exogenously provide H2O2. The residual H2O2 is determined colori metrically by an enzymatic reaction which involves the conversion of 3, 5-dichloro- 2-hydroxybenzene sulphonate to a colored product [35]. SOD activity was assessed relying on the ability of the enzyme to inhibit the phena zinemethosulphate mediated reduction of nitro bluetetrazolium dye; the increase in absorbance at 560 nm for 5 min is measured [36].
Determination of AchE activity
In the brain tissue homogenate, AchE activity was assessed using ELISA Kits (Ray Biotech, Inc., USA) according to the instructions of the manufacturer.
Histo pathological examination of the brain
Brain specimens were fixed in 10% formalin for 24 h then washed with tap water; the specimens were prepared and stained for light microscopy [37]. For dehydration, serial dilutions of alcohol (methyl, ethyl and absolute ethyl) were used. Specimens were cleared in xylene embedded in paraffin at 56 ºC in hot air oven for 24 h. Paraffin bees wax tissue blocks were prepared for sectioning at 4 microns thickness by microtome. The obtained tissue sections were collected on glass slides and deparaffinised, they were stained with Hematoxylin and Eosin stain for routine histological examination.
Statistical analysis
Data was expressed as the mean + SEM. and statistical analysis was carried out by one way ANOVA followed by Tukey multiple comparisons test to calculate significance of the difference between treatments. Values of P < 0.05 were considered significant. All statistical analyses were performed and graphs were sketched using Graph Pad Prism (ISI®, USA) software (version 5) computer program.
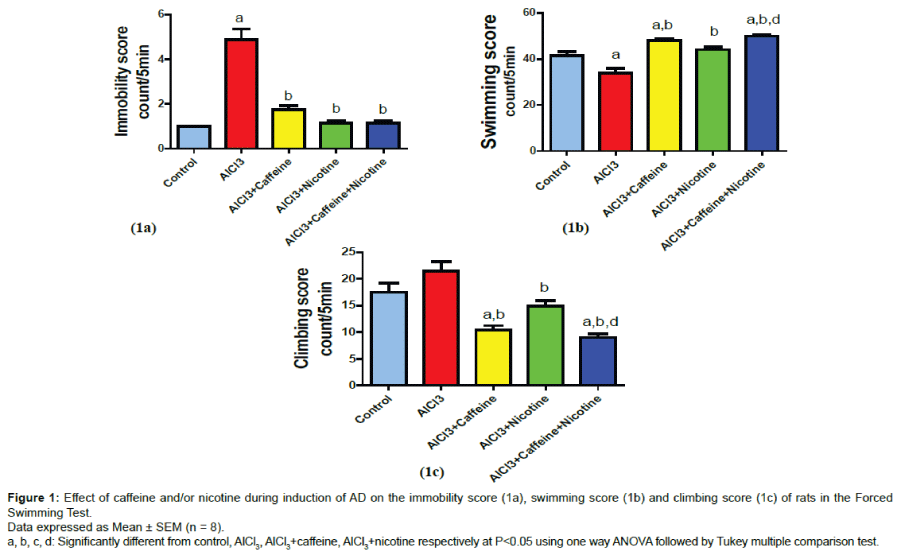
Behavioral changes in the forced swimming test
As shown in (Figure 1a), AD mimic model induced by injection of AlCl3 significantly increased the immobility score reaching 428.5% of the normal control value. Treatment with caffeine or nicotine during induction of AD significantly decreased the immobility score reaching 35.4% and 28.5% as compared to AlCl3 group respectively. Co-treatment with caffeine and nicotine during induction of AD significantly decreased the immobility score reaching 26.04% as compared to AlCl3 group. AD group showed significant decrease in the swimming score reaching 81.2% of the normal control value (Figure 1b). Treatment with caffeine or nicotine significantly increased swimming score reaching 141.8% and 130.5% as compared to AlCl3 group respectively. Cotreatment with both caffeine and nicotine significantly increased the swimming score reaching 147.7% as compared to AlCl3 group and 113.1% as compared to nicotine group. On the other hand, climbing score non-significantly changed by AlCl3 (Figure 1c) however, treatment with caffeine or nicotine during significantly decreased climbing score reaching 48.3% and 68.5% respectively as compared to AlCl3 group. Caffeine and nicotine co-treated group showed significant decrease in climbing score reaching 41.3%, and 60.2% as compared to AlCl3 group and nicotine group respectively.
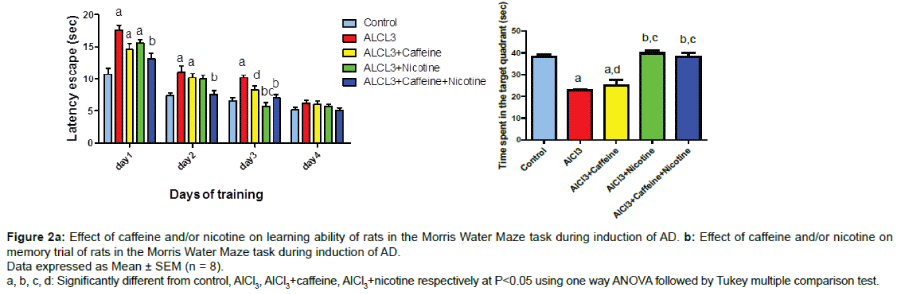
Behavioral changes in the Morris water maze task
Efficiency of the learning ability in animals treated with AlCl3 was decreased. The results in (Figure 2a) showed a significant increase in escape latency from the first to the third day of training reaching approximately 164.7%, 150.2%, and 156.5% respectively as compared to control values. Co-treatment with caffeine and nicotine increased the efficiency of the learning ability in animals which was indicated by a significant decrease in escape latency from the first to the third day of training reaching approximately 74.5%, 67.89%, and 68.49% respectively as compared to AlCl3 group.
Results in (Figure 2b) showed that rats treated with AlCl3 significantly decreased the time spent in the target quadrant reaching approximately 59.5% as compared to the control group. Co-treatment with caffeine and nicotine significantly increased the time spent in the target quadrant reaching approximately 167.4% as compared to AlCl3 received group and 151.3% as compared to Caffeine- treated group.
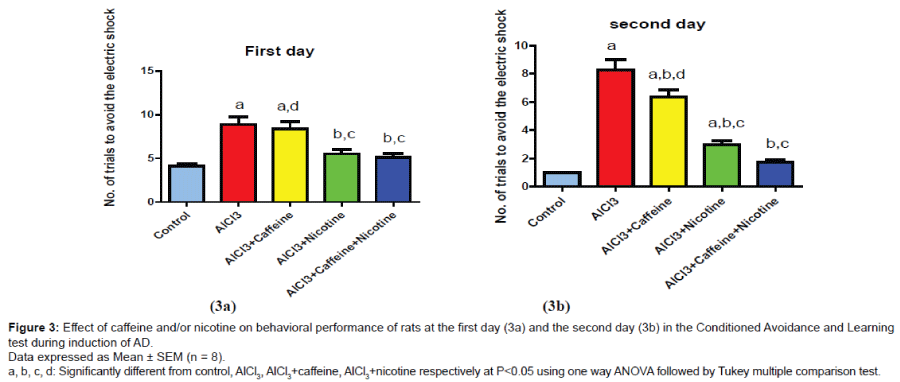
Behavioral changes in the conditioned - avoidance and learning test
As shown in (Figure 3a), AlCl3-received group showed a significant increase in the number of trials to avoid the electric shock at the 1st day of the experiment reaching approximately 214.6% as compared to control group. Co-treatment with caffeine and nicotine showed a significant decrease in the number of trials to avoid the electric shock at the 1st day of the experiment reaching approximately 57.9% as compared to AlCl3 group and 61.4% as compared to caffeinetreated group while nicotine- treated group showed non-significant difference than co-treated group.
Group received AlCl3 showed a significant increase in number of trials to avoid the electric shock at the 2nd day of the experiment (Figure 3b) reaching approximately 745% as compared to control group. Co-treatment of caffeine and nicotine significantly decreased number of trials at the 2nd day reaching approximately 20.7% as compared to AlCl3 group and 26.9% as compared to caffeine group while nicotine- treated group showed non-significant difference than co-treated group.
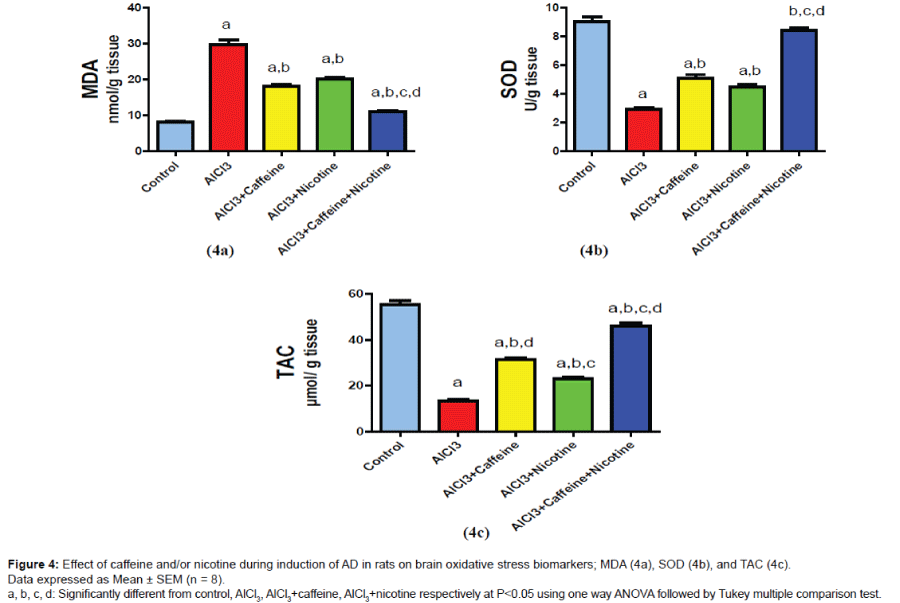
Brain oxidative stress biomarkers (MDA, SOD and TAC)
Results are shown in (Figure 4a-4c); treatment with caffeine or nicotine alone or in combination resulted in a significant decrease in MDA level reaching 60.96%, 67.86% and 36.9% as compared to AlCl3 group respectively. On the other hand, administration of Caffeine or nicotine alone or in combination showed a significant increase in SOD reaching 174.65%, 153.08% and 287.67% as compared to AlCl3 group respectively. Moreover, caffeine or Nicotine-treated group showed a significant increase in TAC level reaching 233.4% and 171.8% as compared to AlCl3 group respectively. However, cotreatment with caffeine and nicotine significantly increased TAC level reaching 341.2% as compared to AlCl3 group while reaching 146.2% and 198.5% as compared to caffeine and nicotine groups respectively.
Brain acetylcholine esterase (AchE) activity
As shown in (Figure 5), AlCl3-injected group showed a significant increase in AchE activity reaching 730.7% as compared to control group. Caffeine or Nicotine-treated group showed a significant decrease in AchE activity reaching 53.9% and 73.15% as compared to AlCl3 group respectively. Co-treatment with caffeine and nicotine showed a significant decrease in AchE activity reaching 27.89% as compared to AlCl3 group, 51.7% as compared to caffeine group and 38.1% as compared to nicotine group.
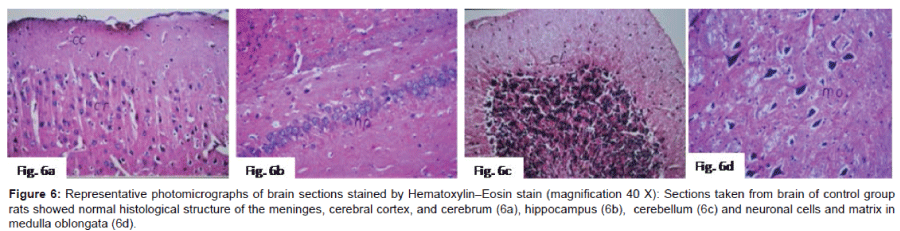
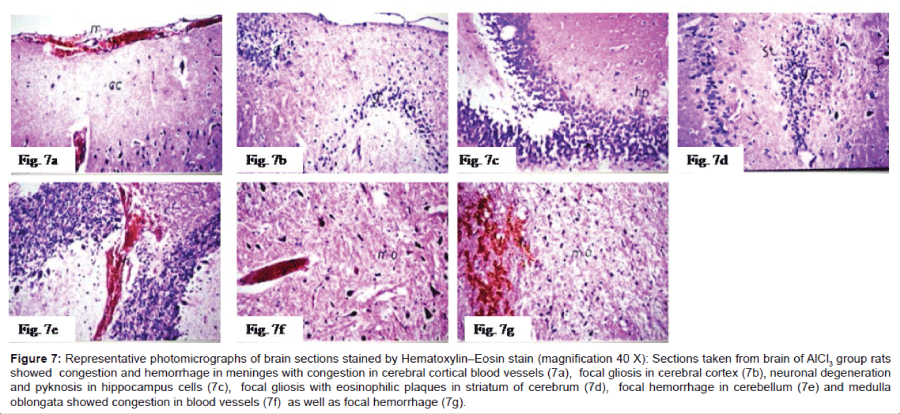
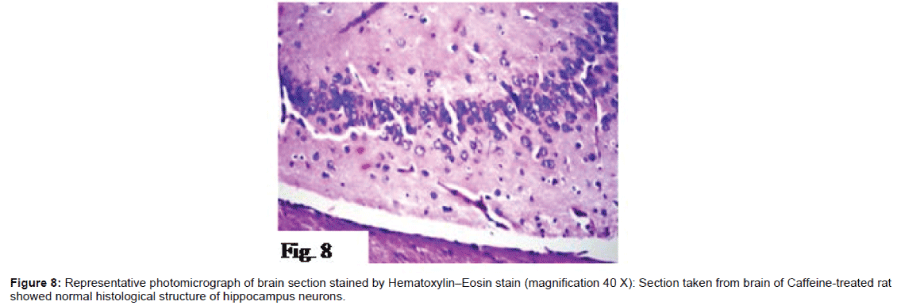
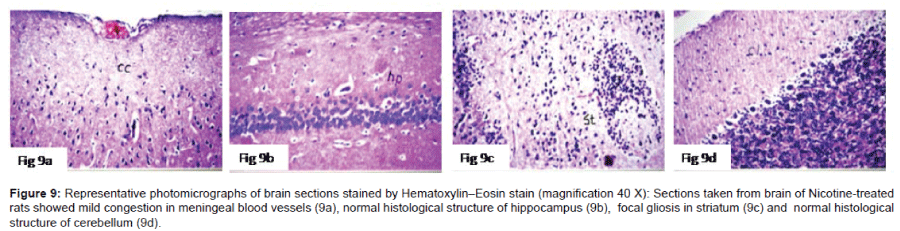
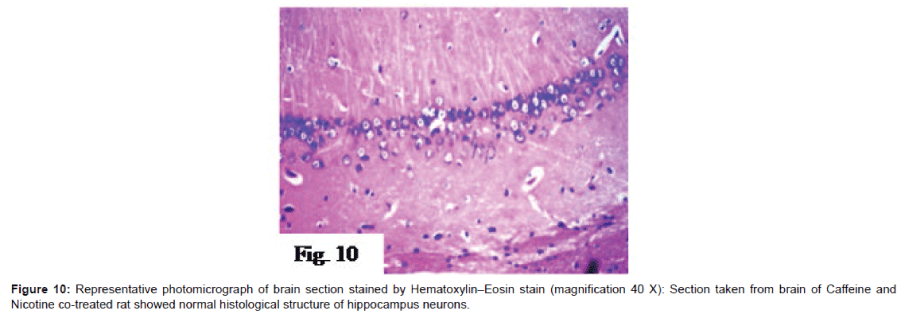
Histo pathological examination of the brain: The severity of histo pathological alterations in the brain of rats is shown in (Table 1). Control group: There was no histo pathological alteration in meninges, cerebral cortex, cerebrum striatum, hippocampus, cerebellum, and medulla oblongata (Figure 6). AlCl3 group: Congestion was observed in the blood vessels of the meninges and cerebral cortex while the haemorrhage was noticed only in the meninges. The cortex showed also focal gliosis. Neuronal degeneration and pyknosis were detected in the round and pyramidal cells of the hippocampus. Focal gliosis as well as focal eosinophilic plagues were detected in the striatum. The cerebellum showed focal haemorrhage while the medulla oblongata had congestion in the blood vessels as well as focal haemorrhages (Figure 7). Caffeinetreated group: There was no histopathological alteration in the hippocampus as recorded in (Figure 8). Nicotine-treated group: Mild congestion was noticed in the meningeal blood vessels. The hippocampus was histologically intact, while focal gliosis was noticed in striatum. There was no histopathological alteration in the cerebellum (Figure 9). Caffeine and Nicotine co-treated group: There was no histopathological alteration in the hippocampus as showen in (Figure 10).
| Histopathological alterations | Control | AlCl3 | AlCl3+ Caffeine |
AlCl3+ Nicotine |
AlCl3+Caffeine +Nicotine |
|---|---|---|---|---|---|
| Degeneration &pyknosis in hippocampus neurons | - | +++ | - | - | - |
| Eosinophillic plaque formation in stratum | - | + | - | - | - |
| Gliosis | - | ++ | - | + | - |
| Congestion | - | + | - | + | - |
+++ sever; ++ moderate; + mild; - Nil
Table 1: Effect of Caffeine and/or Nicotine during induction of AD in rats on the severity of histopathological alterations in the brain of rats.
Figure 6: Representative photomicrographs of brain sections stained by Hematoxylin–Eosin stain (magnification 40 X): Sections taken from brain of control group rats showed normal histological structure of the meninges, cerebral cortex, and cerebrum (6a), hippocampus (6b), cerebellum (6c) and neuronal cells and matrix in medulla oblongata (6d).
Figure 7: Representative photomicrographs of brain sections stained by Hematoxylin–Eosin stain (magnification 40 X):7a) Sections taken from brain ofAlCl3 group rats showed congestion and hemorrhage in meninges with congestion in cerebral cortical blood vessels,7b) focal gliosis in cerebral cortex, 7c)neuronal degeneration andpyknosis in hippocampus cells,7d)focal gliosis with eosinophilic plaques in striatum of cerebrum, 7e)focal hemorrhage in cerebellum and 7f) medulla oblongata showed congestion in blood vessels as well as7g) focal hemorrhage.
Figure 9: Representative photomicrographs of brain sections stained by Hematoxylin–Eosin stain (magnification 40 X):9a) Sections taken from brain ofNicotine-treated rats showed mild congestion in meningeal blood vessels, 9b)normal histological structure of hippocampus,9c) focal gliosis in striatum and9d)normal histological structure of cerebellum.
Discussion
In the present study, AD mimics model was induced by injection of AlCl3 (70 mg/kg, IP) daily for five weeks. It is known that AD is a neurodegenerative disorder, initially characterized by memory loss and followed by functional impairment in a multitude of cognitive domains [38]. There are many studies which have linked aluminium to AD in both animals [13,39] and humans [12]. Exposure to Al causes neuronal degeneration affecting mainly the cholinergic cells in the brain and results in production of free radicals [40] being responsible for neurotoxicity that causes damage of the neuronal membrane [41].
AlCl3-received group in this study showed a significant increase in immobility score and a significant decrease in swimming score as compared to control group in forced swimming test. This indicates a state of lowered mood or hopelessness with no effect on climbing score. It is common to find neuropsychiatric co-morbidities such as depression, schizophrenia and bipolar disorder during the course or development of AD [42]. The presence of depression associated with dementia appears primarily in the elderly [43]. Thus, it is probable that depression is the most common neuropsychiatric disorder in AD [44]. Results of the current study also showed that AlCl3 injection induced a significant increase in escape latency to reach the hidden platform accompanied by a significant decrease in the time spent in target quadrant in the MWM as compared to control group. These results indicate impairment of learning ability and spatial memory. It is well known that Al accumulates in all regions of rat brain following chronic exposure; maximum being in hippocampus which is the site of memory and learning [45]. In the present study, administration of AlCl3 resulted in marked elevation in the number of trials to avoid the electric shock at the 1st and 2nd days of the conditioned-avoidance and learning test as compared to control group. These resulting data could be attributed to the deficits in the cognitive functions as learning, memory and retrieval abilities and are in parallel with the impaired learning and memory capacities in AD mice model evidenced by reduction in latency to enter the dark chamber in the passive avoidance test in which they learned to avoid a dark chamber after exposure to an electrical foot shock followed by a retention test on the next day [46]. Results of the present study also showed that injection of AlCl3 significantly increased brain AchE activity as compared to control group. The current result is supported by the results indicating that Al is cholinotoxin that causes degeneration of cholinergic terminals in cortex and hippocampus and AchE is a marker of the loss of cholinergic neurons [13]. In addition, AlCl3- received group showed marked increase in the brain MAD associated with marked decrease in SOD and TAC as compared to control group indicating marked oxidative stress. These results are in accordance with the effect of Al exposure on the development of oxidative stress related damage to lipids, membrane associated proteins and endogenous antioxidant enzyme activity [47]. Results of the AlCl3- received group were also confirmed by the results of the histopathological examination of the brain in the present study which showed degeneration and pyknosis in hippocampus neurons, focal gliosis as well as focal eosinophilic plagues in the striatum in addition to congestion in blood vessels. Aluminium toxicity is also associated with reduced axonal length and dendritic branches in hippocampus [48].
In the present study, caffeine-treated group showed marked decrease in immobility and climbing score as well as increase in swimming score in the FST as compared to AlCl3 group indicating mood elevation. It is known that, caffeine can immediately facilitate the release of serotoninergic and dopaminergic transmissions which contribute to antidepressant effects [49]. Caffeine-treated rats also showed insignificant changes in escape latency and time spent in the target quadrant as compared to AlCl3 group in the MWM indicating non- improvement in learning and memory impairment induced by AlCl3. Moreover, in the CAL test, caffeine showed insignificant change in the number of trials to avoid the electric shock in the 1st day but significant decrease in the 2nd day of the experiment as compared to AlCl3 group which indicates improvement in memory but not in learning ability. This disruption of acquisition or retrieval induced by caffeine (a non-selective A1 and A2A adenosine receptor antagonist) might be due to the concomitant participation of both A1 and A2A receptors with various effects of different brain regions that control the different phases of memory processing. Inactivation of both A1 and A2A receptors has been found to counteract the age related cognitive decline [50,51]. Because of these cognition enhancement properties from combined blockade of A1 and A2A receptors, caffeine has been proposed as a potential therapeutic agent to reverse age-related cognitive decline [50]. The effects of caffeine on memory retrieval showed mixed results, some studies showing improvement [51] while others have indicated either no response [52] or even impairment in retrieval ability [53].
In addition, caffeine treatment in this study showed decrease in brain AchE activity as compared to AlCl3 group. These results are similar to the results which indicate that administration of caffeine (both short-and long-term) to rats showed increased acetylcholine concentration in the prefrontal cortex [54] since, AchE is a marker of the loss of cholinergic neurons [13]. Caffeine also showed decrease in the brain MDA and increase in SOD and TAC as compared to AlCl3 group as it has antioxidant properties. It is well known that caffeine is one of the major contributors to the dietary antioxidants, which prevent neurons, and other cells from free radical-induced oxidative damage and thus reduce the risk of chronic degenerative diseases [17]. No histopathological alterations in the hippocampus were shown upon treatment with caffeine in this study indicating its ability to prevent neuronal damage caused by AlCl3. Previous results showed that caffeine can prevent neuronal damage and cognitive deficit caused by Aβ, an effect mimicked by A2A receptor antagonists [55].
Data of the present study showed that nicotine treatment decreased immobility and climbing score and increased swimming score in FST as compared to AlCl3 group. Similar results concerning antidepressant effect of nicotine showed that nicotine has antidepressant effect which can be observed following its acute and chronic administration in depressed rats [28]. Nicotine also showed significant decrease in escape latency in the third day of training only and increase the time spent in the target quadrant as compared to AlCl3 group in MWM test indicating mild improvement in learning and memory abilities in this condition. On the other hand, nicotine showed marked decrease in the number of trials to avoid the electric shock in the 1st and 2nd days of the CAL experiments as compared to AlCl3 group which indicates improvement in cognitive functions (learning, memory, and retrieval abilities) in this stressful condition. Nicotine was found to attenuate the impairment of learning and memory associated with chronic stress [56] and other conditions including aging [57].
The present study also showed that nicotine caused decrease in AchE activity as compared to AlCl3 group. Previous study showed that chronic nicotine exposure unregulated nAChRs through post-translational mechanisms and/or by modification of binding characteristics of these receptors in the central nervous system which may be responsible for the neuro protective action of nicotine [58]. In addition, nicotine showed decrease in MDA and increase in SOD and TAC as compared with AlCl3 group as it has antioxidant properties. The protective effect of nicotine could be also mediated through nAChRs related mechanisms, including antioxidant effects [59]. In the present study, treatment with nicotine showed no histo pathological alteration in the hippocampus but still showed focal gliosis in the striatum. Nicotinic-acetylcholine receptors are emerging as a promising candidate of targets for drug treatment of central nervous system disorders associated with neuro degeneration and neuro inflammation [60]. Stimulation of these receptors may provide direct protective effects on neurons [61].
Co-treatment with caffeine and nicotine in the present study showed significant decrease in immobility and climbing score and increase in swimming score as compared to AlCl3 group in the FST. However, results of co-treatment indicate that the effect of co-treatment with caffeine and nicotine is better than nicotine alone in the FST. Data of the present study also showed that co-treatment with caffeine and nicotine increased the efficiency of learning ability in animals in the MWM test as compared to AlCl3 group from the first to the third day of training, this effect couldn’t be achieved by each drug alone. In addition, co-treatment with caffeine and nicotine significantly increased time spent in the target quadrant as compared to AlCl3 group and caffeine group but not to nicotine group. On the other hand, co-treatment with caffeine and nicotine showed significant decrease in the number of trials to avoid the electric shock at the 1st and 2nd days of the CAL test as compared to AlCl3 group. The effect was more pronounced than caffeine alone which indicates that co-treatment with caffeine and nicotine improves cognitive functions more than caffeine alone. Consequently, it is clear that co-treatment with caffeine and nicotine improves the memory impairment induced by AlCl3 and this improvement is better than using caffeine alone in the present study. Co-treatment with caffeine and nicotine also showed significant decrease in brain AchE activity as compared to AlCl3 group. This effect was more pronounced than treating with each of them alone. In addition, co-treatment with caffeine and nicotine showed significant increase in TAC and SOD as well as significant decrease in MDA as compared to AlCl3 and as compared with each drug alone. Moreover, co-treatment with caffeine and nicotine showed no histopathological alteration in the hippocampus and showed more protective effect on neurons than using nicotine alone. However there is no published data regarding the effect of co-treatment of caffeine and nicotine during induction of AD.
Conclusion
In the light of what was mentioned, it is clear that co-administration of caffeine and nicotine to rats during exposure to AlCl3 neurotoxicity (a rat model that suggested to mimic human AD) shows more pronounced improving effect on learning and memory abilities, marked decrease in AchE activity as well as oxidative stress and can also protect against neuronal degeneration in the hippocampus than each one alone. Finally, it could be concluded that co-treatment with caffeine and nicotine has more pronounced protecting effect than each drug alone during induction of AD in rats.
References
- Alzheimer's Association (2011) 2011 Alzheimer's disease facts and figures. Alzheimers Dement 7: 208-244.
- Hardy J (2006) Alzheimer's disease: the amyloid cascade hypothesis: an update and reappraisal. J Alzheimers Dis 9: 151-153.
- Reitz C, Brayne C, Mayeux R (2011) Epidemiology of Alzheimer disease. Nat Rev Neurol 7: 137-152.
- Mattson MP (2004) Pathways towards and away from Alzheimer's disease. Nature 430: 631-639.
- Myers RH, Schaefer EJ, Wilson PW, D'Agostino R, Ordovas JM, et al. (1996) Apolipoprotein E epsilon4 association with dementia in a population-based study: The Framingham study. Neurology 46: 673-677.
- Tanzi RE, Bertram L (2005) Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell 120: 545-555.
- Cervellati C, Romani A, Seripa D, Cremonini E, Bosi C, et al. (2014) Oxidative balance, homocysteine, and uric acid levels in older patients with Late Onset Alzheimer's Disease or Vascular Dementia. J NeurolSci 337: 156-161.
- Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. CurrNeuropharmacol7: 65–74.
- Shiraki H, Yase Y (1991) Amyotrophic lateral sclerosis and Parkinsonism-dementia in the Kii peninsula: comparison with the same disorders in Guam and with Alzheimer’s disease: Handbook of Clinical Neurology, North-Holland Pub. Co.
- Roskams AJ, Connor JR (1990) Aluminum access to the brain: a role for transferrin and its receptor. ProcNatlAcadSci U S A 87: 9024-9027.
- Kawahara M, Kato-Negishi M (2011) Link between Aluminum and the Pathogenesis of Alzheimer's Disease: The Integration of the Aluminum and Amyloid Cascade Hypotheses. Int J Alzheimers Dis 2011: 276393.
- McLachlan DR, Bergeron C, Smith JE, Boomer D, Rifat SL (1996) Risk for neuropathologically confirmed Alzheimer’s disease and residual aluminum in municipal drinking water employing weighted residential histories. Neurology 46: 401-405.
- Gulya K, Rakonczay Z, Kása P (1990) Cholinotoxic effects of aluminum in rat brain. J Neurochem 54: 1020-1026.
- Lopes LV, Cunha RA, Ribeiro JA (1999) Increase in the number, G protein coupling, and efficiency of facilitatory adenosine A2A receptors in the limbic cortex, but not striatum, of aged rats. J Neurochem 73: 1733-1738.
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE (1999) Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51: 83-133.
- Sarter M, Bruno JP (2000) Cortical cholinergic inputs mediating arousal, attentional processing and dreaming: differential afferent regulation of the basal forebrain by telencephalic and brainstem afferents. Neuroscience 95: 933-952.
- Svilaas A, Sakhi AK, Andersen LF, Svilaas T, Ström EC, et al. (2004) Intakes of antioxidants in coffee, wine, and vegetables are correlated with plasma carotenoids in humans. J Nutr 134: 562-567.
- Zoli M (2000) Distribution of cholinergic neurons in the mammalian brain with special reference to their relationship with neuronal nicotinic acetylcholine receptors: Handbook of Experimental Pharmacology, Springer, Berlin.
- Picciotto MR, Caldarone BJ, King SL, Zachariou V (2000) Nicotinic receptors in the brain. Links between molecular biology and behavior. Neuropsychopharmacology 22: 451-465.
- Zanardi A, Leo G, Biagini G, Zoli M (2002) Nicotine and neurodegeneration in ageing. ToxicolLett 127: 207-215.
- Perry E, Martin-Ruiz C, Lee M, Griffiths M, Johnson M, et al. (2000) Nicotinic receptor subtypes in human brain ageing, Alzheimer and Lewy body diseases. Eur J Pharmacol 393: 215-222.
- Ali AA, Ahmed HI, Abolfotoh K (2015) Modeling stages of Alzheimer's disease induced by different doses of aluminum in rats, focus on progression of the disease in response to time. J Alzheimers Dis Parkinsonism 5: 94.
- Vila-luna S, Cabrera-isidoro S, Vila-luna L, Juárez-Díaz I, Bata-García JL , et al. (2012) Chronic caffeine consumption prevents cognitive decline from young to middle age in rats, and is associated with increased length, branching, and spine density of basal dendrites in CA1 hippocampal neurons. Neuroscience 202: 384-395.
- Alkadhi KA, Srivareerat M, Tran TT (2010) Intensification of long-term memory deficit by chronic stress and prevention by nicotine in a rat model of Alzheimer's disease. Mol Cell Neurosci 45: 289-296.
- Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266: 730-732.
- Kirby LG, Lucki I (1997) Interaction between the forced swimming test and fluoxetine treatment on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the rat. J PharmacolExpTher 282: 967-976.
- Parale MP, Kulkarni SK (1986) Clonidine--induced behavioural despair in mice: reversal by antidepressants. Psychopharmacology (Berl) 89: 171-174.
- Vazquez-Palacios G, Bonilla-Jaime H, Velazquez-Moctezuma J (2005) Antidepressant effects of nicotine and fluoxetine in an animal model of depression induced by neonatal treatment with clomipramine. ProgNeuropsychopharmacolBiol Psychiatry 29:39-46.
- Detke MJ, Rickels M, Lucki I (1995) Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 121:66-72.
- Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47-60.
- Bornschein RL, Hastings L, Manson JM (1980) Behavioral toxicity in the offspring of rats following maternal exposure to dichloromethane. ToxicolApplPharmacol 52: 29-37.
- Arnt J (1982) Pharmacological specificity of conditioned-avoidance response inhibition in rats: Inhibition by neuroleptics and correlation to dopamine receptor blockade. ActaPharmacolToxicol 51: 321-329.
- Garofalo P, Colombo S, Lanza M, Revel L, Makovec F (1996) CR 2249: a new putative memory enhancer. Behavioural studies on learning and memory in rats and mice. J Pharm Pharmacol 48: 1290-1297.
- Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86: 271-278.
- Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V (2001) Method for the measurement of antioxidant activity in human fluids. J ClinPathol 54: 356-361.
- Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazinemethosulfate and molecular oxygen. BiochemBiophys Res Commun 46: 849-854.
- Bancroft JD, Gamble M (2008) Theory and Practice of Histological Techniques. Churchill Livingstone, Elsevier, China.
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, et al. (1991) Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30: 572-580.
- Thippeswamy AH, Rafiq M, Viswantha GL, Kavya KJ, Anturlikar SD, et al. (2013) Evaluation of Bacopamonniera for its synergistic activity with rivastigmine in reversing aluminum-induced memory loss and learning deficit in rats. J Acupunct Meridian Stud 6: 208-213.
- LeBel CP, Bondy SC (1991) Oxygen radicals: common mediators of neurotoxicity. NeurotoxicolTeratol 13: 341-346.
- Donald JM, Golub MS, Gershwin ME, Keen CL (1989) Neurobehavioral effects in offspring of mice given excess aluminum in diet during gestation and lactation. NeurotoxicolTeratol 11: 345-351.
- Garcez ML, Falchetti AC, Mina F, Budni J (2015) Alzheimer's Disease associated with Psychiatric Comorbidities. An Acad Bras Cienc 87: 1461-1473.
- Steffens DC, Fisher GG, Langa KM, Potter GG, Plassman BL (2009) Prevalence of depression among older Americans: the Aging, Demographics and Memory Study. IntPsychogeriatr 21: 879-888.
- Contador-Castillo I, Fernández-Calvo B, Cacho-Gutiérrez LJ, Ramos-Campos F, Hernández-Martín L (2009) [Depression in Alzheimer type-dementia: is there any effect on memory performance]. Rev Neurol 49: 505-510.
- Julka D, Vasishta RK, Gill KD (1996) Distribution of aluminum in different brain regions and body organs of rat. Biol Trace Elem Res 52: 181-192.
- Yun HM, Park KR, Kim EC, Kim S, Hong JT (2015) Serotonin 6 receptor controls alzheimer's disease and depression. Oncotarget 6: 26716-26728.
- Sethi P, Jyoti A, Singh R, Hussain E, Sharma D (2008) Aluminium-induced electrophysiological, biochemical and cognitive modifications in the hippocampus of aging rats. Neurotoxicology 29: 1069-1079.
- Sreekumaran E, Ramakrishna T, Madhav TR, Anandh D, Prabhu BM, et al. (2003) Loss of dendritic connectivity in CA1, CA2, and CA3 neurons in hippocampus in rat under aluminum toxicity: antidotal effect of pyridoxine. Brain Res Bull 59: 421-427.
- Ferre S, Ciruela F, Borycz J, Solinas M, Quarta D, et al. (2008) Adenosine A1-A2A receptor heteromers: new targets for caffeine in the brain. Front Biosci 13: 2391-2399.
- Riedel WJ, Jolles J (1996) Cognition enhancers in age-related cognitive decline. Drugs Aging 8: 245-274.
- Angelucci ME, Vital MA, Cesário C, Zadusky CR, Rosalen PL, et al. (1999) The effect of caffeine in animal models of learning and memory. Eur J Pharmacol 373: 135-140.
- Corodimas KP, Pruitt JC, Stieg JM (2000) Acute exposure to caffeine selectively disrupts context conditioning in rats. Psychopharmacology (Berl) 152: 376-382.
- Acquas E, Tanda G, Di Chiara G (2002) Differential effects of caffeine on dopamine and acetylcholine transmission in brain areas of drug-naive and caffeine-pretreated rats. Neuropsychopharmacology 27: 182-193.
- Dall'Igna OP, Fett P, Gomes MW, Souza DO, Cunha RA, et al. (2007) Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25-35)-induced cognitive deficits in mice. ExpNeurol 203: 241-245.
- Dall'Igna OP, Porciúncula LO, Souza DO, Cunha RA, Lara DR (2003) Neuroprotection by caffeine and adenosine A2A receptor blockade of beta-amyloid neurotoxicity. Br J Pharmacol 138: 1207-1209.
- Aleisa AM, Alzoubi KH, Alkadhi KA (2006) Nicotine prevents stress-induced enhancement of long-term depression in hippocampal area CA1: electrophysiological and molecular studies. J Neurosci Res 83: 309-317.
- White HK, Levin ED (2004)Chronic transdermal nicotine patch treatment effects on cognitive performance in age-associated memory impairment. Psychopharmacology (Berl.) 171: 465-471.
- Vallejo YF, Buisson B, Bertrand D, Green WN (2005) Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. J Neurosci 25: 5563-5572.
- Soto-Otero R, Méndez-Alvarez E, Hermida-Ameijeiras A, López-Real AM, Labandeira-García JL (2002) Effects of (-)-nicotine and (-)-cotinine on 6-hydroxydopamine-induced oxidative stress and neurotoxicity: relevance for Parkinson's disease. BiochemPharmacol 64: 125-135.
- Mudo G, Belluardo N, Fuxe K (2007) Nicotinic receptor agonists as neuroprotective/neurotrophic drugs. Progress in molecular mechanisms. J Neural Transm (Vienna) 114: 135-147.
- Takada-Takatori Y, Kume T, Sugimoto M, Katsuki H, Sugimoto H, et al. (2006) Acetylcholinesterase inhibitors used in treatment of Alzheimer's disease prevent glutamate neurotoxicity via nicotinic acetylcholine receptors and phosphatidylinositol 3-kinase cascade. Neuropharmacology 51: 474-486.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 17071
- [From(publication date):
June-2016 - Jul 15, 2025] - Breakdown by view type
- HTML page views : 15764
- PDF downloads : 1307