Review Article Open Access
The Pharmacology of Acute Respiratory Distress Syndrome
| Andrea Chamberlain1 and Brian M Varisco2* | |
| 1Division of Pharmacy, Cincinnati Children’s Hospital Medical Center, USA | |
| 2Division of Pediatric Critical Care Medicine, Cincinnati Children’s Hospital Medical Center, USA | |
| Corresponding Author : | Brian M Varisco, MD Assistant Professor Division of Critical Care Medicine Cincinnati Children's Hospital Medical Center 3333 Burnet Avenue, Cincinnati, OH 45229-3039, USA Tel: 513-803-2485 Fax: 513-803-3311 E-mail: Brian.Varisco@cchmc.org |
| Received August 26, 2014; Accepted September 18, 2014; Published September 23, 2014 | |
| Citation: Chamberlain A, Varisco BM (2014) The Pharmacology of Acute Respiratory Distress Syndrome. Clin Pharmacol Biopharm 3:120. doi:10.4172/2167-065X.1000120 | |
| Copyright: © 2014 Chamberlain A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Acute Respiratory Distress Syndrome (ARDS) is a common respiratory complication of critical severe illness or injury. Despite steady improvement in outcomes over the last three decades, ARDS remains a common cause of morbidity and mortality in pediatric and adult intensive care units. Due to its extensive burden, ARDS has been the focus of numerous multi-center clinical trials which have attempted to translate animal, ex vivo, and small single center studies into wider practice. Since the results of several of these large studies have been recently published, we sought to review the pharmacology of ARDS, summarize the major non-pharmacologic interventions shown to improve outcome, and update the reader on evolving therapies which may enter clinical practice in the coming decade.
| Keywords |
| Acute lung injury; Acute respiratory distress syndrome; Critical care |
| Abbreviations |
| ALI: Acute Lung Injury; ARDS: Acute Respiratory Distress Syndrome; ICU: Intensive Care Unit; iNO: Inhaled Nitric Oxide |
| Introduction |
| Acute lung injury (ALI) and the more severe acute respiratory distress syndrome (ARDS) are common complications in intensive care units (ICUs) being present in approximately 40% of admissions [1]. Although there is wide variability in reported mortality rates in the literature, the most recent comprehensive assessment determined that the average mortality from ARDS is approximately 40% [2]. Due to its high economic and societal burden, many pharmacologic and nonpharmacologic trials have been undertaken to reduce its associated morbidity and mortality. Due to the recent publication of several large trials focused on new pharmacologic therapies for ARDS, we sought to provide an updated guide for intensive care physicians and pharmacists. |
| What are Acute Lung Injury and Acute Respiratory Distress Syndrome? |
| Acute lung injury (ALI) is a syndrome of acute-onset hypoxemia and non-cardiogenic pulmonary edema in the setting of critical illness or injury. Acute respiratory distress syndrome (ARDS) is a more severe form of ALI. Despite a progressive decline in mortality rate reported in observational trials [3], ARDS is still associated with significant mortality in adult and pediatric intensive care units. |
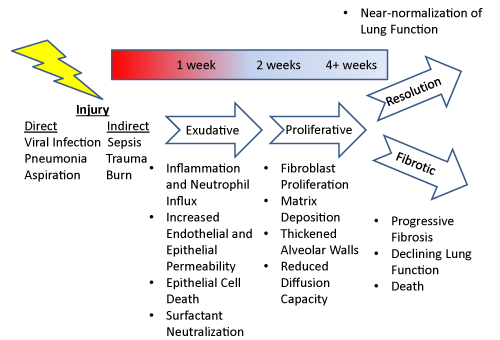
| Traditionally, ARDS has been separated into three stages (Figure 1). In the exudative phase, reduced capillary and lung epithelial cell integrity permits the influx of protein-rich fluid into the alveolar space leading to surfactant neutralization [4-6]. In this phase inflammatory cells potentiate lung injury by release of inflammatory mediators [7,8]. In the proliferative phase, proliferation of lung interstitial cells thickens airspace walls and reduces diffusion capacity [9,10]. The proliferative stage can be followed either by resolution with near-normalization of pulmonary function [11] or progression to the third stage--the fibrotic stage. In this stage, lung interstitial cells synthesis increasing amounts of extracellular matrix leading to a progressive decline in lung function and often death [12]. In reality, there is substantial overlap of these three stages making it impossible to clearly define which is predominant in any given patient. This heterogeneity of disease phenotype likely accounts for why therapies targeted to specific aspects of the disease such as surfactant dysfunction, oxidative injury, or inflammation have shown limited effectiveness in large clinical trials. |
| How are ALI and ARDS Diagnosed? |
| ARDS was first described by Ashbaugh et al. in 1967 as syndrome of acute onset hypoxemia with bilateral chest infiltrates [13], but a commonly accepted definition of ALI and ARDS was not available until 1994 with the American-European Consensus Conference definition [14]. The AECC definition required: |
| 1. Acute onset hypoxemia with an arterial partial pressure of oxygen (PaO2) to fraction of inspired oxygen (FiO2) ratio of ≤ 300 (ALI) or ≤ 200 (ARDS). |
| 2. Bilateral lung infiltrates on chest X-ray. |
| 3. Absence of left atrial hypertension as evidenced by a pulmonary capillary wedge pressure of ≤ 18 cm mm Hg. |
| The AECC report further described ALI and ARDS as either direct (e.g. pneumonia, inhalational injury, or aspiration) or indirect (e.g. burns, trauma, sepsis). |
| In response to criticisms regarding the invasiveness of left arterial atrial pressure measurements, the failure to account for positive end expiratory pressure (PEEP), and a high sensitivity but low specificity compared to autopsy findings [15], in 2012 the European Society of Intensive Care Medicine created the Berlin Definition of ARDS [16] which eliminated the term ALI, incorporated PEEP, and allowed for exclusion of cardiogenic pulmonary edema on less stringent grounds. The Berlin Definition of ARDS required: |
| 1. Onset of hypoxemia within one week of insult. |
| 2. Bilateral chest opacities not explained by lung collapse, effusion or nodules. |
| 3. Pulmonary edema not fully explained by fluid overload or cardiac failure. |
| 4. Mild, Moderate and Severe classification with. |
| a. Mild: PaO2/FiO2 200-299 with PEEP ≥ 5 cm H2O. |
| b. Moderate: PaO2/FiO2 100-199 with PEEP ≥ 5 cm H2O. |
| c. Severe: PaO2/FiO2<100 with PEEP ≥ 5 cm H2O. |
| The Berlin Criteria provide a more accurate classification scheme which will be useful in future clinical trials. All of the clinical trials described in this review utilized the AECC criteria. In evaluating each study, clinicians should be cognizant of the study group and consider whether treatment effect could have been masked by group heterogeneity. |
| What are the Non-Pharmacologic Therapies for ALI and ARDS? |
| Arguably, the improvement in ARDS outcomes over the past three decades can be principally attributed to improved treatments for the triggers of ARDS and the development of strategies to minimize ventilator induced lung injury [17,18]. |
| Methods aimed at minimizing ventilator-induced lung injury are termed the “Lung Protective Strategy.” In this strategy, PEEP is used to maintain alveolar recruitment (keep the alveoli open) while peak inspiratory pressures are limited by using smaller tidal volumes and tolerating elevated arterial carbon dioxide levels (termed permissive hypercapnia). One of the few positive trials in ARDS demonstrated that 6 mL/kg tidal volume reduced ARDS mortality by 9% compared to 12 mL/kg tidal volumes [19]. Use of higher levels of PEEP or oscillatory ventilation improved oxygenation [20-22] but did not improve outcome with a trend towards increased mortality in adult patients randomized to oscillatory ventilation [21]. Likewise, both prone positioning and lung recruitment maneuvers (a temporary increase in airway pressure to open atelectatic alveoli) improved oxygenation but not ventilator days or mortality [23-25]. The fluid and catheters treatment trial (FACTT) demonstrated that a restrictive fluid strategy reduced ventilator days but not mortality compared to a more liberal fluid management strategy [26]. Extracorporeal membranous oxygenation (ECMO) was shown to improve mortality in an adult trial where ARDS patients were randomized to conventional management or transport to a central ECMO center; however, there was no difference in ECMO and control groups within that referral center [27]. |
| In summary, the only proven non-pharmacologic therapies for ARDS are the use of low tidal volume ventilation and a restrictive fluid management strategy. |
| What Local-Acting Pharmacologic Therapies Are Available for ARDS? |
| Surfactant Replacement Therapy |
| Acute lung injury leads to surfactant neutralization and reduced lung compliance. The surfactant recovered from BAL fluid in patients with ARDS demonstrates alterations in both the phospholipids and protein profiles which reduce its ability to decrease surface tension [28]. Exogenous surfactant has a long history and documented benefits for use in neonates with surfactant deficiency secondary to prematurity [29], and numerous animal trials demonstrating improvement in induced lung injury models led to a series of randomized trials in adult and pediatric ARDS. |
| Despite promising initial studies involving small numbers of patients, large randomized clinical trials of surfactant replacement therapy in adult ARDS have been negative. The pooled data of two multicenter, randomized, double-blind trials performed in North America, South Africa, and Europe (448 patients) with ARDS found that surfactant protein c-enriched recombinant surfactant did not improve survival or increase ventilator-free days. However, the authors observed an improvement in gas exchange during the 24 hour treatment period [30]. The use of nebulized synthetic surfactant in a multicenter, prospective, randomized, double-blind, placebo controlled study of 725 adults with sepsis-associated ARDS demonstrated no improvement in oxygenation, 30 day survival, length of ICU stay, or ventilatorfree days [31]. The most recent adult surfactant trial randomized 418 ARDS patients to placebo or endotracheal administration of a porcinederived surfactant and demonstrated trend towards worse outcomes in adults receiving surfactant [32]. |
| The role of surfactant replacement in pediatric ARDS has recently become clear. While preliminary trials suggested benefit of surfactant replacement in pediatric ARDS due to direct lung injury, a recent larger trial failed to show benefit. In 1999, Willson et al. [33] performed a multi-center, randomized, and controlled trial that included 42 pediatric patients that were given Calfactant® at a dose of 80 ml/m2 endotracheally for the treatment of acute hypoxic respiratory failure. They observed improvement in oxygenation, reduced ventilator days, and decreased length of stay in the intensive care unit [33]. This study was included in a 2007 meta-analysis that included a total of 314 pediatric patients enrolled in six randomized controlled trials in which subjects were given surfactant for either ALI/ARDS and found a reduction in mortality (RR=0.7; 95% CI=0.4 to 0.97, P=0.04) and a 2.5 day increase in ventilator free days (95% CI=0.3 to 4.6 days; P=0.02) [34]. However, a recent randomized, blinded, placebo-controlled trial performed in 24 children’s hospitals in six different countries was terminated early for futility after interim analysis of the data collected from 110 patients was evaluated. The trial included children from 37 weeks post-conceptual age to 18 years with acute lung injury or acute respiratory distress syndrome due to direct lung injury. Subjects were given up to three doses of 30 mg/cm of height of Calfactant® or placebo within 48 hours of intubation. At the second interim analysis, the only significant difference between the groups was hospital-free days (10.4 in treatment vs. 6.4 in surfactant; p=0.01). There was no immediate difference in oxygenation and mortality did not differ between groups [35]. |
| Although surfactant replacement therapy clearly has a role in the care of neonates with respiratory distress syndrome, it does not have a role for routine use in pediatric and adult patients with ARDS. This difference is likely due to the surfactant neutralization of ARDS compared to the surfactant deficiency of neonatal respiratory distress syndrome. |
| Inhaled Beta-2 Agonists |
| In patients with acute lung injury and the acute respiratory distress syndrome, lung endothelial cell damage and inflammation increase vascular permeability leading to pulmonary edema. Treatment with β2-agonists may increase the rate of clearance of pulmonary edema by increasing cyclic-AMP which increases chloride and sodium transport and improves the reabsorption of alveolar fluid. This effect has been well documented in in animals and human lung explants [36]. |
| Despite promising animal and single-center human studied, β2- agonist therapy was not shown to be beneficial in two randomized trials. The first was a multicenter, placebo-controlled, parallel-group, randomized trial of intubated and mechanically ventilated adult patients. Within 72 hours of ARDS onset, the subjects were treated with intravenous salbutamol 15 mcg/kg/hour (n=162) or placebo (n=164) for up to 7 days. Recruitment was stopped after the second interim analysis because of safety concerns. The investigators found that salbutamol increased 28 day mortality with increased rates of tachycardia and arrhythmia [37]. In 2011, a randomized, placebocontrolled trial evaluating the use of aerosolized albuterol for the treatment of acute lung injury that included 282 patients on mechanical ventilation found that aerosolized albuterol did not reduce ventilator free days or improve mortality. The authors did note an increase in ventilator free days in patients who presented with shock, but there was no change in mortality rate in this subset of patients [38]. |
| Despite the promising data observed in animal and ex vivo lung models, β2-agonists have not shown benefit in the treatment of ALI and ARDS in clinical trials. However, there may be a subset of patients, including those with a history of airway reactivity and those presenting in shock, who may benefit from this therapy. Furthermore, efficacy in a younger patient population without the cardiovascular risks of adult ICU patients has not been evaluated. |
| Inhaled Nitric Oxide |
| Inflammation and disruption of the alveolar-capillary membrane results in ventilation and perfusion mismatch in the setting of ARDS. Inhaled nitric oxide selectively vasodilates vascular smooth muscle in ventilated areas of the lung to help redistribute blood flow away from areas of low ventilation and therefore decrease mismatching [39]. |
| Case reports and small trials have found that iNO improved oxygenation and pulmonary vascular resistance in patients with acute lung injury. However, no differences in mortality or duration of mechanical ventilation were demonstrated in studies of large numbers of patients. In a meta-analysis of 1,237 patients with ALI or ARDS from 12 trials receiving iNO between 1 and 40 ppm, investigators found no significant reduction in mortality, duration of ventilation, or ventilator-free days. Interestingly the authors did not find a reduction in pulmonary arterial pressure. However, iNO did improve oxygenation [40]. A systematic review of five randomized, controlled trials of inhaled nitric oxide vs placebo for acute hypoxemic respiratory failure (including ALI, ARDS, and other diagnoses) in adults and children concluded that inhaled nitric oxide improved oxygenation for up to 72 hours but had no effect on mortality or the duration of mechanical ventilation [41]. |
| The current body of evidence does not support the use of inhaled nitric oxide as standard therapy for the treatment of ALI or ARDS. However, it can improve oxygenation and reduce pulmonary arterial pressure and may be beneficial in patients with severe hypoxemia or concurrent pulmonary hypertension. |
| What Systemic Pharmacologic Therapies are Available for ALI? |
| Corticosteroids |
| Corticosteroids have multiple mechanisms by which they can slow the inflammatory cascade associated with ALI and ARDS. By binding to the glucocorticoid receptor, corticosteroids suppress gene transcription of pro-inflammatory cytokines, enhance expression of anti-inflammatory proteins, and inhibit neutrophil activation and adhesion to endothelial cells [42]. Early studies utilized corticosteroids in an attempt to prevent progression of ALI to ARDS and potentially reverse the inflammatory damage to prevent fibrosis. |
| The results of clinical trials evaluating the use of corticosteroids have had mixed results. Several studies have concluded that corticosteroids reduce the duration of mechanical ventilation but have no positive effect on mortality or prevention of ARDS, and other trials have shown improvement in survival and reduction in the duration of mechanical ventilation. |
| A randomized, double-blind, placebo controlled trial of 90 patients by Meduri et al. [43] evaluated the administration of methylprednisolone within 72 hours of the onset of ARDS for up to 28 days of therapy and found a significant reduction in inflammatory markers, improvement in lung injury, reduction in duration of mechanical ventilation, ICU stay, and ICU mortality. There was no increased rate of infection in the treatment arm. The steroid protocol used was a loading dose of 1 mg/kg followed by 1 mg/kg/day from day 1 to day 14, 0.5 mg/kg/day from day 15 to day 21, 0.25 mg/kg/day from day 22 to day 25, and 0.125 mg/kg/day from day 26 to day 28. The taper was advanced to 0.5 mg/kg/day if the patient was successfully extubated within the first 14 days of therapy [43]. A randomized, double-blind, placebo controlled trial performed in 180 patients with ARDS diagnosed for at least 7 days compared 21 days of methylprednisolone to placebo and found no 60 day mortality benefit. This study utilized a similar treatment protocol to that used in the Meduri trial. In patients who were started on steroids on day 14 or later of illness, there was an increase in mortality. However, methylprednisolone did increase ventilator-free days and improved oxygenation, lung compliance, and blood pressure. They found no increase in infection rates, but steroids were associated with a higher rate of neuromuscular weakness [44]. However, a secondary analysis of the 60 day survivors of this study found no association with methylprednisolone and the incidence of neuromuscular weakness [45]. A systematic review and meta-analysis that included five cohort studies and four randomized, controlled trials for a total of 648 total patients showed a trend toward mortality reduction. The review also noted improvement in length of ventilatorfree days, length of ICU stay, multiple organ dysfunction scores, lung injury scores, and improvement in oxygenation. The authors found no increase in infection or neuromuscular weakness. The authors did note significant heterogeneity between the studies which is important when interpreting the results of meta-analysis especially those including a small number of trials [46]. |
| The routine use of corticosteroids in patients with persistent acute respiratory distress remains controversial. If corticosteroids are initiated, it may be most beneficial to do so before day 14 of ARDS onset. Other medications that increase the risk of neuromuscular weakness, such as neuromuscular blockers, should be avoided if possible in patients on prolonged course of corticosteroids. Steroids should also be used with caution in patients confirmed or presumed infection. |
| Neuromuscular Blockade |
| The principal rationale for the use of neuromuscular blockers in patients with ALI and ARDS is to facilitate mechanical ventilation and reduce asynchrony with the ventilator. In general, as the severity of ARDS increases the use of neuromuscular blockers tends to increase [47]. |
| A randomized, controlled, multicenter trial that included 56 adult ICU patients with ARDS compared 48 hours of neuromuscular blockers to placebo and found a higher PaO2/FiO2 ratio that was sustained for up to 120 hours after randomization. The investigators also found a reduction in ventilator support requirements and concluded that NMBs are associated with a sustained improvement in oxygenation in patients with ARDS [48]. These results were replicated in a larger randomized by Papazain et al. [49] in a double blind, multicenter trial that included 340 adult patients within 48 hours of onset of severe ARDS. The investigators randomly assigned patients to cisatracurium or placebo for 48 hours. They found a decrease in 90 day mortality after adjusting for baseline severity of lung injury, and there was a statistically significant decrease in 28 day mortality in the NMB group. They found no difference in ICU-acquired neuromuscular weakness [49]. |
| Neuromuscular blockers should be considered to facilitate mechanical ventilation and reduce asynchrony with mechanical ventilation. The majority of trials report use for only 48 hours early in the course of ARDS. The safety and sustained benefit has not been fully investigated, so their use for extended periods may cause increased long term complications especially if used with corticosteroids or aminoglycoside antibiotics [50,51]. |
| Statins |
| HMG CoA-reductase inhibitors are known to modulate the inflammatory response [52,53] and are associated with reduced ICU mortality in patients with infective endocarditis [54] and in elderly patients admitted to the ICU [55]. The ARDSnet investigators evaluated whether rosuvastatin therapy improved clinical outcomes in critically ill patients with sepsis-associated ARDS. This was a randomized, placebo-controlled, double-blind, multicenter trial in which patients either received enteral rosuvastatin 40 mg once followed by 20 mg daily or placebo. The study was stopped for futility after 745 patients were enrolled. They found no significant difference in 60 day mortality or ventilator-free days. Rosuvastatin therapy was associated with increased renal failure and hepatic failure [8]. Despite the antiinflammatory effects of HMG CoA-reductase inhibitors, they are not associated with improved outcomes in patients with sepsis mediated ARDS and may increase the incidence of renal and hepatic failure. |
| What Pharmaconutrition Strategies are Available in ARDS? |
| Omega-3 Fatty Acid Supplementation |
| The omega-3 fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) may increase the production of antiinflammatory prostaglandins and leukotrienes [56]. Patients with ALI have lower than normal levels of omega-3 fatty acids [57]. |
| The OMEGA study randomized 272 adults from 44 hospitals who were within 48 hours of onset of ALI requiring mechanical ventilation that were starting on enteral nutrition to either receive omega-3 fatty acids, gamma-linolenic acid, and antioxidant supplementation or placebo in a double blind fashion. The study was stopped early for futility after 143 were enrolled in the treatment arm and 129 were randomized to placebo. The investigators found a significant increase in plasma levels of EPA, but subjects in the treatment arm required longer mechanical ventilation and ICU stay, had more non-pulmonary organ failure, and resulted in more days with diarrhea. There was no difference in adjusted 60 day mortality [58]. Enteral supplementation with omega-3 fatty acids, gamma-linolenic acid, and antioxidants cannot be routinely recommended for patients with ARDS. |
| What Future Pharmacologic Therapies May Become Available for ARDS? |
| Current therapies and strategies for ARDS largely focus on mitigating ongoing injury and slowing the inflammatory cascade. Emerging therapies focus on improving endothelial and epithelial integrity and using cell-based therapies to reverse lung inflammation. In animals [59] and human lung explants [60] keratinocyte growth factor (KGF) improves epithelial cell survival and reduces the accumulation of alveolar fluid in experimental lung injury. Other therapies aimed at improving endothelial or epithelial barrier integrity have shown promise in animals but have not been tested in human systems [61]. Bone marrow stromal cells that adhere to plastic surfaces after bone marrow aspirate and are often termed mesenchymal stem cells (MSCs). MSCs have shown promise in attenuating injury after myocardial infarction [62] and have been shown to attenuate lung injury in animal models [63-65]. MSCs are currently in Phase 1 trial in adults with ARDS (NCT01775774). Continued improvement in ARDS outcomes will depend upon developing novel therapies targeted to central pathogenic pathways and targeting patient populations more precisely so as to target therapies to sub-populations of ARDS patients most likely to benefit. |
| Conclusions |
| ARDS continues to be associated with high rates of morbidity and mortality in both adult and pediatric intensive care units. To date, the only therapies proven to improve outcomes are low tidal volume ventilation, a conservative fluid administration strategy, initiation of corticosteroid therapy before day 14 of illness, and early, limited use of neuromuscular blockade. A host of therapies have been shown to improve oxygenation but not patient outcomes. |
| How can therapies which significantly impact a meaningful surrogate outcome such as oxygenation not reduce ventilator free days or mortality? As the developers of the Berlin criteria point out, careful selection of a target population is essential to demonstrating the effect of an intervention [16]. Interventions will be unable to demonstrate a meaningful effect in less sick patients, and in extremely ill patient any intervention will fail to alter the eventual outcome. Given the heterogeneity of ARDS, perhaps it is not surprising that many of the therapies found beneficial in small trials failed to show benefit when tested in larger more heterogeneous populations. In any given patient, the extensive literature available on therapies for ARDS can only inform clinical decision making; it cannot dictate which decisions are right or wrong. |
| References |
|
References
- Linko R, Okkonen M, Pettilä V, Perttilä J, Parviainen I, et al. (2009) Acute respiratory failure in intensive care units. FINNALI: a prospective cohort study. Intensive Care Med 35: 1352-1361.
- Zambon M, Vincent JL (2008) Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 133: 1120-1127.
- Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, et al. (2009) Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review. Am J Respir Crit Care Med 179: 220-227.
- Sarker M, Rose J, McDonald M, Morrow MR, Booth V (2010) Modifications to Surfactant Protein B Structure and Lipid Interactions under Respiratory Distress Conditions: Consequences of Tryptophan Oxidation. Biochemistry 50: 25-36.
- Zasadzinski JA, Stenger PC, Shieh I, Dhar P (2010) Overcoming rapid inactivation of lung surfactant: analogies between competitive adsorption and colloid stability. Biochim Biophys Acta 1798: 801-828.
- Markart P, Ruppert C, Wygrecka M, Colaris T, Dahal B, et al. (2007) Patients with ARDS show improvement but not normalisation of alveolar surface activity with surfactant treatment: putative role of neutral lipids. Thorax 62: 588-594.
- Bijli KM, Kanter BG, Minhajuddin M, Leonard A, Xu L, et al. (2014) Regulation of Endothelial Cell Inflammation and Lung PMN Infiltration by Transglutaminase 2. Shock .
- National Heart, Lung, and Blood Institute, ARDS Clinical Trails Network, Truwit JD, Bernard GR, Steingrub J, et al. (2014) Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med 370: 2191-2200.
- Kang D, Nakayama T, Togashi M, Yamamoto M, Takahashi M, et al. (2009) Two forms of diffuse alveolar damage in the lungs of patients with acute respiratory distress syndrome. Hum Pathol 40: 1618-1627.
- Synenki L, Chandel NS, Budinger GR, Donnelly HK, Topin J, et al. (2007) Bronchoalveolar lavage fluid from patients with acute lung injury/acute respiratory distress syndrome induces myofibroblast differentiation. Crit Care Med 35: 842-848.
- Peters JI, Bell RC, Prihoda TJ, Harris G, Andrews C, et al. (1989) Clinical determinants of abnormalities in pulmonary functions in survivors of the adult respiratory distress syndrome. Am Rev Respir Dis 139: 1163-1168.
- [No authors listed] (2000) American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 161: 646-664.
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE (1967) Acute respiratory distress in adults. Lancet 2: 319-323.
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, et al. (1994) Report of the American-European Consensus conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Consensus Committee. J Crit Care 9: 72-81.
- Esteban A, Fernández-Segoviano P, Frutos-Vivar F, Aramburu JA, Nájera L, et al. (2004) Comparison of clinical criteria for the acute respiratory distress syndrome with autopsy findings. Ann Intern Med 141: 440-445.
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, et al. (2012) Acute respiratory distress syndrome: the Berlin Definition. JAMA 307: 2526-2533.
- Adhikari N, Burns KE, Meade MO (2004) Pharmacologic treatments for acute respiratory distress syndrome and acute lung injury: systematic review and meta-analysis. Treat Respir Med 3: 307-328.
- Slutsky AS, Ranieri VM (2000) Mechanical ventilation: lessons from the ARDSNet trial. Respir Res 1: 73-77.
- Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, et al. (2004) Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 351: 327-336.
- Santa Cruz R, Rojas JI, Nervi R, Heredia R, Ciapponi A (2013) High versus low positive end-expiratory pressure (PEEP) levels for mechanically ventilated adult patients with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 6: CD009098.
- Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, et al. (2013) High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 368: 795-805.
- Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, et al. (2013) High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 368: 806-813.
- Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, et al. (2008) Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 299: 637-645.
- Curley MA, Hibberd PL, Fineman LD, Wypij D, Shih MC, et al. (2005) Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA 294: 229-237.
- Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, et al. (2001) Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 345: 568-573.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, et al. (2006) Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354: 2564-2575.
- Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, et al. (2009) Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 374: 1351-1363.
- Günther A, Ruppert C, Schmidt R, Markart P, Grimminger F, et al. (2001) Surfactant alteration and replacement in acute respiratory distress syndrome. Respir Res 2: 353-364.
- Seger N, Soll R (2009) Animal derived surfactant extract for treatment of respiratory distress syndrome. Cochrane Database Syst Rev CD007836.
- Spragg RG, Lewis JF, Walmrath HD, Johannigman J, Bellingan G, et al. (2004) Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med 351: 884-892.
- Anzueto A, Baughman RP, Guntupalli KK, Weg JG, Wiedemann HP, et al. (1996) Aerosolized surfactant in adults with sepsis-induced acute respiratory distress syndrome. Exosurf Acute Respiratory Distress Syndrome Sepsis Study Group. N Engl J Med 334: 1417-1421.
- Kesecioglu J, Beale R, Stewart TE, Findlay GP, Rouby JJ, et al. (2009) Exogenous natural surfactant for treatment of acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 180: 989-994.
- Willson DF, Zaritsky A, Bauman LA, Dockery K, James RL, et al. (1999) Instillation of calf lung surfactant extract (calfactant) is beneficial in pediatric acute hypoxemic respiratory failure. Members of the Mid-Atlantic Pediatric Critical Care Network. Crit Care Med 27: 188-195.
- Duffett M, Choong K, Ng V, Randolph A, Cook DJ (2007) Surfactant therapy for acute respiratory failure in children: a systematic review and meta-analysis. Crit Care 11: R66.
- Willson DF, Thomas NJ, Tamburro R, Truemper E, Truwit J, et al. (2013) Pediatric calfactant in acute respiratory distress syndrome trial. Pediatr Crit Care Med 14: 657-665.
- Sakuma T, Folkesson HG, Suzuki S, Okaniwa G, Fujimura S, et al. (1997) Beta-adrenergic agonist stimulated alveolar fluid clearance in ex vivo human and rat lungs. Am J Respir Crit Care Med 155: 506-512.
- Gao Smith F, Perkins GD, Gates S, Young D, McAuley DF, et al. (2012) Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet 379: 229-235.
- National Heart L. Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network, Matthay MA, Brower RG, Carson S, Douglas IS, et al. (2011) Randomized, placebo-controlled clinical trial of an aerosolized beta(2)-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 184: 561-568.
- Adhikari N, Granton JT (2004) Inhaled nitric oxide for acute lung injury: no place for NO? JAMA 291: 1629-1631.
- Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, et al. (2007) Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ 334: 779.
- Sokol J, Jacobs SE, Bohn D (2003) Inhaled nitric oxide for acute hypoxic respiratory failure in children and adults: a meta-analysis. Anesth Analg 97: 989-998.
- Barnes PJ, Adcock I (1993) Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol Sci 14: 436-441.
- Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, et al. (2007) Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest 131: 954-963.
- Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, et al. (2006) Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 354: 1671-1684.
- Hough CL, Steinberg KP, Taylor Thompson B, Rubenfeld GD, Hudson LD (2009) Intensive care unit-acquired neuromyopathy and corticosteroids in survivors of persistent ARDS. Intensive Care Med 35: 63-68.
- Tang BM, Craig JC, Eslick GD, Seppelt I, McLean AS (2009) Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med 37: 1594-1603.
- Arroliga AC, Thompson BT, Ancukiewicz M, Gonzales JP, Guntupalli KK, et al. (2008) Use of sedatives, opioids, and neuromuscular blocking agents in patients with acute lung injury and acute respiratory distress syndrome. Crit Care Med 36: 1083-1088.
- Gainnier M, Roch A, Forel JM, Thirion X, Arnal JM, et al. (2004) Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med 32: 113-119.
- Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, et al. (2010) Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 363: 1107-1116.
- Fischer JR, Baer RK (1996) Acute myopathy associated with combined use of corticosteroids and neuromuscular blocking agents. Ann Pharmacother 30: 1437-1445.
- Murray MJ, Cowen J, DeBlock H, Erstad B, Gray AW Jr, et al. (2002) Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med 30: 142-156.
- Lindholm MW, Nilsson J (2007) Simvastatin stimulates macrophage interleukin-1beta secretion through an isoprenylation-dependent mechanism. Vascul Pharmacol 46: 91-96.
- Watts KL, Sampson EM, Schultz GS, Spiteri MA (2005) Simvastatin inhibits growth factor expression and modulates profibrogenic markers in lung fibroblasts. Am J Respir Cell Mol Biol 32: 290-300.
- Yang TF, Chu H, Ou SM, Li SY, Chen YT, et al. (2014) Effect of statin therapy on mortality in patients with infective endocarditis. Am J Cardiol 114: 94-99.
- Al Harbi SA, Tamim HM, Arabi YM (2011) Association between statin therapy and outcomes in critically ill patients: a nested cohort study. BMC Clin Pharmacol 11: 12.
- Calder PC (2004) n-3 fatty acids, inflammation, and immunity--relevance to postsurgical and critically ill patients. Lipids 39: 1147-1161.
- Kumar KV, Rao SM, Gayani R, Mohan IK, Naidu MU (2000) Oxidant stress and essential fatty acids in patients with risk and established ARDS. Clin Chim Acta 298: 111-120.
- Rice TW, Wheeler AP, Thompson BT, deBoisblanc BP, Steingrub J, et al. (2011) Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA 306: 1574-1581.
- Chen J, Li C, Gao X, Li C, Liang Z, et al. (2013) Keratinocyte growth factor gene delivery via mesenchymal stem cells protects against lipopolysaccharide-induced acute lung injury in mice. PLoS One 8: e83303.
- Shyamsundar M, McAuley DF, Ingram RJ, Gibson DS, O'Kane D, et al. (2014) Keratinocyte growth factor promotes epithelial survival and resolution in a human model of lung injury. Am J Respir Crit Care Med 189: 1520-1529.
- Lucas R, Verin AD, Black SM, Catravas JD (2009) Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem Pharmacol 77: 1763-1772.
- Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, et al. (2009) A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 54: 2277-2286.
- Rojas M, Xu J, Woods CR, Mora AL, Spears W, et al. (2005) Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 33: 145-152.
- Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, et al. (2003) Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A 100: 8407-8411.
- Gupta N, Su X, Popov B, Lee JW, Serikov V, et al. (2007) Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 179: 1855-1863.
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 16324
- [From(publication date):
November-2014 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11703
- PDF downloads : 4621
