The Paradox of Ectopic Melanin Synthesis in Adipose: Potential Mechanism, Benefits and Perspectives in Abating Obesity Complications
Received: 16-Jan-2018 / Accepted Date: 22-Jan-2018 / Published Date: 25-Jan-2018 DOI: 10.4172/2165-7904.1000363
Abstract
Obesity and related non-communicable diseases (NCDs) are global health challenges prevalent worldwide. Intervention became imperative for creative natural measures to minimize NCDs. The chronic state of inflammation and oxidative stress are the land mark signatures for obesity driven NCDs. In this description study, we try to decipher and rationalize the new puzzle of ectopic melanin synthesis in adipose by formulating analytical view from extensive literature review. Oxidative stressed adipose triggers structural homeostasis program to initiate renovation of adipose tissue for self-healing. These processes are induced by rewiring interactions of signalling pathways for promoting adipocytes survival or replenishing them from their precursors. We postulate that melanogenesis and adipogenesis are co-driven by sharing interactive molecular signalling mechanisms. Cross-talk between melanogenesis and adipogenesis occurs through Wnt/β-catenin pathway and its interaction with Sox signalling molecules; through PPAR-γ and C/EBP-α. Activating these signalling pathways stimulate adipogenesis to relief the oxidative stress induced cell damages. Ectopic melanin synthesis and adipogenesis concurrently occur as adaptative response to hypertrophic induced oxidative stress and ROS as second messengers. Therefore, the activation of melanin synthesis probably is a natural preventive measure to slow down or minimizes hyperplasia and ameliorates consequent obesity complications. In conclusion; before we recommend using analogs of melanin inducers as therapeutic strategy, we advocate the use of the melanin intermediates as antioxidants or antiinflammatory agents in obesity research and therapy. This descriptive study is based on very limited amount of preliminary data and at times several levels of assumptions are made. The hypothesis of melanin production in adipose tissue having a role to prevent complications of obesity is unconventional and challenges current dogma.
Keywords: Adipose tissue; Obesity; Adipogenesis; Melanin; Antioxidant
Introduction
Melanogenesis is a biological process, active in specialized cells called melanocytes. “With the coordination of keratinocytes, they form 'melanin producing units.” Melanogenesis produces polymeric phenolic pigment called melanin in skin, hair, eyes, inner ear, bones, heart, and brain. The UV light absorption properties of melanin principally contribute in photo protection [1,2] thermoregulation and coloring [3]. Melanin is a strong cation chelator, anti-oxidant and free radical scavenger [4-6].
The Biochemical synthesis of melanin starts by oxidation of amino acid tyrosine catalyzed by tyrosinase and results in forming a blackbrown eumelanin and red-yellow pheomelanin [7]. The quantity and the quality of the melanin are controlled by a collection of enzymes mainly tyrosinase (TYR), tyrosine related protein 1 (TYRP 1) and tyrosine-related protein 2 (TYRP 2) [8,9]. The activity of these enzymes is controlled by complex signalling pathways. The main pathway is through cyclic adenosine monophosphate (cAMP) and microphthalmia-associated transcription factor (MITF) which is triggered by the action of α-MSH agonist on melanocortin 1 receptor (MC1R) in the membrane of melanocytes [10]. Genetic elements, hormonal and environmental factors interact intricately affecting over all the melanogenesis process.
Even though melanin can be present in all tissues of human body, it has been thought its synthesis is restricted to melanocytes and that MITF is a lineage specific marker. Interestingly, ectopic expression of MITF can convert 3T3 fibroblasts into cells with characteristics of melanocytes and some of these cells expressed melanogenic marker proteins [11]. Lately, ectopic melanin synthesis has been newly discovered in adipocytes of adipose tissue [12]. A significant overexpression of melanogenesis related genes in visceral fat of obese individuals was also reported [12,13]. However, among studied obese subjects, there was no significant correlation in melanogenic activity and the degree of obesity [13]. Page suggested that melanin could be used to halt oxidative stress and inflammation through its capability to scavenge reactive oxygen species (ROS) in adipose of obese tissue [13].
Page and Randhawa provided inadequate scientific explanations to many questions related to ectopic melanin synthesis [13]. Examples, what are the possible molecular mechanisms that initiates or activates melanogenesis in adipocytes, how could melanin abrogate or stop reactive oxygen species in adipose? When does oxidative stress and inflammation induce ectopic melanogenesis?
This is a description study that tries to illustrate the inflammatory and oxidative nature of adipose status in obesity and seeks to link inflammation and oxidative stress nature in obese adipose with biological mechanisms that could explain ectopic melanogenesis. Furthermore, in this review we intend to discuss the probable functions of ectopic melanin in obesity and critically evaluate the possible physiological role of ectopic melanin and its intermediates in ameliorating obesity complications.
Oxidative stress and inflammation are complementary and interconnected drivers of obesity complications
Obesity and its related complications such as insulin resistance, cardiovascular disease and non-alcoholic fatty liver disease (NAFLD) are prevalent worldwide and became a global health challenge. As a matter of fact, intervention became imperative but for new creative and scientific measures to minimize these non-communicable diseases (NCDs). The pathogenesis mechanisms of NCDs that stem from obesity are complex. However, the chronic state of inflammation and oxidative stress are interconnected but interdependent signatures for most obesity driven complications.
Redox state balance is intricate process but vital for the health of adipocytes and adipose tissues. Redox state imbalance leads to plethora of consequences that lay the ground for the final health status of the cell. Unrestrained oxidative stress mainly results from excess of reactive oxygen species (ROS). Oxidative stress usually is the end result of the dominance of endogenous and external pro-oxidant molecules over their counterpart antioxidants in the adipocyte cells in adipose tissue [14].
The plasma membrane, mitochondria, endoplasmic reticulum and peroxisomes are the primary natural sources of endogenous ROS production. Most of these ROS molecules are a result of biochemical reactions such as oxidative phosphorylation that is catalysed by enzymes such as NADPH oxidase, aldehyde oxidase, xanthine oxidase, and glucose oxidase. These enzymes are more active in people with obesity [15].
High activity of NADPH oxidase in hypertrophic adipocytes and infiltrated macrophages produces most of ROS in obese tissue [15]. Many antioxidant defences are higher in the normal weight subjects and their levels inversely correlate with central adiposity [16]. For example, gene expressions of antioxidant enzymes superoxide dismutase (SOD), glutathione peroxidase (GPX) , and catalase, are lower in white adipose tissue in obese mice [16].
Obese subjects with insulin resistant have reduction in the expression of antioxidant enzyme glutathione S-transferase alpha 4 (GST-A4) [17]. Even though adipose tissue is a favoured storage site for lipid-soluble endogenous antioxidants such as vitamins and carotenoids [18], it is probable that the food quality consumed by obese subjects is poor in antioxidants. Nevertheless, excess fat in adipose can also act as a sequester basin for vitamins and some antioxidants in adipocyte lipid droplets, therefore, limiting their bioavailability [19,20].
Oxidative stress can initiate obesity and drives its complications, but also can be an end result [21]. Excess of ROS in adipose tissue will lead to many damages starting by lipids peroxidation, DNA adducts formation, proteins carbonylation, inflammation of cells and tissues and even death. For example, excess of fat in adipocytes leads to increase of ROS in the hypertrophic adipocytes and ends up in pyroptosis and a proinflammatory programmed cell death [22,23]. Some of the hypertrophic adipocytes also undergo necrotic-like death and spill out many of their inflammatory molecules into the adipose extracellular spaces feeding the vicious cycle of oxidative stress.
Some of the most common oxidative stress biomarkers are endproducts of ROS and pro-oxidants mediated lipid peroxidation. Free radicals attack the methylene group next to the double bonds in polyunsaturated fatty acids (PUFAs) in lipids of the cell and the products of this process are aldehyde derivatives. The main two reactive products are malondialdehyde (MDA) and 4-hydroxy-2- nonenal (4-HNE) [24-26].
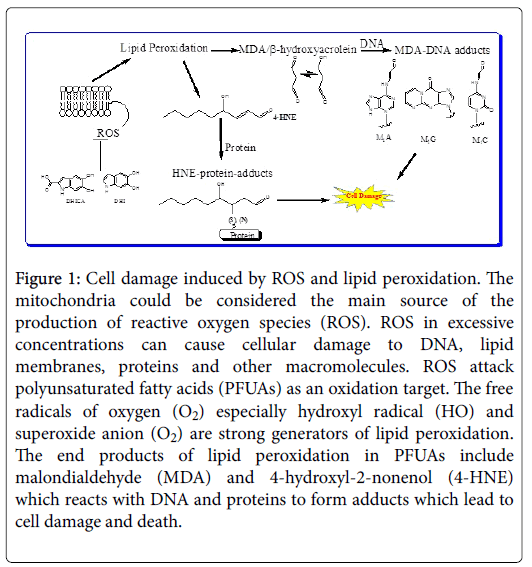
Both are important signalling molecules in stimulating some gene’s expression for cell survival. But they also have cytotoxic role in inhibiting other gene’s expression and promoting cell death (Figure 1).
Figure 1: Cell damage induced by ROS and lipid peroxidation. The mitochondria could be considered the main source of the production of reactive oxygen species (ROS). ROS in excessive concentrations can cause cellular damage to DNA, lipid membranes, proteins and other macromolecules. ROS attack polyunsaturated fatty acids (PFUAs) as an oxidation target. The free radicals of oxygen (O2) especially hydroxyl radical (HO) and superoxide anion (O2) are strong generators of lipid peroxidation. The end products of lipid peroxidation in PFUAs include malondialdehyde (MDA) and 4-hydroxyl-2-nonenol (4-HNE) which reacts with DNA and proteins to form adducts which lead to cell damage and death.
MDA appears to be the most mutagenic product of lipid peroxidation, whereas 4-HNE is the most toxic [27]. Lipids peroxidation of omega-6 fatty acids causes an increase of 4-HNE [28] which is more common complication in obese subjects than lean ones.
HNE leads to carbonylation of many proteins and enzymes such that involved in lipids and carbohydrates metabolism [29]. The rate of lipids peroxidation correlates with the amount of HNE produced and so with the degree of obesity [29]. HNE formation can lead to insulin resistance in adipocytes as it binds to insulin receptor substrate 1 and 2 (IRS-1/-2) generating HNE-IRS adducts impairing IRS function and favour their degradation [30]. For example, stressing adipose cells by exposing them to high concentration of glucose oxidase induces formation of lipid markers as 4-HNE and MDA [31].
This process caused a decrease in adiponectin secretion and an increase in lactate production; both are intermediate markers for progression to insulin resistance [31]. In addition, 4-HNE also stimulated cyclooxygenase-2 (COX-2) expression which directly linked to inflammation [30]. Adipocytes exposed to ROS increased gene expression of proinflammatory cytokines as plasminogen activator inhibitor-1 (PAI-1), Interleukin-6 (IL-6) and macrophage chemo attractive molecule (MCP-1) [16,32]. Increases of such molecules usually promote infiltration of adipose by pro-inflammatory macrophages.
In contrast, obese mice treated with antioxidants corrected and improve diabetes, hyperlipemia, and adipocytokine dysregulation [16]. Moreover, it has been shown that fatty aldehyde dehydrogenase (FALDH ), antioxidant enzyme; improved insulin resistance resulted in 3T3-L1 adipocyte that is driven by HNE exposure. In the same study, rosiglitazone showed to have an antioxidant effect by increasing FALDH gene expression which in turn blocks the poisonous effect of HNE on IRS-1 [33].
Prospects of boasting natural antioxidant defines compounds such as glutathione, melatonin, carotenoids, natural flavonoids, and vitamins, has been an appealing preventive strategies for obesity and minimizing its complications. From therapeutic perspective, we think that directly interfering with production of ROS in adipose is appealing but an intricate process. Because ROS are produced from many different sources in the body with different levels, and the type and quantity vary through obesity progression [34]. However, in this review we focus our discussion and analysis on using ectopic melanin synthesis in intervention and abating the oxidative stress and toxic effects in adipose tissue.
The significant over-expression of melanogenesis-related genes in visceral fat and in variant concentrations in obese subjects is puzzling in many ways. What trigger and activate melanogenesis in adipocytes may also influence adipogenesis, indicating that both processes share interactive molecular signalling mechanisms. ROS act as second messengers in adaptive responses to oxidative stress. Consequently melanin synthesis is also an adaptive response to this oxidative stress especially after hypertrophy of adipocytes. As melanogenesis development in the skin evolved as adaptive selection to counter the detrimental effect of UV radiations [35], ectopic melanin synthesis evolved as adaptive response to counter the effect of oxidative stress in adipose tissue.
Cross talk between melanogenesis and adipogenesis in adipose tissue
Both melanocytes and adipose cell; in addition to others; are generated upon development from neural crest cells (NCCs). It is probable that they share some of the signals that regulate their morphogenetic induction, migration, and fate determination. Some of their precursors’ stem-like cells reside in their specific tissues for regeneration at times of stress and insult for healing and regeneration. For example, adipocytes proliferating progenitors has been found to be residing in the mural cell compartment of the adipose vasculature [36] and melanoblasts in the neural crest.
It has been demonstrated that the expansion of adipose tissue in obesity is mainly characterized by hypertrophy, accompanied by oxidative stress and inflammatory environment influenced by disturbances in energy and lipids storage [37,38]. ROS produced from mitochondrial oxidative stress play an important role in regulating adipocyte differentiation of mesenchymal stem cells (MSCs) [39]. We believe that ROS induced adipocyte differentiation is an adaptative responses to hypertrophy stress. To relief hypertrophy and associated oxidative stress, adipose tissue attempt to reinstate metabolic homeostasis.
Adipose triggers structural homeostasis program to initiate renovation of adipose tissue for self-healing. Restructured homeostasis involves interaction of signalling pathways for promoting adipocytes survival or replenishing them from their precursors either from neural crest cells or from proliferating progenitors in the mural cell compartment of the adipose vasculature [40].
Another alternative is trans differentiation of MSCs and redirecting them from becoming neurogenic and/or chondrogenic to become adipogenic. Trans differentiation of MSCs could be accomplished by enrichment of a specific cocktail of transcription factors that are considered as key cell fate modulators. Another promising strategy comes from the plasticity potential of adipocytes which is reflected by its capability of dedifferentiation to adipo fibroblasts in vitro [41]. This characteristic gives adipofiroblast’s proliferation and multi potent capacities of the potential to repair many organs and tissues.
All mentioned processes, involve selectively activating many signalling pathways and molecules to stimulate adipogenesis to relief the oxidative stress induced cell damages. However, some of these signalling pathways and molecules also influence melanogenesis process in adipose. The activation of Wnt/β-catenin signalling is one of the first molecular responses to hypertrophy, cellular damage, and inflammation [37].
Wnt/β-catenin signalling and low-density lipoprotein (LDL) receptor-related proteins are modulators of inflammatory mechanisms [42,43]. As some researchers suggested we support that melanogenesis and adipogenesis triggered and intertwined by an increase in shared or common signalling molecules.
Wnt signalling could be anti-adipogenic and pro-melanogenic in the same cell or in different cell types. Inhibition of WNT signalling is required to induce mesenchymal stem cells to undergo adipogenesis and differentiation [44,45]. WNT molecules play role in the pathogenesis of human obesity and type 2 diabetes [46]. Wnt3a inhibit the adipogenic differentiation of porcine adipose-derived mesenchymal stem cells in vitro culture [47], while Wnt3a acts on melanoblasts to maintain MITF expression and promote melanoblast differentiation into melanocytes [48].
Wnt10b promotes differentiation of mouse hair follicle melanocytes [49], while expression of Wnt10b is elevated in 3T3-L1 pre-adipocytes and declines upon induction of differentiation. Moreover, ectopic expression of Wnt10b activates the Wnt signalling pathway and potently blocks differentiation [50,51].
Furthermore, over expression of Wnt5a increases adipose tissue inflammation [52] and on the other side, Wnt5A has been promoted to be a therapeutic target for melanoma metastasis [53]. WNT pathway can be an attractive drug-development target to combat obesityassociated metabolic complications [44]. SRY-related HMG-box (SOX) proteins and signalling pathway also interact with Wnt signalling path way in many processes. SOX-Wnt interactions adjust the activity of cAMP response element binding protein (CREB) and its action on MITF activation.
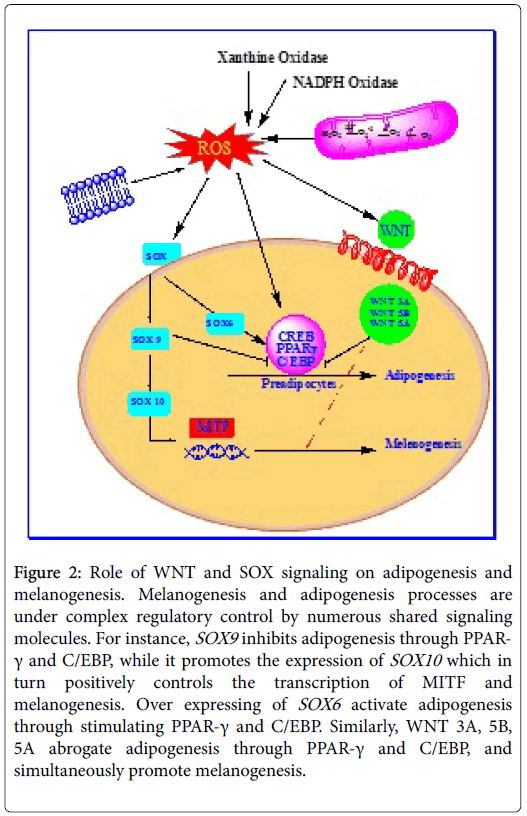
A new research by Leow and his colleagues shed light on the vital role of sox transcription factors in melanogenesis (Figure 2). SOX5 hinders melanogenesis and SOX6 involved in the developmental origins of obesity by promoting adipogenesis [54]. Overexpressing of SOX6 increased cellular triglyceride content and promote adipogenesis through stimulating PPAR-γ and C/EBP-α and inhibition of WNT/β- catenin signaling [54].
Figure 2: Role of WNT and SOX signaling on adipogenesis and melanogenesis. Melanogenesis and adipogenesis processes are under complex regulatory control by numerous shared signaling molecules. For instance, SOX9 inhibits adipogenesis through PPAR- γ and C/EBP, while it promotes the expression of SOX10 which in turn positively controls the transcription of MITF and melanogenesis. Over expressing of SOX6 activate adipogenesis through stimulating PPAR-γ and C/EBP. Similarly, WNT 3A, 5B, 5A abrogate adipogenesis through PPAR-γ and C/EBP, and simultaneously promote melanogenesis
Other recent studies have provided evidence that limiting SOX9 through the up regulating prefadipocyte factor-1 (Pref-1) is necessary for differentiation of pre adipocyte to adipocytes [55] but SOX9 induces the expression of SOX10 which in turn controls the transcription of MITF and melanogenesis proteins [56,57]. Such findings suggest that the main cross talk between melanogenesis and adipogenesis processes occurs through Wnt/β-catenin pathway and its interaction with SOX signaling molecules.
Furthermore, there is growing evidence that the inflammatory molecules and ROS’s stimulate both melanogenesis and adipogenesis. Many studies established that post-inflammation released cytokines such as interleukin 1 (IL-1), interleukin 1 (IL-6), tumour necrosis factor (TNF-α) stimulate melanin synthesis in the epidermis [58-60]. ROS also promotes adipocyte differentiation from MSCs by activating peroxisome proliferator-activated receptor gamma (PPARγ), and the antioxidant N-acetyl-L-cysteine inhibits adipocyte differentiation through ROS [39]. The cross talk mediator of melanogenesis and adipogenesis apparently occur through PPAR-γ and C/EBP-α [31-36]. PPAR-γ regulates both MITF and some inflammatory molecules. PPAR-γ down regulates the MITF gene expression and so tyrosinaserelated proteins [61,62]. Activation of PPAR γ negatively influences the production and localization of TNF-α, IL-6, and IL-1beta by macrophages [63,64]. Macrophages and lymphocytes infiltrating adipose also contribute to the signalling circuits of linking melanogenesis and adipogenesis. Lysosomal stress in adipose tissue macrophages (ATM) in adipose induces glycoprotein non metastatic melanoma protein B (Gpnmb) expression and both positively correlate with obesity and insulin resistance [65]. Gpnmb identified as a novel marker for obesity-induced ATM infiltration. Interestingly, Gpnmb influence nuclear MITF localization and activity [65].
Moreover, a nuclear protein that is expressed in lymphocytes called lymphoid enhancing factor (LEF-1) is a mediator of Wnt signalling. LEF-1 and MITF interact and regulate dopachrome tautomerase, a key protein in melanin synthesis [66]. MITF, the main marker of melanocytes, is a transcription factor and regulator of many other genes. Activation of melanogenesis through MITF not only activates melanocytes related proteins, but also may have a role in lipids metabolism. For example, MITF recognizes and binds promoter of phospholipase A1 and apolipoprotein l domain containing 1 (APOLD1) genes [67]. MITF is also found that it controls attractin gene (ATRN) . ATRN in human is ortholog to mahogunin gene in mice produced by some white blood cells in human. It affects melanogenesis through MC1R signaling by a cAMP-independent pathway [68]. Attractin contributes to the skin color of Europeans and East Asians through convergent evolution [69]. In connection, a membrane-bound isoform of attractin has been found to promote obesity. Mutation in mahoguim gene (ATRN) keep mice lean even after fed with high amounts of fat rich diet [68]; attractin expression also was higher in circulating monocytes in people with obesity [69-71]. While other isoform secreted by t-lymphocytes influences inflammatory responses and immune cells interactions [72].
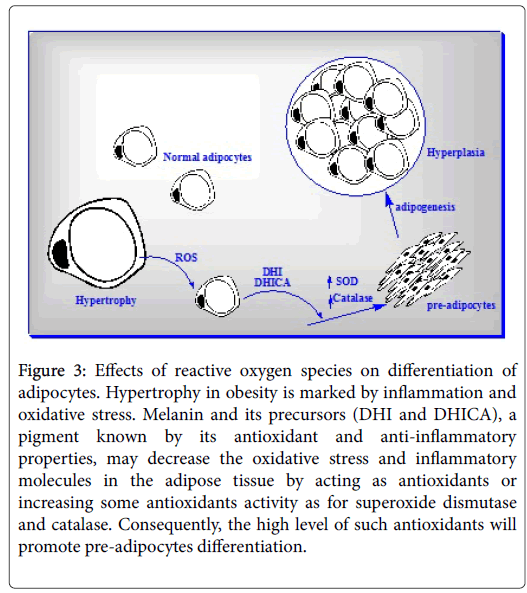
Like hypertrophy in obesity is marked by inflammation and oxidative stress, also melanogenesis process itself produces ROS and is influenced by some inflammatory molecules. Even though, the melanin’s anti-oxidative and scavenging power for ROS much more than the ROSs produced through the melanogenesis process. We think that if the oxidative stress and inflammation accompanied or consequent to hypertrophy in obese exceeds to ROS’s that result from melanogenesis process in adipocytes, the melanogenesis is rewired and activated. The indirect effect of melanogenesis could be through dihydroxyindole (DHI) and 5,6-dihydroxyindole-2-carboxylic acid (DHICA) melanin precursors which further stimulate expression of antioxidants genes [73]. High level of antioxidants genes has been shown to be promoting pre-adipocytes differentiation [74,75]. In this mechanism, we can assume that melanogenesis can co-drive adipogenesis to relief hypertrophy (Figure 3).
Figure 3: Effects of reactive oxygen species on differentiation of adipocytes. Hypertrophy in obesity is marked by inflammation and oxidative stress. Melanin and its precursors (DHI and DHICA), a pigment known by its antioxidant and anti-inflammatory properties, may decrease the oxidative stress and inflammatory molecules in the adipose tissue by acting as antioxidants or increasing some antioxidants activity as for superoxide dismutase and catalase. Consequently, the high level of such antioxidants will promote pre-adipocytes differentiation.
Subsequently adipocytes undergone hypertrophy probably rather melanin production as it will slow down or stop the momentum of oxidative stress and inflammatory environment in obesity. This may explain why melanogenesis rate varies in people with obesity but is not correlated with obesity level; and why ectopic melanin is much less in lean subjects compared to people with obesity.
Therapeutic potential of melanin and intermediates in ameliorating NCDs
Melanin protective characteristic against UV harmful effects is sound and solid. Although melanogenesis is an oxygen dependent process, it has both antioxidant and ROS-dependent cytotoxic properties [76]. Melanin synthesis process itself generates ROS as superoxide anion (O2) and hydrogen peroxide (H2O2), which could subject other cells in tissues to oxidative stress [77,78].
However, there is still uncertainty about whether the melanin or the melanin intermediates can be used as antioxidants and/or antiinflammatory agent especially in obesity research and therapy. Melanin has antimicrobial properties as it can neutralize their enzymes and toxins [7]. Natural human melanin is insoluble in water and so nondiffusible. However, treatment of 3T3-L1 adipocytes with water-soluble melanin complex extracted from some fungi showed significant increase in insulin-stimulated glucose uptake and adiponectin gene expression [79]. Same treatment improved insulin sensitivity and reduced adiposity in high fat fed obese mice [79]. The soluble and diffusible melanin intermediates such as DHI and DHICA could have better anti-inflammatory and anti-oxidant capabilities [80,81].
L-dopa, one of the melanin intermediates, can thwart production of the inflammatory cytokines from T lymphocytes and monocytes [8,81]. DHI has scavenging character for free radicals [82]. It has been demonstrated that DHI and DHICA substantially decreased malondialdehyde (MDA) formation from lipid peroxidation in rat brain cortex homogenates [83]. DHICA increased the activity and expression of antioxidant enzymes such as SOD and catalase and protect cells from UVA harm and apoptosis [73]. Authors even stipulated that “DHICA is a messenger in the cross-talk among epidermal cells”. Also few researchers showed that some melanin precursors may has pro-oxidant characteristic based on results from in vitro experiments on cell cultures [84,85].
Nevertheless, great caution must be taken in extrapolating on such conclusions due to the innate nature of the oxidative stress and ROS that are often produced from chemical reactions in the culture media [86]. It is also important to take into consideration the insoluble nature of melanin and its confinement in melanosomes could undermine the significance of some of their conclusions. Also, the same researchers neglected or underestimated of possible secondary role of melanin or its intermediates, which can stimulate anti-oxidative enzymes such as SOD and catalases in other cells in paracrine fashion [73]. What's more is that the concentration of the used melanin or its intermediates may have different effect on the cell viability and type.
For example, (DHI) protected retinal cells in cultures from UVAdamage but only at low concentrations [87]. Therefore, evaluation of the melanogenesis process as a whole is more central than focusing on partial components alone as its end product or specific intermediates. The multi-biological effect of melanogenesis depends on the relative type of melanin, the levels of melanin intermediates, the concentrations of reactive metals within the melanosome microenvironment and its influence on anti-oxidative enzymes.
Baranova has advocated that melanocortins especially α- melanocyte stimulating hormone (α-MSH) or its synthetic analogues could have therapeutic potential by stimulating ectopic melanogenesis and so ameliorating or minimizing obesity complications [88]. The author cited many supporting research. Mainly, certain concentrations of α-MSH and analogs have been used for reducing body weight or preventing body weight gains in mice [89]. Also in one human trial, by using intranasal administration of α-MSH, showed it is effective in reducing weight of lean subjects but not that of people with obesity [90,91].
It is also true that much research has shown that α- MSH and other melanocortins suppresses the expression or secretion of many proinflammatory cytokines. So α- MSH can be used in treatments of inflammatory diseases and bacterial infections. For example, it has been demonstrated that α-MSH can inhibit nuclear factor-κB (NF-κB) that regulates the expression of genes of pro-inflammatory cytokines linking mediator of the melanocortin system with inflammatory responses [92]. As Baranova deduced, “α-MSH may prevent or delay the onset of the secondary complications of obesity” and mentioned that still there is no solid proof of correlation of serum levels of α-MSH and obesity level. So, there is no one reliable bioassay that can be used to link α-MSH and the relief of obesity complications collectively.
However, the risks associated in using α-MSH and the uncertainties in its therapeutics dose effect on different people with obesity individuals are still worrying. α-MSH analogues might increase blood pressure [93]. Also, α-MSH analogues promoting of melanogenesis and melanocytes proliferation might cause melanoma [94]. In addition, melanotan, especially II, raises some concerns of its usage especially on long terms and in high dosages. MTII causes rapidly growing of moles in a male with a malignant melanoma [95] and concerns of higher risk of cussing new melanomas [96,97]. Not to forget the possible secondary disturbing endocrinological consequences.
Brennan in 2004 suggested in part of his patent; the idea of combination of α-MSH and leptin may have better effect in treatment of obesity and reducing body weight [89]. Though the authors ignored the leptin’ resistance implication in obesity development as the main issue to fix rather than the serum level of leptin.
In addition, α-MSH itself may increases leptin release from adipocytes and so serum levels [98]. From our point of view, we advocate and encourage animal trials to use α-MSH in combination with adiponectin instead of leptin. Adiponectin and its serum level is a better reliable marker negatively correlates with obesity and its complications. Adiponectin also tightly linked to adipogenesis and cell differentiation which can relief the stress of hyperplasia by inducing more adipocytes. More importantly, adiponectin helps in ameliorating secondary complication of obesity in other tissues as liver by preventing LPS-induced ROS production [99].
Nevertheless, the right magic formula and combinations of molecules as adiponectin to be used in human trial need more study. Moreover, whether the ultimate and final effect of such suggested treatment would have anti-inflammatory effect, anti-oxidative effect or both; still need to be examined. Also it needs to be clarified that if the treatment leads to fine tuning of the metabolic process in systematic or localized way; or whether can it be excreted on adipose or fat cells only. All such inquiries need further studies.
Conclusions
We may postulate that the activation of melanin synthesis is a natural preventive measure or adaptive response to oxidative stress to slow down or minimizes hyperplasia and ameliorate consequent obesity complications. Oxidative stress in hypertrophy drives melanogenesis and adipogenesis by sharing interactive molecular signalling mechanisms. Cross-talk between melanogenesis and adipogenesis occur through Wnt/β-catenin pathway and its interaction with SOX signaling molecules; through PPAR-γ and C/EBP-α.
Solid research quiet still needed to explain why melanogenesis process in adipose happen in various levels in different levels of obesity but not correlated. This can be checked based on the idea that the momentum of melanogenesis may be is related to the stage and time of hypertrophy. Moreover, in vivo and in vitro studies are still necessary to determine whether melanin or the melanin intermediate metabolites are better anti-oxidants and scavengers for ROS in adipose tissue to be used as therapeutic potential molecules.
More research still needed on different animals to strengthen the ectopic melanin finding in adipose and more animal trials to determine the right doses, mode of administration and possible side effects when using α-MSH alone or in combination with other molecules preferably with adiponectin. Such proposed explanatory studies are based on very limited amount of preliminary data and at times several levels of assumptions are made.
The assessments of melanin regulation of ROS production and inflammatory mediators (cytokines and macrophages) in adipose cell models are relevant and may generate novel pharmacological concepts, especially that pharmacological tool are already available. Melanin itself as well as available melanocortin receptor agonists can be tested in vitro and also in vivo in mice. It is also possible that such compounds could be used for human proof-of-principle studies in the future.
Limitations of such proposed studies also exist. The hypothesis challenges previous research findings in adipose tissue biology and the current support is fairly weak. Thus, there is some likelihood that it is proven wrong, which nonetheless will be important knowledge.
The high levels of the polymorphism in human genes regulating melanin biosynthesis provide a basis for the highly individual melanogenic response of adipocytes that may account for the differences in an individual’s propensity to develop secondary complications of obesity. Accordingly, one of the pitfalls for induction of ectopic melanogenesis by using the melanocortin analogues, in animal models or even on cellular level, makes the translation of the results of such project to the clinical practice is a little challenging for the need of tailored application. In addition, unclear consequences of possible global induction of melanogenesis in animal models could undermine the values of systematic ectopic melanogenesis value.
The hypothesis of melanin production in adipose tissue having a role to prevent complications of obesity is unique and challenges current dogma. Although, the current evidences supporting such local melanin effects are rather scarce.
Conflict of Interests
The authors do not have any potential conflict of interest.
References
- Kollias N (1995) The spectroscopy of human melanin pigmentation in melanin: Its role in human photoprotection. Valden Pub: 31-38.
- Meredith P, Riesz J (2004) Radiative relaxation quantum yields for synthetic eumelanin. Photochem Photobiol 79: 211-216.
- Brenner M, Hearing VJ (2008) The protective role of melanin against uv damage in human skin. J Photochem Photobiol 84: 539-549.
- Sarna T (1992) Properties and function of the ocular melanin: A photobiophysical view. J Photochem Photobiol B 12: 215-258.
- Hill HZ (1992) The function of melanin or six blind people examine an elephant. BioEssays 4: 49-56.
- Wang Z, Dillon J, Gaillard ER (2006) Antioxidant properties of melanin in retinal pigment epithelial cells. Photochemistry and Photobiology 82: 474-479.
- Mackintosh JA (2001) The antimicrobial properties of melanocytes, melanosomes and melanin and the evolution of black skin. J Theor Biol 211: 101-113.
- Yamaguchi Y, Brenner M, Hearing VJ (2007) The regulation of skin pigmentation. J Biol Chem 282: 27557-27561.
- Lin JY, Fisher DE (2007) Melanocyte biology and skin pigmentation. Nature 445: 843-850.
- Schallreuter KU, Kothari S, Chavan B, Spencer JD (2008) Regulation of melanogenesis-controversies and new concepts. Exp Dermatol 17: 395-404.
- Tachibana M, Takeda K, Nobukuni Y, Urabe K, Long, JE, et al. (1996) Ectopic expression of MITF, a gene for Waardenburg syndrome type 2, converts fibroblasts to cells with melanocyte characteristics. Nat Genet 14: 50-54.
- Randhawa M, Huff T, Valencia JC, Younossi Z, Chandhoke V, et al. (2009) Evidence for the ectopic synthesis of melanin in human adipose tissue. FASEB J 23: 835-843.
- Page S, Chandhoke V, Baranova A (2011) Melanin and melanogenesis in adipose tissue: possible mechanisms for abating oxidative stress and inflammation. Obes Rev 12: 21-31.
- Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5: 9-19.
- Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94: 909-950.
- Furukawa S, Fujita T, Shimabukuro M, Masanori IM, Yamada Y, et al. (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114: 1752-1761.
- Curtis JM, Grimsrud PA, Wright WS, Xu X, Foncea RE, et al. (2010) Downregulation of adipose glutathione S-tansferase A4 leads to increased protein carbonylation, oxidative stress and mitochondrial dysfunction. Diabetes 59: 1132-1142.
- Landrier JF, Marcotorchino J, Tourniaire F (2012) Lipophilic micronutrients and adipose tissue biology. Nutrients 4: 1622-1649.
- Galinier A, Carriere A, Fernandez Y, Carpene C, Andre M, et al. (2006) Adipose tissue proadipogenic redox changes in obesity. J Biol Chem 281: 12682-12687.
- Traber MG, Kayden HJ (1987) Tocopherol distribution and intracellular localization in human adipose tissue. Am J Clin Nutr 46: 488-495.
- Marseglia L, Manti S, D’Angelo G, Nicotera A, Parisi E, et al. (2015) Oxidative stress in obesity: A critical component in human diseases. Int J Mol Sci 16: 378-400.
- Giordano A, Murano I, Mondini E, Perugini J, Smorlesi A, et al. (2013) Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J Lipid Res 54: 2423-2436.
- Kusminski CM, Scherer PE (2012) Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol Metab 23: 435-443.
- Esterbauer H, Cheeseman KH, Dianzani MU (1982) Separation and characterization of the aldehydic products of lipid peroxidation stimulated by ADP-Fe2+ in rat liver microsomes. Biochem J 208: 129-140.
- Poli G, Dianzani MU, Cheeseman KH, Slater TF, Lang J, (1985) Separation and characterization of the aldehydic products of lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in isolated rat hepatocytes and rat liver microsomal suspensions. Biochem J 227: 629-638.
- Benedetti A, Comporti M, Esterbauer H (1980) Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim Biophys Acta 620: 281-296.
- Esterbauer H, Eckl P, Ortner A (1990) Possible mutagens derived from lipids and lipid precursors. Mutat Res 238: 223-233.
- Schneider C, Porter NA, Brash AR (2008) Routes to 4-hydroxynonenal: Fundamental issues in the mechanisms of lipid peroxidation. J Biol Chem 283: 15539-15543.
- Frohnert BI, Sinaiko AR, Serrot FJ, Foncea RE, Moran A, et al. (2011) Increased adipose protein carbonylation in human obesity. Obesity 19: 1735-1741.
- Zarrouki B, Soares AF, Guichardant M, Lagarde M, Geloen A (2007) The lipid peroxidation end-product 4-HNE induces COX-2 expression through p38MAPK activation in 3T3-L1 adipose cell. FEBS Lett 58: 2394-2400.
- Soares AF, Guichardant M, Cozzone D, Bernoud-Hubac N, Lagarde M (2005) Effects of oxidative stress on adiponectin secretion and lactate production in 3T3-L1 adipocytes. Free Radic Biol Med 38: 882-889.
- Volk T, Hensel M, Schuster H, Kox WJ (2000) Secretion of MCP-1 and IL-6 by cytokine-stimulated production of reactive oxygen species in endothelial cells. Mol Cell Biochem 206: 105-112.
- Demozay D, Mas J, Rocchi S, Van Obberghen E (2008) FALDH reverses the deleterious action of oxidative stress induced by lipid peroxidation product 4-Hydroxynonenal on insulin signaling in 3T3-L1 adipocytes. Diabetes 57: 1216-1226.
- Tangvarasittichai S (2015) Oxidative stress, insulin resistance, dyslipidemia, and type 2 diabetes mellitus. World J Diabetes 6: 456-480.
- Muehlenbein MP (2010) Human Evolutionary Biology. Cambridge University Press.
- Zeve D, Tang W, Graff J (2009) Fighting fat with fat: The expanding field of adipose stem cells. Cell Stem Cell 5: 472-481.
- Kim Y, van de Wall E, Laplante M, Azzara A, Trujillo E, et al. (2007) Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Investig 117: 2621-2637.
- Weyer C, Foley E, Bogardus C, Tataranni A, Pratley E (2000) Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 43: 1498-1506.
- Wang W, Zhang Y, Lu W, Liu K (2015) Mitochondrial reactive oxygen species regulate adipocyte differentiation of mesenchymal stem cells in hematopoietic stress induced by arabinosylcytosine. PLoS ONE 10: e0120629.
- Garraway LA, Sellers WR (2006) Lineage dependency and lineage-survival oncogenes in human cancer. Nat Rev Cancer 6: 593-602.
- Fernyhough ME, Helterline DI, Vierck JL, Hausman GJ, Hill RA, et al. (2005) Dedifferentiation of mature adipocytes to form adipofibroblasts: More than just a possibility. Adipocytes 1: 17-24.
- Ma B, Hottiger MO (2016) Crosstalk between Wnt/β-Catenin and NF-κB signaling pathway during inflammation. Frontiers Immunol 7: 1-14.
- Borrell-Pages M, Romero JC, Badimon L (2015) LRP5 and plasma cholesterol levels modulate the canonical Wnt pathway in peripheral blood leukocytes. Immunol Cell Biol 93: 653-661.
- Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A (2009) Adipogenesis and WNT signaling. Trends Endocrinol Metab 20: 16-24.
- Lagathu C, Christodoulides C, Tan CY, Virtue S, Laudes M, et al. (2010) Secreted frizzled-related protein 1 regulates adipose tissue expansion and is dysregulated in severe obesity. Int J Obes (Lond) 34: 1695-1705.
- Laudes M (2011) Role of WNT signaling in the determination of human mesenchymal stem cells into preadipocytes. J Mol Endocrinol 46: 65-72.
- Li HX, Luo X, Liu RX, Yang YJ, Yang GS (2008) Roles of Wnt/β-catenin signaling in adipogenic differentiation potential of adipose-derived mesenchymal stem cells. Mol Cell Endocrinol 291: 116-124.
- D’Mello S, Finlay GJ, Baguley BC, Askarian-Amiri ME (2016) Signaling pathways in melanogenesis. Int J Mol Sci 17: 1144.
- Ye J, Yang T, Guo H, Tang Y, Deng F, et al. (2016) Wnt10b promotes differentiation of mouse hair follicle melanocytes. Int J Med Sci 10: 691-698.
- Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, et al. (2002) Regulation of Wnt signaling during adipogenesis. J Biol Chem 277: 30998-31004.
- Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, et al. (2000) Inhibition of adipogenesis by Wnt signaling. Science 289: 950-953.
- Fuster JJ, Zuriaga MA, Ngo DT, Farb MG, Aprahamian T, et al. (2015) Noncanonical wnt signaling promotes obesity-induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes 64: 1235-1248.
- Hoek KS, Schlegel NC, Brafford P, Sucker A, Ugurel S, et al. (2006) Metastatic potential of melanomas defined by specific gene expression profiles with no BRAF signature. Pigment Cell Res 19: 290-302.
- Leow SC, Poschmann J, Too PG, Yin J, Joseph R, et al. (2016) The transcription factor SOX6 contributes to the developmental origins of obesity by promoting adipogenesis. Development 143: 950-961.
- Wang Y, Sul HS (2009) Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9. Cell Metab 9: 287-302.
- Hou L, Arnheiter H, Pavan WJ (2006) Interspecies difference in the regulation of melanocyte development by SOX10 and MITF. Proc Natl Acad Sci 103: 9081-9085.
- Aoki Y, Saint-Germain N, Gyda M, Magner-Fink E, Lee YH, et al. (2003) Sox10 regulates the development of neural crest-derived melanocytes in Xenopus. Dev Biol 259: 19-33.
- Chang MW, Bolognia JL, Jorizzo JL, Rapini RP (2009) Disorders of hyperpigmentation. Dermatology 2nd edn: 333-389.
- Tomita Y, Maeda KC, Tagami, H (1992) Melanocyte-stimulating properties of arachidonic acid metabolites: Possible role in postinflammatory pigmentation. Pigment Cell Res 5: 357-361.
- Ortonne J (1992) Retinoic acid and pigment cells: A review of in-vitro and in-vivo studies. Br J Dermatol 127: 43-47.
- Jang JY, Lee JH, Shin HK, Choi YH, Lee JD, et al. (2010) Partially purified asiasari radix inhibits melanogenesis through extracellular signal-regulated kinase signaling in B16F10 cells. Int J Mol Med 25: 287-292.
- Grabacka M, Placha W, Urbanska K, Laidler P, Płonka PM, et al. (2008) PPAR γ regulates MITF and β-catenin expression and promotes a differentiated phenotype in mouse melanoma S91. Pigment cell Melanoma Res 21: 388-396.
- Gosset P, Charbonnier AS, Delerive P, Fontaine J, Staels B, et al. (2001) Peroxisome proliferator activated receptor activators affect the maturation of human monocyte-derived dendritic cells. Eur J Immunol 3: 2857-2865.
- Nencioni A, Grunebach F, Zobywlaski A, Denzlinger C, Brugger W, et al. (2002) Dendritic cell immunogenicity is regulated by peroxisome proliferator-activated receptor gamma. J Immunol 169: 1228-1235.
- Gabriel TL, Tol MJ, Ottenhof R, Van Roomen C, Aten J, et al. (2014) Lysosomal stress in obese adipose tissue macrophages contributes to mitf-dependent gpnmb induction. Diabetes 63: 3310-3323.
- Yasumoto K, Takeda K, Saito H, Watanabe K, Takahashi K, et al. (2002) Microphthalmia-associated transcription factor interacts with LEF-1, a mediator of Wnt signaling. EMBO J 21: 2703-2714.
- Regard JB, Scheek S, Borbiev T, Lanahan AA, Schneider A, et al. (2004) A novel vascular early response gene. J Neurosci 24: 4092-4103.
- Hida T, Wakamatsu K, Sviderskaya EV, Donkin AJ, Montoliu L, et al. (2009) Agouti protein, mahogunin, and attractin in pheomelanogenesis and melanoblast-like alteration of melanocytes: A cAMP-independent pathway. Pigment Cell Melanoma Res 22: 623-634.
- Norton HL, Kittles RA, Parra E, McKeigue P, Mao X, et al. (2006) Genetic evidence for the convergent evolution of light skin in Europeans and east Asians. Mol Biol Evol 24: 710-722.
- Nagle D, McGrail SH, Vitale J, Woolf EA, Dussault BJ, et al. (1999) The mahogany protein is a receptor involved in suppression of obesity. Nature 398: 148-152.
- Laudes ML, Oberhauser F, Schulte DM, Schilbach K, Freude S, et al. (2010) Dipeptidyl-peptidase 4 and attractin expression is increased in circulating blood monocytes of obese human subjects. Exp Clin Endocrinol Diabetes 118: 473-477.
- Duke-Cohan JS, Gu J, McLaughlin DF, Xu Y, Freeman GJ, et al. (1998) Attractin (DPPT-L), a member of the CUB family of cell adhesion and guidance proteins, is secreted by activated human T lymphocytes and modulates immune cell interactions. Proc Natl Acad Sci 95: 11336-11341.
- Kovacs D, Flori E, Maresca V, Ottaviani M, Aspite N, et al. (2012) The eumelanin intermediate 5,6-dihydroxyindole-2-carboxylic acid is a messenger in the cross-talk among epidermal cells. J Invest Dermatol 132: 1196-1205.
- Reiners JJ, Hale MA, Cantu AR (1988) Distribution of catalase and its modulation by 12-O-tetradecanoylphorbol-13-acetate in murine dermis and subpopulations of keratinocytes differing in their stages of differentiation. Carcinogenesis 9: 1259-1263.
- Baker SS, Baker RD (1992) Antioxidant enzymes in the differentiated Caco-2 cell line. In Vitro Cell Dev Biol 28: 643-647.
- Wood JM, Jimbow K, Boissy RE, Slominski A, Plonka PM, et al. (1999) What’s the use of generating melanin? Exp Dermatol 8: 153-164.
- Simon JD, Peles D, Wakamatsu K, Ito S (2009) Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res 22: 563-579.
- Koga S, Nakano M, Terokubota S (1992) Generation of superoxide during the enzymatic action of tyrosinase. Arch Biochem Biophys 292: 570-575.
- Lee J, Hyun C (2014) Insulin-sensitizing and beneficial lipid-metabolic effects of the water-soluble melanin complex extracted from inonotus obliquus. Phytother Res 28: 1320-1328.
- Plonka P, Grabacka M (2006) Melanin synthesis in microorganisms-biotechnological and medical aspects. Acta Biochim Pol 53: 429-443.
- Slominski A, Zbytek B, Slominski R (2009) Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int J Cancer 124: 1470-1477.
- Schmitz S, Thomas PD, Allen TM, Poznansky MJ, Jimbow K (1995) Dual role of melanins and melanin precursors as photoprotective and phototoxic agents: Inhibition of ultraviolet radiation-induced lipid peroxidation. Photochem Photobiol 61: 650-655.
- Memoli S, Napolitano A, d'Ischia M, Misuraca G, Palumbo A, et al. (1997) Diffusible melanin-related metabolites are potent inhibitors of lipid peroxidation. Biochim Biophys Acta 1346: 61-68.
- Kipp C, Young AR (1999) The soluble eumelanin precursor 5,6-dihydroxyindole-2-carboxylic acid enhances oxidative damage in human keratinocyte DNA after UVA irradiation. Photochem Photobiol 70: 191-198.
- Tomita Y, Hariu A, Kato C, Seiji M (1984) Radical production during tyrosinase reaction, dopa-melanin formation, and photoirradiation of dopa-melanin. J Invest Dermatol 82: 573-576.
- Halliwell B (2014) Cell culture, oxidative stress, and antioxidants: Avoiding pitfalls. Biomed J 37: 99-105.
- Heiduschka P, Blitgen-Heinecke P, Tura A, Kokkinou D, Julien S, et al. (2007) Melanin precursor 5,6-dihydroxyindol: Protective effects and cytotoxicity on retinal cells in vitro and in vivo. Toxicol Pathol 35: 1030-1038.
- Baranova (2011) Preventing obesity-related metabolic syndrome with melanogenesis. Patent Application Publication, Pub. No.: US 2011/0206642 A1.
- Brennan, Ute Hochgeschwender (2010) Composition and method for regulation of body weight and associated conditions. Patent No.: US 7,655,622 B2.
- Fehm HL, Smolnik R, Kern W, McGregor GP, Bickel U, et al. (2001) The melanocortin melanocyte-stimulating hormone/adrenocorticotropin (4-10) decreases body fat in humans. J Clin Endocrinol Metab 86: 1144-1148.
- Hallschmid M, Smolnik R, McGregor G, Born J, Fehm HL (2006) Overweight humans are resistant to the weight-reducing effects of melanocortin 4-10. J Clin Endocrinol Metab 91: 522-525.
- Nordlund JJ (2007) The melanocyte and the epidermal melanin unit: An expanded concept. Dermatol Clin 25: 271-281.
- Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, et al. (2009) Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med 360: 44-52.
- Hadley ME, Dorr RT (2006) Melanocortin peptide therapeutics: Historical milestones, clinical studies and commercialization. Peptides 27: 921-930.
- Cardones AR, Grichnik JM (2009) Alpha-Melanocyte-stimulating hormone-induced eruptive nevi. Arch Dermatol 145: 441-444.
- Langan EA, Nie Z, Rhodes LE (2010) Melanotropic peptides: More than just 'Barbie drugs' and 'sun-tan jabs'. Br J Dermatol 163: 451-455.
- Thestrup-Pedersen K, Sondergaard K (2011) Melanotan-induced lentigines and nevi. Ugeskr Laeger 173: 975.
- Bradley RL, Kokkotou EG, Maratos-Flier E, Cheatham B (2000) Melanin-concentrating hormone regulates leptin synthesis and secretion in rat adipocytes. Diabetes 49: 1073-1077.
- Shrestha A, Park PH (2016) Globular adiponectin attenuates LPS-induced reactive oxygen species production in HepG2 cells via FoxO3A and HO-1 signaling. Life Sci 148: 71-79.
Citation: Jarrar M, El-Shafey A (2018) The Paradox of Ectopic Melanin Synthesis in Adipose: Potential Mechanism, Benefits and Perspectives in Abating Obesity Complications. J Obes Weight Loss Ther 8: 363. DOI: 10.4172/2165-7904.1000363
Copyright: © 2018 Jarrar M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4980
- [From(publication date): 0-2018 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 4152
- PDF downloads: 828