Research Article Open Access
The Neonicotinoid Insecticide Imidacloprid: A Male Reproductive System Toxicity Inducer-Human and Experimental Study
Essam M Hafez*, Sahar Y Issa, Maha K AI-Mazroua, Karem T Ibrahim and Safaa M Abdel RahmanMinia University Faculty of Medicine, Minia, Egypt
- *Corresponding Author:
- Essam M. Hafez
Minia University Faculty of Medicine, Minia, Egypt
Tel: 00966545990785
E-mail: essamtox@yahoo.com
Received date: Jan 10, 2016 Accepted date: Feb 10, 2016 Published date: Feb 18, 2016
Citation: Hafez EM, Issa SY, AI-Mazroua MK, Ibrahim KT, Rahman SMA (2016) The Neonicotinoid Insecticide Imidacloprid: A Male Reproductive System Toxicity Inducer-Human and Experimental Study. Toxicol open access 1:109. doi:10.4172/2476-2067.1000109
Copyright: © 2016 Hafez EM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Toxicology: Open Access
Abstract
Objective: This study was undertaken to explore relationships between level of imidacloprid in the serum and semen quality among men farmers in addition to investigating histopathological findings in treated mature male rats.
Methods and results: Our research entailed two parts; firstly, human part done on farm workers (n=35) with age between (Mean ± SD 34.3±6.4) and healthy volunteers (n=25) their ages were (35.6±8.2) years old asked to provide semen and blood samples. A significant negative correlation between sperm concentration, motility, with serum and seminal Imidacloprid (IMI), its main metabolite 6-chloronicotinic acid (6- CINA) and cotinine , was identified in farmers (r= − 0.489; p<0.05). Second part of research was done on adult male rats that had been divided into a total of five groups 10 each. Two groups served as control one as negative and the other as positive. The other three experimental groups were given 45, 90 and 450 mg/kg body weight Imidacloprid in corn orally by a gavage process for fifteen days respectively. There was a significant difference in luteinizing hormone (LH), follicular stimulating hormone (FSH), testestrone (tes), Estradiol 2 (E2), prolactin and semen quality in IMI treated rats when compared to control groups. Vacuolar degeneration of germinal epithelium and loss of spermatids, seminiferous tubules suggesting inhibition of spermatogenesis was recorded in IMI treated rats.
Conclusion: Toxic effects to male reproductive system can be considered as an outcome of IMI exposure and infertility problems can be expected in chronically exposed subjects.
Introduction
Imidacloprid (IMI) [1-(6-chloro-3-pyridylmethyl)-Nnitroimidazolidin -2-ylideneamine] is a member of a relatively new class of insecticidal chemistry, the chloronicotinyl neonicotinoid compounds. It was introduced into commercial use only in the last decade and is increasingly used worldwide. It is the most important systemic insecticide and has a wide diversity of uses: in agriculture, on turfs, on pets and for household pests [1].
According to the world health organization (WHO) and United States Environmental Protection Agency this compound is categorized as a “moderately toxic” Class II or III requiring a Warning or Caution labels on marketed products [2]. IMI is rapidly and almost completely absorbed from the gastrointestinal tract, and eliminated via urine and feces. The most important metabolic steps include the degradation to 6-chloronicotinic acid (6-CINA), neurotoxic agent [3].
There are many previous epidemiologic studies about insecticides exposures and semen quality that mainly focused on occupational exposures among pesticide sprayers [4].
There are some reports that show IMI has an adverse effect on the reproductive system. Histopathological changes have been widely used as significant biological markers for environmental toxicity [5].
In vivo studies demonstrated that IMI and other neonicotinoid pesticides could adversely affect mammalian reproductive organs leading to retardation of testicular development, damage to spermatogenesis, decrease in sperm quality and change of ovary morphology [6,7]. Added to that the developmental retardation in mammalian fetus [8].
Aim of the work
This study was undertaken to explore relationships between level of imidacloprid (IMI) and its main metabolite 6 chloronicotinc acid (6- CINA) in the serum, seminal plasma and semen quality among smokers and non-smokers men in the occupational pesticides exposed population working in rural areas in addition to investigating their effect in terms of quality, quantity and morphology of sperm content and histopathological findings in subchronic exposed mature male rats.
Materials and Methods
Human part
Inclusion criteria: Farm workers with age between 21 to 44 years were informed about this study. Approved participants either participates signed or finger printed an informed consent. Both regular smoker and non-smoker farmers were included. Socio-demographic characteristics, reproductive history, lifestyle habits, and potential pesticides exposure of recruited cases were documented. Healthy volunteers with age ranged between 23 to 49 years not exposed to pesticides were selected from the general population of Maghagha city- Minia governorate after their approval to participate in this study were asked to provide semen and blood samples. The Human Subjects Review Committee of Minia faculty of medicine approved the study protocol.
Exclusion criteria: Subjects with any of the following findings were excluded: current systemic diseases, infection of the genital tract, varicocele, cryptorchidism or acute orchitis, hypogonadism, digitorectal indication of prostatitis, indication of chronic orchoepididymitis, history of genital region trauma, abnormal location and size of the testis, genital inflammation, recent hormonal treatment or previous diagnosis of sperm abnormalities.
Human part experimental design: Study subjects were divided into two groups; Group I representing healthy volunteers n=25, Group II representing farmers n = 35. Each group was then divided into smokers and non-smokers. All smokers in the studied groups smoked less than 20 cigarettes per day for a duration exceeding three years smoking.
Sampling: Semen samples are collected from all candidates by masturbation after at least 3 days of sexual abstinence after ensuring washing hands and use of sterile wide mouth containers. Venous blood (5 mL) was also drawn from all subjects in heparinized vacutainer tubes on the same day of semen collection and stored in metal-free containers at −20°C until analysis of IMI, 6-CINA and cotinine.
Sample Care: After collection, semen specimens were allowed to liquefy at room temperature for 30 minutes and used for analysis. Traditional semen analysis focused on measurements for sperm concentration, motility and morphology was evaluated according to standards set by WHO, 2010 [9].
Animal experimental design
Fifty healthy adult male albino rats weighting about 120-150 grams were obtained from the animal house in faculty of agriculture, Alexandria University. All animals were allowed free access to distilled water and laboratory chow ad libitum. To avoid stress of isolation or overcrowdings, 5 rats were housed per cage. They were left freely wandering in their cage for two weeks with 12 hour dark to light cycle for acclimatization before starting the experiment.
Experimental procedures were performed in accordance with the guide of the care and use of laboratory animals approved by the committee of Alexandria University, the fewest number of animals estimated to obtain valid results were used and painful procedures were conducted with appropriate sedation to avoid pain and stress.
Doses selection
Imidacloprid doses were selected based on its LD50, which is reported to be 450 mg/kg body weight [10]. Low dose 1/10th of LD50 (45 mg/kg) and high dose, 1/5th of LD50 (90 mg/kg) were selected for sub-acute toxicity study.
The adult male rats were randomly divided into a total of five groups 10 in each group. Two groups served as control one as negative and the other as positive control. The other three groups that served as experimental groups were given 45 mg/kg, 90 mg/kg and 450 mg/kg body weight Imidacloprid in corn orally by a gavage process for fifteen days respectively. The animals were maintained under conditions of controlled temperature (22 ± 3) and humidity (30%-70%) with 12 hr light and dark cycle.
Group I (negative control): (10 rats) were kept in a quite group nonstressful environment, provided with food ad libitum and free access to water.
Group II (positive control): (10 rats) each animal received 0.2 ml/day corn oil orally. They were kept throughout the experiment under the same conditions.
Group III (10 rats-low doses): Each animal received Imidacloprid 45 mg/kg body weight for fifteen days.
Group IV (10 rats -High dose): Each animal received Imidacloprid 90 mg/kg body weight for fifteen days.
Group V (Imidacloprid acute toxicity group 10 rats): Each animal received a single dose Imidacloprid 450 mg/kg body weight.
At the end of the experiment animals of the all groups were sacrificed by cervical dislocation at 24 hours after a single dose for group V (acute toxicity group) and the last dose at the end of the fifteen days for all groups.
Chemicals
6-CINA and IMI 95% technical grade were obtained from Jiangzoue Agrostar Company, China and was purchased from AL-ASPANIA Company for producing and trading natural fertilizer in Egypt.
Samples (whole blood from the heart and femoral vein and semen) for analysis were collected at the time of autopsy. All samples were stored at -30°C prior to analysis. Prolactin, Leutinizing hormone(LH), Estradiol (E2) ,testosterone (Tes) level was measured by ELISA method using DRG Elisa kit (ELISA EIA-1559, 96 Wells kit, DRG Instruments, GmbH, Marburg, Germany) according to the standard protocol supplied by the kit manufacturer.
Detection of Cotinine, IMI and 6-CINA were assessed in both serum and semen by The Agilent 6230B - Q-TOF LC/MS/MS system, USA in the central laboratory Alexandria, Egypt.
Histopathology
Collection of Spermatozoa: Caudal epididymides were isolated, gently squeezed out and placed in a 2.0 ml eppendorf tube with HTFBSA. ‘‘Swim-up’’ spermatozoa were obtained after incubation at 37°C for 10 min.
All specimens were fixed in 10% formalin fixative for histological investigations and subsequently embedded in paraffin. Sections (5-6 μm) were stained with iron-weigert for histo-pathological assessment of germinal cell nuclei in the testes. All specimens were studied by multiple magnification (H& E * 200).
Results
Human part
Demographics of the studied subjects were shown in table 1; group I (n = 25), mean ages were (Mean ± SD, 35.6 ± 8.2).
| Studied Subjects | Group I | Group II | ||||
|---|---|---|---|---|---|---|
| Total | Non Smoker | Smoker | Total | Non Smoker | Smoker | |
| Number of Subjects | 25 | 14 | 11 | 35 | 14 | 21 |
| Age in years Mean (SD) | 35.6±8.2 | 35.4±8.7 | 33.6±8.1 | 34.3±6.4 | 33.4±6.5 | 34.8±6.4 |
Table 1: Demographics of the Studied Subjects.
Non-smoker (n = 14 had ages 35.4 ± 8.7 years old and smokers n = 11, their ages were 33.6 ± 8.1 years old. Group II subjects n=35, their ages were 34.3 ± 6.4 years old, non-smokers n=14 had ages 33.4 ± 6.5 years old, smoker n=21 and their ages were 34.8 ± 6.4 years old.
Group II had significantly higher blood plasma concentrations of (IMI), and its main metabolite (6- CINA) and cotinine than did group I subjects for both smokers and non-smokers (P<0.001) as shown in table 2.
| Elements | G I | G II | ||||
|---|---|---|---|---|---|---|
| Total N = 25 | NS (n = 14) | S (n = 11) | Total N = 35 | NS (n = 14) | S (n = 21) | |
| Cotinine (ng/mL) | 72.55±72. 97 | 0.95±0.03 | 144.25±3.67*** | 284.68 ± 3.04* | 0.96±0.21 | 493.58±3.04** |
| IMI (ng/mL) | 2.13 ± 1.15 | 2.99±1.79 | 2.56±1.47 | 9.71±5.87* | 9.04±6.37 | 10.38±6.12 |
| 6- CINA( ng/mL) | 0.36±0.19 | 0.52±0.31 | 0.43±0.24 | 1.65±0.98 | 1.53±1.08 | 1.76±1.04 |
*p<0.001 when compared to Group I (ANOVA, Tukey’s Range [HSD] test)
**p<0.001 for direct comparison of smoking and no smoking (among GII)
***p<0.001 for direct comparison of smoking and no smoking (among GI)
****p<0.001 when compared to Group I (among nonsmokers)
Table 2: Concentration of Cotinine, Imidacloprid (IMI) and its main metabolite 6-chloronicotinic acid (6- CINA) in the sera of studied groups.
Also, Group II had significantly higher semen concentrations of (IMI), and its main metabolite (6- CINA) and cotinine than did non exposed subjects for both smokers and non-smokers (P<0.001) as shown in table 3.
| Elements | G I | G II | ||||
|---|---|---|---|---|---|---|
| Total N = 30 | NS (n = 14) | S (n = 11) | Total N = 35 | NS (n = 14) | S (n = 21) | |
| Cotinine(ng/mL) | 114.57±115.81 | 0.85±0.03 | 228.29±8.15*** | 344.19±306.44* | 1.06±0.14 | 597.75±46.52** |
| IMI (ug/mL) | 0.79±0.43 | 1.11±0.66 | 0.95±0.54*** | 3.59±2.17* | 3.34±2.35**** | 3.84±2.26 ** |
| 6- CINA(ug/mL) | 0.133±0.07 | 0.19±0.24 | 0.15±0.19*** | 1.32±0.81* | 0.57±0.39**** | 0.39±0.38** |
*p<0.001 when compared to Group I (ANOVA, Tukey’s Range [HSD] test)
**p<0.001 for direct comparison of smoking and no smoking (among GII)
***p<0.001 for direct comparison of smoking and no smoking (among GI)
****p<0.001 when compared
Table 3: Concentration of Cotinine, Imidacloprid (IM) and its main metabolite 6-chloronicotinic acid (6- CINA) in of seminal plasma studied groups.
Mean values of sperm parameters in group I subjects and group II (farm workers) can be seen in table 4.
| Variable | G I | G II | ||||
|---|---|---|---|---|---|---|
| Total N = 25 | NS (n = 14) | S (n = 11) | Total N = 35 | NS (n = 14) | S (n = 21) | |
| Volume(ml) | 6.86±1.41 | 4.54±1.31 | 4.51±1.11 | 5.72±1.61 | 4.16±1.91 | 3.13±0.91 |
| Sperm count (×106 ml) | 122.81±14.34 | 887.87±13.33 | 78.37±16.56*** | 54.55±26.66* | 38.63±30.51**** | 31.81±19.69** |
| Motility (%) | 99.38±8.98 | 68.07±8.68 | 65.34±8.78 | 63.83±24.84* | 42.47±24.84**** | 42.72±21.01 |
| Normal morphology (%) | 20.09±3.93 | 15.93±3.83 | 12.16±4.84 | 7.77±3.63* | 5.75±3.59**** | 4.11±2.22** |
*p<0.001 when compared to Group I (ANOVA, Tukey’s Range [HSD] test)
**p<0.001 for direct comparison of smoking and no smoking (among GII)
***p<0.001 for direct comparison of smoking and no smoking (among GI)
****p<0.001 when compared to Group I (among nonsmokers)
Table 4: Semen parameters of subjects in the studied groups.
No significant differences between the groups were observed in semen volume. While Sperm count, motility, and normal morphology in group II smokers and non-smokers were significantly lower than those in group I smokers and non-smokers.
A trend toward a higher quality of sperm was seen for non-smokers compared with smokers in group I subjects. Also these comparisons can be seen in subjects of group II (exposure groups) smokers had significantly low quality of sperm than non-smokers (P<0.001) as shown in table 4. A significant negative correlation between sperm concentration, motility, and seminal Imidacloprid (IMI), its main metabolite 6-chloronicotinic acid (6-CINA) and cotinine was identified in group II subjects (r= − 0.489; p<0.05).
Animal part
As shown in table 5 there is significant difference in LH and FSH in group (III), when compared with all other groups.
| Test | Control Groups | Group III | Group IV | Group V | Reference Interval |
|---|---|---|---|---|---|
| LH (mlU/mL) | 2.25±0.15 | 2.0±0.1 | 1.4±0.1* | 1.9±0.14 | 0.7-7.4 |
| FSH(mlU/mL) | 0.7±0.11 | 0.9±0.1 | 1.0±0.1* | 0.65±0.1 | 0.6-0.9 |
| Tes(ng/mL) | 5.0±0.4 | 2.6±0.1* | 2.2±0.1** | 3.0±0.1* | 3.6-9.9 |
| E2 (pg/mL) | 44.2±1.1 | 51.3±1.0* | 61.5± 1.2** | 52.4±1.1* | 10-50 |
| Prolactin(ng/ml) | 6.6±1.0 | 10.0±0.1* | 12.9±0.11* | 9.0±0.2 | <20 |
Table 5: Effect of Imidacloprid administration on gonadal activity in adult male rats.
Regarding testestrone (tes) and Estradiol 2 (E2) there was highly significant difference in group IV while significant difference in both groups III and V. Also there was significant increase in prolactin level in group III and IV.
In the table 6, the obtained data showed that the sperm count in groups IV showed highly significant decreased when compared all other groups.
| Test | Control Groups | Group III | Group IV | Group V | Reference Interval |
|---|---|---|---|---|---|
| Count(x106) | 69.22± 4.56 | 61.12± 3.74 | 58.38± 1.16** | 66.54± 4.52 | ≥ 15 x 106 |
| Motility (%) | 78.71± 0.85 | 68.44± .91* | 61.49± 1.53* | 71.64± 0.91* | ≥ 60% |
| Vitality (%) | 78.24± 3.06 | 74.61± .35* | 71.74± 2.56* | 60.14± 1.4** | ≥ 58 % |
| Normal sperm morphology (%) | 84.5 ± 4.38 | 68.88±6.15** | 64.28± 4.07** | 83.81± 5.74 | > 4 % |
Table 6: Mean ± SD of sperm motility, sperm count and sperm vitality following administration of imidacloprid in rats groups.
Sperm motility and vitality were significantly lowered in groups III, IV, V. Sperm morphology was highly significantly affected in groups III and IV.
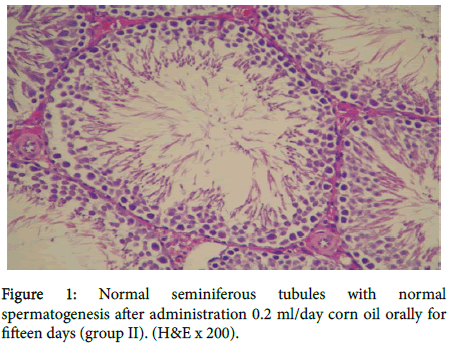
As shown in figure 1 group II rats showed normal seminiferous tubules with normal spermatogenesis.
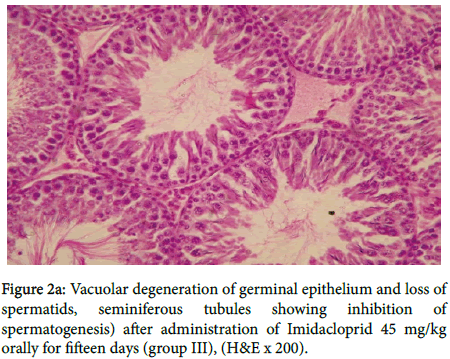
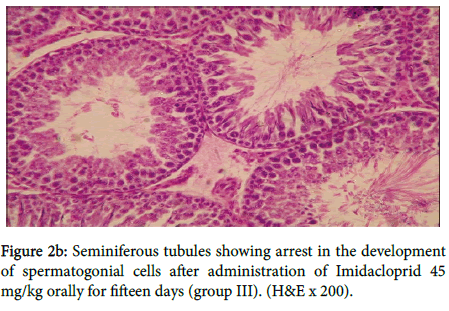
In group III, figure 2a showed vacuolar degeneration of germinal epithelium and loss of spermatids, seminiferous tubules suggesting inhibition of spermatogenesis, also in figure 2b
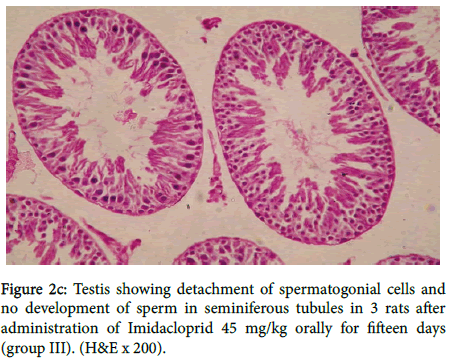
Seminiferous tubules showing arrest in the development of spermatogonial cells. Testis showing detachment of spermatogonial cells and no development of sperm in seminiferous tubules in three rats as shown in figure 2c.
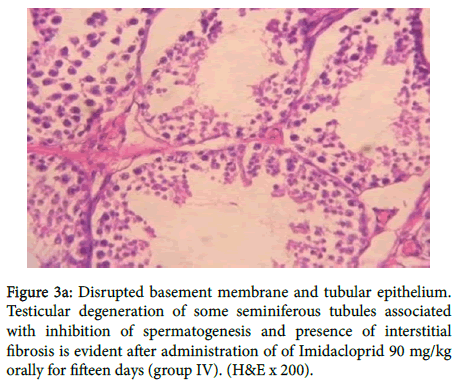
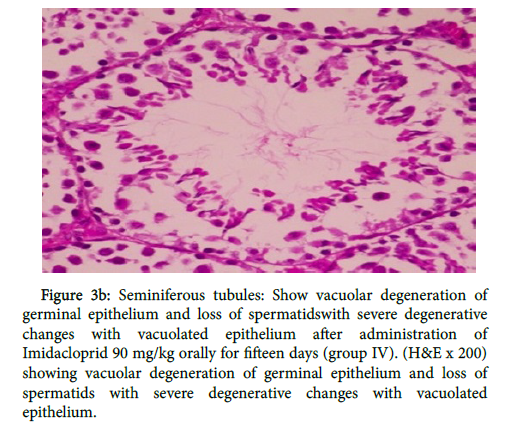
In group (IV) as shown in figure 3a there was disrupted basement membrane and tubular epithelium. Testicular degeneration of some seminiferous tubules associated with inhibition of spermatogenesis and presence of interstitial fibrosis, also in figure 3b
Figure 3a: Disrupted basement membrane and tubular epithelium. Testicular degeneration of some seminiferous tubules associated with inhibition of spermatogenesis and presence of interstitial fibrosis is evident after administration of of Imidacloprid 90 mg/kg orally for fifteen days (group IV). (H&E x 200).
Figure 3b: Seminiferous tubules: Show vacuolar degeneration of germinal epithelium and loss of spermatidswith severe degenerative changes with vacuolated epithelium after administration of Imidacloprid 90 mg/kg orally for fifteen days (group IV). (H&E x 200) showing vacuolar degeneration of germinal epithelium and loss of spermatids with severe degenerative changes with vacuolated epithelium.
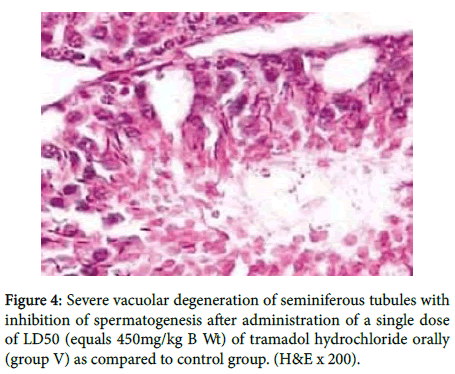
While in figure 4 there was severe vacuolar degeneration of seminiferous tubules with inhibition of spermatogenesis.
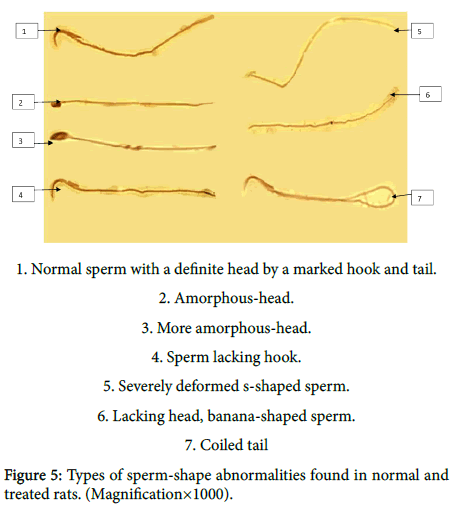
Figure 5 showing different morphological changes recovered in sperms of normal and treated rats in the form of; normal sperm with a definite head by a marked hook and tail, amorphous-head, more amorphous-head, sperm lacking hook, severely deformed s-haped sperm, lacking head, banana-shaped sperm and coiled tail.
Discussion
The effect of multi-compound pesticide exposure had been addressed by a large series of studies involving in most instances a limited number of subjects. A positive association between exposures and alterations of semen parameters was observed in the majority [11-14], but not all the published studies [15,16].
It is not surprising that 6-CINA was found in lower concentrations in comparison with IMI found in exposed subjects (Tables 2 and 3) because it is only one of IMI metabolites.
The inhibition of testicular androgenic enzymes due to pesticides exposure as observed in this study could be attributed to lower LH which is a prime regulator of testicular androgenic enzyme activities [17]. The testosterone in testis is essential for normal spermatogenesis as well as the maintenance of structural morphology and normal physiology of seminiferous tubules [18]. Decrease in the level of intratesticular testosterone may lead to detachment of germ cells from seminiferous epithelium and may initiate germ cell/testicular cell apoptosis and degeneration [19]. This leads to low total sperm count, increased sperm abnormalities, histopathologically defective spermatogenesis/lack of spermatogenesis along with testicular and epididymal changes which were clearly noticed in our study.
Similarly, exposure to IM has been observed to cause increase in cell death, production of immature sperms, and decreased sperm velocity with decrease in serum testosterone in experimental animals [20].
Our results showed that Testesterone (Tes) in the animals treated with IMI was reduced in group IV more than group III i.e., dose dependent, in accordance with Mahgoub and El- Medany [21].
The decreased epidydimal sperm concentration and increased abnormal sperm morphology are common observations in the experimental studies with many other pesticides [22].
Sperm count and sperm abnormality are the prominent factors affecting fertility. Suppressions of gonadotrophins might be an underlying mechanism for the decreased sperm density [23,24].
IMI treatments caused a significant decrease (p<0.05) in total epididymal sperm count, motility, and live sperm count (Table 5). A significant increase (p<0.05) in abnormal sperm count in terms of head and tail abnormalities, was also noted. This is in accordance with results that reported by Naryana et al. [25] and Bustos et al. [26]. Insecticides and pesticides act as reproductive toxicants in male rats leading to severe histopathological focal necrosis of germinal cells associated with tubular atrophy. Oxidative stress is a major causative factor responsible for the male reproductive failure [27].
Perry et al. [28] reported that using a onetime semen sample to evaluate motility and morphology gives a limited characterization of how an environmental exposure may impact testis function.
A decrease in sperm motility may seriously reduce fertilizing ability and is related to low level of ATP content [29]. For the normal forward movement of spermatozoa, full ATP pool is required and a slight deprivation of ATP leads to reduction in motility, which may cause infertility. Therefore sperm motility can be hindered by altered enzymatic activities of oxidative phosphorolytic process, which is required for ATP production [30-32].
Conclusion
Male reproductive system can be considered as a target for IMI exposure in treated rats manifested by histopathological damage on testicular tissue, sperm mortality, morphology and decreased testosterone level in mature male rats. IMI can cause infertility problems in chronically exposed subjects. Repeated semen analysis with longer abstinence time should to be used to asses more toxic environmental exposures for such pesticides.
Declaration of interest
The authors report no conflict of interest.
Funding
This research received no specific grant from any funding.
References
- A Elbert H,Overbeck K,Iwaya S (1990)Tsuboi, Imidacloprid, a novel systematic nitromethylene analogue insecticide for crop protection, in: Proceedings of Brighton Crop Protection Conference, Pests and Diseases 1: 21-28.
- L. Sheets (2001)Imidacloprid: a neonicotinoid insecticide, second ed., in: R. Kreiger (Ed.), Handbook of Pesticide Toxicology, Academic Press, New York pp. 1-8.
- Meister RT (1994) Farm Chemicals Handbook. 2nd ed. New York. Meister Publishing Company 301-305.
- Recio-Vega R, Ocampo-Gomez G, Borja-Aburto VH (2008)Organophosphorous pesticide exposure decreases sperm quality: association between sperm parameters and urinary pesticide levels. J ApplToxicol 28:674-680.
- Bhuiyan AS, Nesa B, Nessa Q (2001) Effect of sumithion on the histological changes of spotted murrel, Channapunctatus (Bloch). Pak J BiolSci4: 1288-1290.
- Kapoor U, Srivastava MK, Srivastava LP (2011) Toxicological impact of technical midacloprid on ovarian morphology, hormones and antioxidant enzymes in female rats. Food ChemToxicol 49: 3086-3089.
- Bal R, Naziroaylu M, Tark G, YilmazA, Kuloaylu T, et al. (2012) Insecticide imidacloprid induces morphological and DNA damage through oxidative toxicity on the reproductive organs of developing male rats. Cell BiochemFunct 30: 492-499.
- Mohammad Nabiuni, KazemParivar, RooholahNoorinejad, Zahra Falahati, FaezehKhalili, et al. (2015) The reproductive side effects of Imidacloprid in pregnant Wistar rat. International Journal of Cellular & Molecular Biotechnology 1: 10-18.
- World Health Organization (2010) WHO Laboratory Manual for the Examination and Processing of Human Semen. Fifth ed. WHO Press; Geneva.
- (1997) C.D.S. Tomlin (Ed.), the Pesticide Manual, 11th ed., British Crop Protection Council, Surrey, UK 706-708.
- Oliva A, Spira A, Multigner L (2001) Contribution of environmental factors to the risk of male infertility. Hum Reprod 16: 1768-1776.
- Swan SH, Brazil C, Drobnis EZ, Liu F, Kruse RL, et al. (2003) Study For Future Families Research Group.Geographic differences in semen quality of fertile U.S. males. Environ Health Perspect 111: 414-420.
- Kamijima M, Hibi H, Gotoh M, Taki K, Saito I, et al. (2004) A survey of semen indices in insecticide sprayers. J Occup Health 46: 109-118.
- Yucra S, Rubio J, Gasco M, Gonzales C, Steenland K, et al. (2006) Semen quality and reproductive sex hormone levels in Peruvian pesticide sprayers. Int J Occup Environ Health 12: 355-361.
- Tielemans E, Burdorf A, teVelde ER, Weber RF, van Kooij RJ, et al. (1999) Occupationally related exposures and reduced semen quality: a case-control study. FertilSteril 71: 690-696.
- Sa´nchez-Pen˜a LC, Reyes BE, Lo´pez-Carrillo L,Recio R, Moran-Martinez, et al. (2004)Organophosphorous pesticide exposure alters sperm chromatin structure in Mexican agricultural workers. ToxicolApplPharmacol 196:108-113.
- MilindmitraLonare, Manoj Kumar, Sachin Raut, Amar More, SagarDoltade,et al. (2015) Environmental Toxicology, Evaluation of Ameliorative Effect of Curcumin on Imidacloprid-Induced Male Reproductive Toxicity in Wistar Rats.
- Russell LD, Kershaw M, Borg KE, El Shennawy A, Rulli SS, et al. (1998) Hormonal regulation of spermatogenesis in the hypophysectomized rat: FSH maintenance of cellular viability during pubertal spermatogenesis. J Androl 19: 308-319.
- Blanco-Rodriguez J, Martinez-Garcia C (1998) Apoptosis precedes detachment of germ cells from the seminiferous epithelium after hormonal suppression by short-term oestradiol treatment of rats. Int J Androl 21:109-115.
- Najafi G, Razi M, Hoshyar A,Feyzi S, ShahmohamadlooS, et al. (2010) The effect of chronic exposure with imidacloprid insecticide on fertility in mature male rats. Int J FertilSteril 4:9- 16.
- Mahgoub AA, El-Medany AH (2001) Evaluation of chronic exposure of the male rat reproductive system to the insecticide methomyl.Pharmacol Res 44: 73-80.
- Matsumoto M, Hirose A, Ema M (2008) Review of testicular toxicity of dinitrophenolic compounds, 2-sec-butyl-4,6-dinitrophenol, 4,6-dinitro-o-cresol and 2,4-dinitrophenol. ReprodToxicol 26: 185-190.
- Bebb R.A,Anawalt BD, Christensen RB, Paulsen CA, Bremner WJ, et al. (1996) Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: a promising male contraceptive approach. J ClinEndocrinolMetab 81: 757-762.
- Sinha N, Narayan R,Shanker R,Saxena DK (1995)Endosulfan induced biochemical changes in the testis of rats.Vet. Hum. Toxicol 37: 547-549.
- Narayana K, Prashanthi N, Bairy LD,Narayana A, D'Souza UJ(2006) An organophosphate insecticide methyl parathion (0-0- dimethyl0-4-nitrophenylphosphorothioate) induces cytotoxic damage and tubular atrophy in the testis despite elevated testosterone level in rats. J ToxicolSci 31: 177-189.
- Bustos-Obregón E, González-Hormazabal P (2003) Effect of a single dose of malathion on spermatogenesis in mice. Asian J Androl 5: 105-107.
- Kaur P, Bansal MP (2004) Effect of experimental oxidative stress on steroidogenesis and DNA damage in mouse testis. J Biomed Sci 11: 391-397.
- Perry MJ, Venners SA, Chen X, Liu X, Tang G, et al. (2011) Organophosphorous pesticide exposures and sperm quality. ReprodToxicol 31: 75-79.
- Bai JP, Shi YL (2002) Inhibition of Ca(2+) channels in mouse spermatogenic cells by male antifertility compounds from Tripterygiumwilfordii Hook. f. Contraception 65: 441-445.
- Rodriguez H, Bustos-Obregon E (2000) An in vitro model to evaluate the effect of an organophosphoricagropesticide on cell proliferation in mouse seminiferous tubules. Andrologia 32: 1-5.
- Contreras HR, Bustos-Obregón E (1999) Morphological alterations in mouse testis by a single dose of malathion. J ExpZool 284: 355-359.
- Akbarsha MA,Latha PN, Murugaian P (2000) Retention of cytoplasmic droplet by rat caudaepididymal spermatozoa after treatment with cytotoxic and xenobiotic agents. JReprodFertil 120: 385-390.
Relevant Topics
- Aflatoxins
- Cardiac Toxicity
- Chemical Toxicology
- Developmental Toxicology
- Drug Toxicity
- Heavy Metal Toxicity
- Heavy Metal Toxins
- Industrial Hygiene Toxicology
- Insecticides Toxicology
- Metal Toxicology
- Nano Toxicology
- Pesticidal Toxicology
- Renal Toxicity
- Reproductive Toxicology
- Skin Toxicology
- Tetanus Toxin
- Toxicogenomics
- Toxicology Reports
- Toxicology Testing
Recommended Journals
Article Tools
Article Usage
- Total views: 14082
- [From(publication date):
May-2016 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 12982
- PDF downloads : 1100