Research Article Open Access
The Need for Herbicide Use in Direct Seeding, Promotes More Sustainable and Implementation of Integrated Systems
| Luna V1*, Masciarelli O1, Travaglia C2, Marchetti G1, Lucero M1 and Reinoso H2 | |
| 1Laboratory of Plant Physiology, Spain | |
| 2Plant Morphology, Department of Natural Sciences Faculty of Exact, Physical and Natural Chemistry, National University of Río Cuarto, Ruta 36 Km 601, Rio Cuarto, Córdoba | |
| Corresponding Author : | Luna V Laboratory of Plant Physiology, Spain Tel: +543584676230; +543584676103 E-mail: vluna@exa.unrc.edu.ar; omasciarelli@exa.unrc.edu.ar |
| Received May 11, 2015; Accepted July 08, 2015; Published July 15, 2015 | |
| Citation: Luna V, Masciarelli O, Travaglia C, Marchetti G, Lucero M, et al. (2015) The Need for Herbicide Use in Direct Seeding, Promotes More Sustainable and Implementation of Integrated Systems. Adv Crop Sci Tech 3:176. doi:10.4172/2329-8863.1000176 | |
| Copyright: © 2015 Luna V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Advances in Crop Science and Technology
Abstract
Technological advances the Argentine has been in recent years such that now stands at the forefront worldwide. The explosion of agriculture, tillage, GM crops and the widespread use of glyphosate have raised controversy, especially regarding the toxicity of the latter. Before weeds are controlled with tillage and these involve serious problems of erosion and soil degradation. Tillage and herbicides have changed the situation and consequently the sustainability of production systems. Glyphosate plays an important role in this transformation, but were allowed to use, would be replaced by more expensive and dangerous herbicides. Under that is essential to achieve these objectives through high yields very tech practice, it is necessary to introduce new technologies to assist the sector mitigation tools and can coexist using those chemicals that may mitigate high exposure to them through technology capabilities to detoxify crops. This tech package is just something known by the scientific-technical and production industry, but that has innovation in its application. The use of PGPR as biofertilizers is a widespread technique that has been installed as a enhancer of crops and environmentally friendly. Our study is based on countering the abuse of chemicals through a complementary technology to mitigate residual glyphosate in crops.
| Keywords |
| PGPR; Glyphosate; Foliar fertilizer application |
| Agricultural Prospect Argentine |
| In the Province of Córdoba over 50 million liters of glyphosate applied per year. The figure only includes the amount of herbicide used, on average, to fumigate more than six million acres of soybeans and corn planted last season. Based on the same estimate in Río Cuarto department over 6 million liters of this agrochemical apply. |
| This is actually in line with what happens at the national level: “The consumption of agrochemicals in Argentina continually increases,” warns a report analyzing the market for pesticides in the country prepared by Physicians Fumigated Towns from the University Network Environment and Health (Reduas). With alarming figures, professionals emphasize the impact these practices have on the environment and population. |
| Based on data from the Chamber of Agricultural Health and Fertilizers (CASAFE), the report reveals that pesticide use nationwide increased 858% in the past 22 years did acreage by 50%, while the yield crops only grew 30%. According to the document, in the campaign 2012/2013 317 million liters of pesticides were applied in the fields of the country, of which 200 million are glyphosate, the most used product in Argentina. Thus, agrochemical sales increase year to year: in the last period this service reached a turnover of 2,381 million dollars. “The strength of the business would not worry so much if they are fumigated with this huge amount of chemical intensive monoculture areas where more than 12 million people live. These same communities are exposed every year, during the same months, the same products, but every year the same dose is increased gradually and the mixture even more dangerous toxic”. |
| The expansion of GM crops in the last two decades in Argentina has led to increasing use of agrochemicals. But in turn, the data show that current production model needs to apply increasing amounts of these products to cultivate the same area. While in 1996 -when begins to transgenic soybeans planted in the country it is recommended to apply up to 3 liters of glyphosate per hectare per year, according Reduas today in the same hectare get to apply up to 12 liters annually. |
| This data, say from the university network, “demonstrates the inability of the model to address toxic agriculture adaptive responses of nature, and the emergence of resistance in plants. The only reflex response is to increase the dose of the chemical, question. Meanwhile, various experts consulted by this newspaper indicate that, on average, currently 8 liters of glyphosate applied per hectare per year. Thus, in the province of Córdoba during the last year more than 50 million gallons of the herbicide were applied to cover 6,340,840 acres of soybeans and corn planted in 2012. |
| In the department Río Cuarto, the latest available data indicate that the 2011/2012 cultivated with soybean and corn area was 760,000 hectares. This means that, each year, the region more than 6 million gallons of the herbicide Roundup apply. Even those amounts is compounded fumigation carried out in other crops before planting, and even in home gardening, although both uses represent a small percentage compared to the large amounts used in soybean fields and transgenic corn. These two crops focus not only the main demand of glyphosate but also the rest of the agrochemicals used: of the 317 million liters of pesticides applied in the last campaign in the country, 258 million were used in corn and soybeans. |
| In 2012 the total pesticide consumption reached 335 million liters and forth continuously increasing, based on data taken up by the Medical CASAFE fumigated Peoples. “In a few years, mostly by weather issues, fumigated volumes decreased slightly, but the extended series shows a consistent upward trend in the consumption of pesticides”, expressed in the report. Furthermore, citing the chambers of industry, explained that last year the volume used slightly decreased (5%), because it is sold prepared with higher concentrations of active ingredients. |
| However, growth in the use of chemicals in recent decades reveals great disproportion to the increase in cultivated areas in Argentina. “In 20 years, from 1991 to 2012 the cultivated grain and oilseed area increased by 50%, 20 to 30 million hectares, and consumption of pesticides rose from 39 million to 335 million liters per year, an 858% more volume used”, synthesized in the document. |
| Given these figures, agronomist at the National University of Rio Cuarto Claudio Sarmiento explained that although the area planted is growing every year, the amount of chemicals needed is increasing due to the characteristics of the production model. On the one hand, he said, “some weeds are developing resistance to the herbicide glyphosate and are being implemented more liters each year.” However, he stressed that the consumption of fertilizers is that has increased due to the loss of soil fertility and the tillage system does not remove the soil as conventional tillage, preventing it from oxygen and nutrients are released organic matter. |
| The concept of sustainable agriculture, which involves a set of production systems with few inputs and integrated pest and disease management, has growing importance in our country, promoting innovation and improving agricultural production. Priority was thus given to ecologically safer methods, minimizing unwanted agrochemicals and emphasizing environmental protection and human health [1,2] side effects. |
| The effectiveness of controls requires the development of qualitative and quantitative regulations that address the relationships between pesticide residues and the factors affecting their level, such as: a) physical and chemical properties of compounds. b) Conditions of use (speed, dose, frequency, etc.) c) weather condition d) structural and chemical characteristics of the sites or objects of treatment e) production processes and maintenance [3]. |
| Among other measures, the European Union created in 2002, the Rapid Alert System for Food and Feed [4] designed to ensure the exchange of information and facilitate control by the authorities on the detection pollutant and the implementation of measures to ensure food safety. Through this system the formation of a network of Member States, which requires, among other things, the immediate notification to the other states of any pollution incident in a batch, container or cargo is set where contaminants are detected above the Maximum Residue Limits (LRMs) allowed in the rules, which involve direct or indirect health risks (European Commission Regulation 466/2001, European Commission Regulation 178/2002, European Commission Regulation 882/2004). |
| The LMR is the maximum concentration of a pesticide residue that can legally contain a product intended for human consumption or when used for animal feed in mg pesticide/kg food [5]. The maximum residue limits are set by national regulatory agencies based on the recommendations of the JMPR (Joint FAO/WHO Meetings on Pesticide Residues) and good farming practices, which ensure that the pesticide residue in food is kept as low as possible practically. |
| For the National Health Service and Food Quality (SENASA) the LMR relates to the maximum concentration of a residue of plant protection products, which are legally permitted or recognized as acceptable in or on a food, agricultural commodity or animal feed. Through Resolution No. 507/2008 the Secretariat of Agriculture, Livestock, Fisheries and Food (SAGPyA) has set LMRs currently in effect. |
| Biotechnology as a tool for agricultural sustainability |
| According to the Convention on Biological Diversity of 1992, biotechnology could be defined as “any technological application that uses biological systems, living organisms or derivatives thereof, to make or modify products or processes for specific use”. |
| The promises of agricultural biotechnology reside on increasing productivity and reducing costs, generating innovations and improvements in food and lead to more “green” agricultural practices; contribute, in short, sustainable agriculture, which uses resources with respect for the environment without mortgaging future generations. |
| The entrance of biotechnology in agriculture is characterized by three main features: the redefinition of the public and private sectors in research and development (R&D) agronomic; increasing integration or cooperation with the food industry, chemical and pharmaceutical; displacement of agricultural innovations from classic Industrial substrate (machinery, agrochemicals) to the biological substrate, based on a better understanding and manipulation of living systems [6]. |
| Since the concept of agricultural sustainability cannot be measured directly, indicators are required to determine the levels and temporal variations especial presenting the sustainability of a given activity. This paper presents and analyzes agricultural sustainability from the perspective of the cropping system and production and how it impacts on practice, which will ultimately have an impact on the food, we eat. |
| Herbicide |
| Glyphosate [N-(phosphonomethyl) glycine] is a nonselective herbicide, post-emergence, broad-spectrum, widely used for weed control. Introduced by Monsanto in the 70s as the active ingredient of Roundup formulated and is perhaps the most widely used herbicide in the world. |
| It is used in the range of 10 days prior to harvest, is a nonselective systemic broad-spectrum herbicide that when translocated throughout the plant, inhibits the production of some aromatic amino acids essential for plant growth. The main natural metabolite of the plant and the soil is amino methyl phosphonic acid (AMPA). By the nature of their physical and chemical properties of glyphosate is not suitable for weed control treatments via the root system. |
| In Argentina, the current model of agriculture is based on direct seeding; use a technology package that includes genetically modified crops resistant to glyphosate (Roundup Ready) is widespread. This tolerance of crops leads to the widespread use of this herbicide for weed control because it can be applied throughout the crop cycle without causing damage to it. According to CASAFE [7] in the domestic market in 2007 more than 160 million liters were sold. The two main crops with these characteristics are soybean (Glycine max) and corn (Zea mays). |
| The supposed low bioaccumulation of glyphosate in these species has been called into question by works such as Arregui et al. [8] in which three fields in the province of Santa Fe were monitored with two or three applications of glyphosate isopropylamine in different concentrations, yielding glyphosate residues ranging from 1.9 to 4.4 mg.kg-1 in leaves and stems and from 0.1 to 1.8 grains. Lorenzatti et al. [9] found low concentrations of green soy beans and some processed foods from soybeans. |
| The National Commission for Scientific and Technological Research (CONICET) in the report made under decree 21/2009 on the assessment of scientific information related to glyphosate in their impact on human health and the environment partially concludes that “despite paucity of data on the levels of glyphosate and AMPA residues in foodstuffs, food consumption containing residues of glyphosate and its metabolite, when applying good agricultural practices, contribute a minimum portion of the Acceptable Daily Intake (ADI) as would not constitute a risk to consumer health. “With respect to this last statement, which are not specific good agricultural practices (GAP) in relation to applications of glyphosate. |
| For the degradation of glyphosate, the microbial transformation is perhaps the main track, to transient intermediates as AMPA (highly toxic amino methyl phosphonic acid),sarcosine and formaldehyde to carbon dioxide, in which the largest amount of detected radioactivity recovered in different studies with 14C-glyphosate. Various bacterial strains have the ability to decompose and degrade glyphosate. In most laboratory experiments, the rate of degradation of glyphosate in soil appears to be rapid. In most cases, the process can be described as linear first order kinetics (Weed Science Society of America, Herbicide Handbook, Gliphosate, p150, 7th. Ed 1994). Also, studies that Monsanto has said that glyphosate as an herbicide is inactivated and degrades rapidly in the soil. The United States Environmental Protection Agency has reported that the half-life of glyphosate in soil may be up to 60 days or more, according to The EPA (Washington US, environmental Protection Agency) adds that the waste field studies are often the following year. |
| Some commercial formulations of glyphosate incorporate a surfactant known as POEA, in a proportion of about 15%. This compound, according to various toxicological investigations, can cause gastrointestinal damage, certain central nervous system disorders, and some breathing problems and be able to destroy red blood cells in human blood. The POEA is said, too, that may contain an impurity identified as 1-4 dioxane, which has demonstrated carcinogenicity to animals and cause damage to the liver and kidneys of humans [10]. |
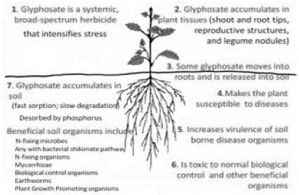
| As shown in Figure 1, glyphosate interacts with a broad range of health determinants, which intensifies the stress and reduces the yields. Not only accumulate in plant tissues (stem and root tips, reproductive structures and legume nodules), accumulates in the roots from which then seeps into the soil and damages the beneficial soil microorganisms including acting as a biological control of pathogens the obvious consequence is the increased virulence of soil borne pathogens that lead to disease. |
| Herbicide interactions/biological |
| A rhizosphere level, there are studies conducted with transgenic soybean resistant to glyphosate, Zablotowicz and Reddy [11] found that nitrifying bacteria Bradyrhizobium japonicum, which fix nitrogen in soybean roots, has a sensitive enzyme glyphosate when it is exposed to this herbicide, shikimic acid accumulates and hydroxybenzoic acids, causing growth inhibition and death of the bacteria at high concentrations. It was also found that glyphosate accumulates in the root nodules of soybeans. Everything described affects growth of all leguminous plants (which establish symbiotic relationships with nitrogen-fixing bacteria) and soil health in general. |
| This herbicide affects especially the agro-ecosystem nitrogen cycle phenomenon reported by Hutchinson [12] and Nielsen and Favilli [13]. A study in India with degraded soil from tea plantations treated with glyphosate, demonstrated a reduction in colonies of nitrogen-fixing bacteria Bezbaruah et al. [14] has also been reported an inhibition on root nodulation in soils clover glyphosate levels between 2 and 2000 mg / kg soil, the effect persisted for 120 days after the Eberbach et al. [15] application. |
| Moreover, the PGPR (growth promoting rhizobacteria plants) have demonstrated ability to tolerate the presence of herbicides [16], to degrade xenobiotic, such as phenolic compounds and benzoates [17]. Furthermore, it has demonstrated its ability to metabolize root exudates, the biodegradation byproducts intermediates of aromatic, such as lignin, tannins and xenobiotic halogenated [18] molecules. Also, its ability to be used as remedial waste of oil and aniline Murakami was demonstrated. Yet very little has been studied on the effect of herbicides on growth of Azospirillum and some strains of the genus Pseudomonas in vitro, no data on the decline of nitrogenase activity in Azospirillum roots associated with the presence of glyphosate alone 2,4-D, and others like Mepro and Dipro [19]. |
| The use of PGPR bacteria |
| Microorganisms, especially Azospirillum and Pseudomonas, have a critical or leading role in activities that affect the development, nutrition and health of plants, and also produce a profit on the floor. |
| No symbiotic bacteria fix atmospheric nitrogen, and living in the rhizosphere [20,21], those often cause increased crop yields. Many species of N2-fixing bacteria possess these properties, including Bacillus spp, Azotobacter spp, Azospirillum spp, Beijerinckia, Pseudomonas spp, etc [20,22]. |
| One explanation of the effect of the use of PGPR on plant growth stimulation is the production of growth regulators [23], several of them having been identified in culture supernatants of A. brasilense and Pseudomonas sp (auxins, zeatin, gibberellin, abscisic acid and ethylene). In quantitative terms, the most important plant hormone produced by these bacteria is the auxin indole-3-acetic acid (IAA) acid. It is assumed that the production of this hormone by the bacteria is causing radical changes in the system after inoculation, which is associated with greater absorption of nutrients. Bacterial IAA interacts with the plant and has the potential to influence some of these processes through changes in the spatial path of auxin pool plant. The impact of exogenous auxins on plant development oscillates between positive and negative effects, usually being a function of the available amount of IAA and plant tissue sensitivity to changes in the concentration of IAA. |
| Masciarelli [24] showed that at least in part, the ability to promote the growth of maize root system Azospirillum sp. synthesis dependent AIA, and this hormone is part of an assembly consisting of the complete yeast culture medium=+ bacteria + adding Trp (dl-tryptophan). The mere participation of bacteria is not sufficient to promote early seedling growth (measured as shoot dry weight), but it produced by the AIA and released to the medium (supernatant) is also required. It was also evident that the AIA in the supernatants is the developer of changes in exo-morphology and anatomy of the root apex [24,25] Bacterial AIA would be used primarily for the development of roots and likely to generate a microcosm around roots favoring a greater affinity with the bacteria. Here, Perrig et al. [26] could confirm that two strains of A. brasilense compounds produce plant growth promoters, and can be quantified in vitro ethylene, indole-3-acetic acid, gibberellic acid, zeatin, abscisic acid cultures and polyamines. They also observed that some content increased when it was added the biosynthetic precursor. |
| Agricultural practices |
| Extensive agricultural practices that’s seeks high performance, requiring extensive use of chemical fertilizers, which are costly and create environmental problems. Therefore, more recently there has been a resurgence of interest in a sustainable environment, for example, organic farming practices [27] The use of biological fertilizer containing beneficial microorganisms, rather than chemical synthetic products, provide the advantage of improving plant growth by direct supply of nutrients or to facilitate the incorporation of the same, providing growth regulators, as well as, because they are biological products are environmentally friendly. |
| While this practice of using biofertilizers is widespread in our country, it has always been to apply the product as seed inoculants. Few studies on the effects these products may have on plant growth by foliar application. |
| Por ejemplo, estudios de la aplicación foliar de Bacillus OSU- 142 y Pseudomonas BA-8 sobre maíz evidenciaron un aumento en el porcentaje de micronutrientes sobre las no tratadas [28]. |
| The information gap regarding the accumulation of glyphosate in the corn kernels, which are used both for direct human consumption and for livestock consumption, coupled with the lack of information regarding the influence of different doses and times of application on the concentration of residue in them as well as the potential of using PGPR bacteria (Pseudomonas and Azospirrillum) as promoters of growth and nutritional facilitators detoxifying chemical waste are the reasons for this proposed work. |
| Considering the chemical structure of glyphosate and its derivatives, and the types of carbonaceous sources can be used PGPR bacteria of the genera Pseudomonas and Azospirrillum for metabolism, we considered the possibility that the use of growth regulators is foliar a tool, as it has been studied by several authors and has been proven that the answer varies depending on the tissue, the sensitivity, the concentration of PGRs (Plant Growth Regulators). Cytokines are known to cause an increase in stomatal aperture, while abscisic acid on the contrary; gibberellins cause stem elongation and movement of photo-assimilates; auxins and thickening of the walls of the stem and root, salicylic enhancement improves leaf area [29,30]. |
| Proposed Panorama |
| The use of these bacteria PGPR, at leaf level can detoxify grains and corn plant tissue contaminated by the use of glyphosate and residual chemical compounds, thus improving their quality. This effect would be additive to the already known increase in crop yields, give sustainability to the system and introduce innovative purposes healthier as is the use of biological foliar fertilizers agricultural practice. We may also get their crops with improved tolerance to different responses to airborne pathogens, targeting health thereof. This, could be replaced, at least in part, imported inputs used in the customary practices of foliar fertilization with synthetic chemicals and the use of growth regulators for its high cost. Furthermore, we are aiming for a more environmentally friendly practice, and to allow crops to withstand marginal areas and / or conditions of abiotic stress, and help detoxification of xenobiotic by the PGPR. |
| Hypothesis |
| H1: There is a correlation between the amounts of glyphosate added to bacterial culture media (PGPR) in Vitro with accumulated at the end of fermentation (residual). |
| H2: Glyphosate application level or seedlings will have an adverse effect initial phyto-toxic and could be reversed by application of PGPR at leaf level in the early stages of growth. |
| H3: residues of glyphosate were found in RR corn kernels treated with the product. |
| H4: The concentrations of glyphosate found in some treatments exceed the maximum residue limits set forth by the SAGPyA-SENASA. |
| H5: The application of PGPR to allow lower level foliar glyphosate residues. |
| Specific objectives |
| 1-Assess the ability of bacteria PGPR (Azospirillum and Pseudomonas) use the herbicide in vitro by biological growth parameters. |
| 2-Quantify the residual values of the products of chemical synthesis; determine their degradation or metabolisms in vitro by these bacteria. |
| 3-Evaluate growth parameters in RR corn seedlings to glyphosate application and PGPR. |
| 4-Confirm the presence of glyphosate in RR corn seeds after a crop cycle conducted by agricultural practices commonly used in the area. |
| 5-Determine the correlation between the amounts of accumulated glyphosate and different doses and time of application of the herbicide. |
| 6-Check if the concentrations found exceed the maximum limits tolerated by SAGPyA-SENASA. |
| 7-Demonstrate the use and application of PGPR at leaf level decrease residual amounts of the herbicide. |
| Methodology and Results |
| Specific objective 1 and 2 |
| The preparation of inocula strains Azospirillum brasilense strain Az 39 and used Pseudomonas sp. Cultured in liquid LB medium (Luria Broth, Sigma Chem. Co.) in a shaker at 30 ° C at 80 rpm. The purity of the strains by the Gram staining technique and mobility was evaluated in fresh. Growth was assessed by biomass production by measuring optical density (OD 546 nm). |
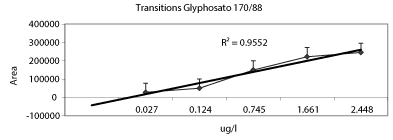
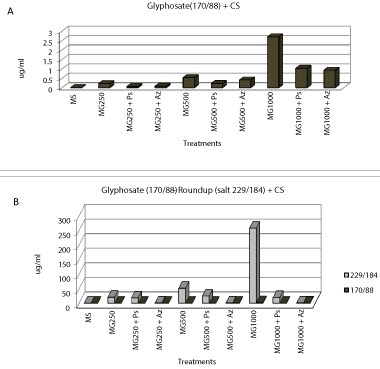
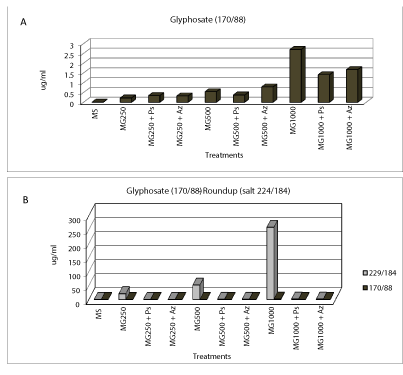
| Tolerance tests in vitro bacterial cultures added to the medium sources of pure glyphosate (Sigma Chem. Co.) and trade (Roundup) were performed. Tolerance tests were performed at different equivalent concentrations of 250, 500 and 1000 μl of a 1.3 ppm solution of each herbicide. On one hand, the treatments consisted of the use of these compounds added to the medium with and without the added carbon source Congo red medium [31], (Figure 2) and monitoring the growth was performed for 120 hours, by measurement of DO 546 nm, see Figures 3 and 4 respectively. Then we proceeded to separate cells from the supernatant by centrifugation at 8000 rpm for 15 min/4ºC. On this supernatant was determined by liquid chromatography-mass spectrometry (LC-MSMS, Waters Corp. and Micromass Ltd.) presence and quantification of herbicides. The extraction method and used prepurification was the organic partition with ethyl ether in acidic (pH=2.5 to 3) medium. Subsequently, the organic phases were collected and evaporated to dryness and re suspended in 100 ul of 100% methanol to be injected into the chromatograph. The analytes corresponding to pure glyphosate study were the following transitions: |
| a) Pure glyphosate (N-phosphonomethyl glycine): 170/88 and 170/124 |
| b) Roundup (isopropylamine salt of (N-phosphonomethyl) glycine): 229/184 and 170/88 |
| Previously, a calibration curve was constructed with both compounds from increasing concentrations and was designed from data obtained in the LC-MSMS, (Figure 2). |
| Treatments used for testing metabolites or degradation in vitro for both bacterial strains were as follows (Table 1). |
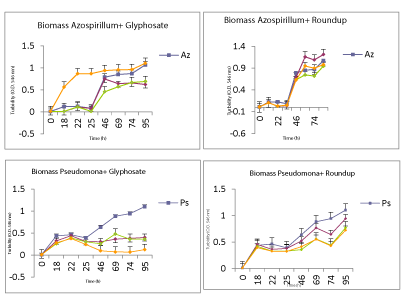
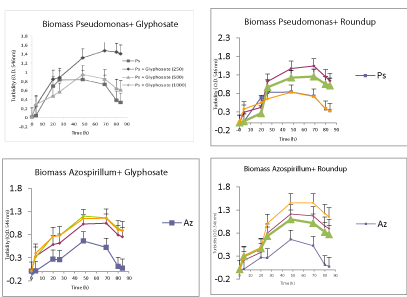
| When the analysis of degradation or loss of substrate (glyphosate or Roundup) PGPR both in vitro conditions for the critical condition with carbon source available, the results obtained were as follows was performed, (Figure 5). |
| As for in vitro condition without available carbon source and essential chemical herbicides, disappearance curves of the same is shown in the following (Figures 6). |
| Test application modeling to blister consisted of placing a tube with 100 μl A Roundup and other tube herbicide B + 100 μl was placed 100 ul of inoculum (1 × 109 cfu/ml) + 100 μl of bacterial/stabilizing protector. After 5 days of testing determinaciσn residual and bacterial survival is realized. Subsequently, upon the determination, the tube A was diluted with 200 μl double distilled water to match the dilution had the pipe B, then the samples according to as described above were processed, 10 μl was injected into the euipo LC-MSMS, and the results were shown in the following (Table 2). |
| Specific objective 3 |
| To demonstrate that the application of glyphosate at leaf level physiological changes generated on the growth of the seedlings developed laboratory experiments in growth chambers controlled by photoperiod at 30°C/16 C and 22 h light/8 h dark with 40% RH (relative humidity). 5 glasses were placed for treatment with three seeds/vessel of 300 cc with ground support perlite (1:1). These were placed on plastic trays that contained Hoagland solution (macro and micronutrients) that capillary irrigation was maintained. These trials lasted 30 days, at 14 and 24 days foliar applications were made. The doses of Roundup were used according to what is proposed in the tag, preparing a solution of 30 ml for sprayado for each treatment, while for the bacteria (inoculants) dose of 1 l/100 l of water was used. The treatments were: |
| a) control Roundup (G) |
| b) Roundup + Azospirillum brasilense Az39 (G+A) |
| c) Roundup + Pseudomonas sp (G+P) |
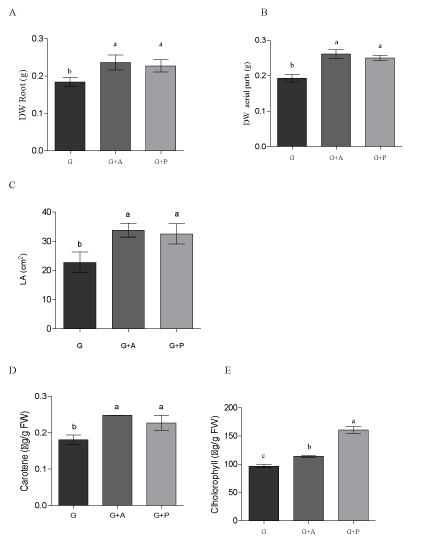
| The dry weight of aerial and root hiatus (b), average leaf area (c), average total chlorophyll content and carotenoids (dye), phytohormone content or profile (f) and residual content in seedlings (g) were evaluatedand are shown in (Figure 7 and Table 3). |
| Specific objectives 4 to 7 |
| To meet these objectives the following field experiment design. |
| Location: The study site was the experimental field of the Faculty of Agronomy and Veterinary UNRC whose coordinates are: 33° 06’ 29.71’’S; 64° 17’55.78’’W. It is located 432 m.s.n.m. and precipitation regime of the area is monsoon type. |
| Field trials: A test with the same variety of RR corn plots subjected to different treatments by land application spray Glyphosate isopropylamine salt 48% (generic Roundup) was performed. |
| Treatments were those who follow and consisted of three application times: |
| -A single application at 3-4 leaves. |
| -A single application to 6-7 leaves. |
| -Two applications at 3-4 leaves and 6-7 leaves. |
| -A test or blank without any treatment |
| In turn, for each dose of the above treatments were applied 2.5 liters to 5 liters per hectare. Among the herbicide application rates were 2.5 lts, replicated the time of application described above to evaluate the effects of the use of PGPR at leaf level at a dose of 2.5 l/ha in two stages of the crop cycle. All plots were planted by direct seeding with fertilizer applications and other commonly used pesticides in cultivation, in order to reflect as closely as possible the actual practices in the area (Table 4). |
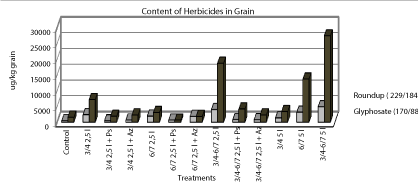
| Spectral/glyphosate quantitative analysis: Once the crop harvested grain samples were analyzed in the laboratory. Glyphosate Quantification was performed by liquid chromatography (Waters Inc., MA, USA) coupled to tandem mass spectrometry (Micromass, MA, UK) (LC-MSMS). Technique of grinding homogenizer beans to a powder was used, then 5 g of grinding weighed and was added 25 ml of H2O deionized and container ultrasound was placed for 10 min, then 10 min and centrifuged at 6000 rpm, then the process was repeated sonication and centrifuged to finally filter and inject 10 ul to LC-MSMS team, Glyphosate isopropylammonium salt (m/z: 229/184) and the derivative of glyphosate metabolism (AMPA or aminomethyl) (m/z: 110/63). Previously, calibration curves were constructed from the different standards to determine and quantify the concentrations found in the material. These analytes were evaluated by electro spray ionization mode at + and through multiple reaction monitoring (MRM), (Figure 8). |
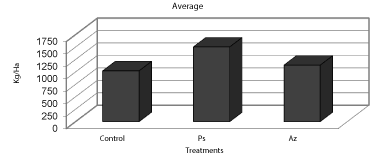
| This figure shows the profile of contamination or residues of the herbicide in the beans according to the treatment, since the commercial product (Roundup) could determine that heavily salt is converted to the active ingredient “glyphosate” on inspections treated without the application of biological. Moreover, when evaluating the returns were taken or gathered fruit for controls 3.4, 6-7 and 3 / 4-6 / 7 to 2.5 liters, and the same for which was added the application of biological (Pseudomonas and Azospirillum respectively). Corresponding to 47 and 11% increase in yield over the control respectively (Figure 9). |
| Discussion and Conclusion |
| Both strains were able to circumvent the growth conditions, Azospirillum showed greater capacity and growth rate of glyphosate when the highest concentration available carbon source; whereas with Roundup growths were much more even, with the best performance with the lowest concentration. Pseudomonas presented a smaller capacity growth compared to controls without glyphosate or Roundup. When broth was absent essential carbon source, the strains showed a greater ability to grow any of the concentrations where the chemical was present, preferably being glyphosate Roundup for Pseudomonas and Azospirillum. It’s like the bacteria Azospirillum is less affected by the chemical ingredients of the commercial product. These data growth, measured as the value of biomass, could be corroborated with the skills and abilities of both strains to metabolize or break the presence of chemicals in the tested conditions. The application of these bacteria PGPR (Azospirillum and Pseudomonas) as bioestimulante or biofertilizers contributing to increased crop production, should be a more widespread practice and assumed by the productive sector, as it is friendly interest in the environment, and is a sustainable and organic farming practice. In those regions where fertilizer application is little or, as in some countries with underdeveloped agriculture, this is a more common practice. In regions where the practice is based on a modern technical agriculture, these tools use would reduce the large amounts of fertilizers and pesticides that generally apply, and would be less costly. This, would reduce both the cost of production and the problems arising from their use, mainly decrease pollution. Therefore, we proposed to extend the applicability of these biological, taking into account its proven effectiveness in yields and plant health; In this sense, the dual purpose was in addition, to evaluate whether the application improved herbicidal effects caused in the first days of development of the plant. So much so, that we achieve certain growth parameters were affected when foliar applied herbicide alone. Mainly by modifying the intrinsic hormonal profile seedling favorable condition to develop and improve the integrity of it despite the herbicide applied. We could also confirm that the application of these biological components mitigate the effects of the herbicide and its residues in tissues. In addition to all these results, we were able to reaffirm the results found by field, where not only observe a lower concentration of the herbicide in grain crop, but we significantly improve yields of the same. |
| The use of PGPR bacteria to detoxify foliar contaminated grain and glyphosate use and residual chemical compounds, thus improving their quality corn plant tissue. This effect would be additive to the already known increase in crop yields, sustainability purposes the system and introduce a healthier as is the use of biological fertilizer agricultural practice. We may also get their crops with improved tolerance to different responses to airborne pathogens, targeting health thereof. We could substitute, at least in part, imported inputs used in the customary practices of foliar fertilization with synthetic chemicals and the use of growth regulators for its high cost. Furthermore, we are aiming for a more environmentally friendly practice, and to allow crops to withstand marginal areas and / or conditions of abiotic stress, and help detoxification of xenobiotics by the PGPR. |
| Herbicides are widely used in agricultural practices. Some of the farmers, because of their ignorance and impatience in achieving high performance, apply more than the expected amount of herbicide application. These chemicals can persist for long periods in the environment, which may affect other organisms which were not addressed. This study reports the capacity and ability of two bacterial species commonly used as inoculants, which can modify the tissue contamination and grain crops treated with herbicides. The ability of these strains to utilize glyphosate or Roundup primarily provides a means of effectively eliminating this compound, not only to the agricultural products, but also to reduce environmental pollution they cause. Therefore the ability of strains to survive and grow in the presence of high concentrations of herbicide marks them as good candidates for bioremediation of these chemicals in different environments. |
| The proposal leaves sitting the basis for establishing traceability rules in grains and vegetables on aspects of food hygiene and safety. In the case of grains will establish control parameters for export and for the food industry, for purposes of applying the rules on marketing. While consumer and vegetables entrants Markets Supply it possible to determine maximum permissible levels of residues and/ or contaminants. |
| Acknowledgements |
| This work was supported by the equipment resources of the University National of Río Cuarto (UNRC) and by funding from the National Agency of Scientific and Technological Promotion (ANPCyT), the National Council for Scientific and Technical Research (CONICET), and UNRC. We also thank Lic. Julia Iparraguirre for help with the figures and tables editing. This research is being adopted to continue research to field level Inbicor Argentina SACompany (Rio Cuarto, Argentina). |
| References |
References
- value="1" id="Reference_Titile_Link">Ricca A (2004) Chemical food safety from the point of view of pesticide residues, research, development, implementation, prevention, control and legislation (ITA- INTA).
- value="2" id="Reference_Titile_Link">Ricca A, Martinez MJ,Bartosick R, PreselloD(2006) Identification of deriesgo situations, development and validation of methods for prevention of pre and post-harvest contamination. INTA Project 3353. PNCER.
- value="3" id="Reference_Titile_Link">Spynu EI (1989) Predicting pesticide residues to reduce crop contamination. Reviews of Environmental Contamination and Toxicology 109:89-107
- value="4" id="Reference_Titile_Link">Rapid Alert System for Food and Feed (2002)the legal basis for the creation of the European Commission RASFF is elReglamento 178/2002, Article 50 RASFF.
- value="5" id="Reference_Titile_Link">Charlotte Hecht-Buchholz (1998)Theapoplast-habitat of endophyticdinitrogen-fixing bacteria and their significance for the nitrogen nutrition of nonleguminous plants. Journal of Plant Nutrition and Soil Science161.
- value="6" id="Reference_Titile_Link">BifaniP(1992) International implications of biotechnology: the war of patents. Considerations after the Uruguay Round. Agriculture and Society 64.
- value="7" id="Reference_Titile_Link">Argentine market of plant protection products CASAFE (2009) Prepared Kleffmann& Partner SRL - kleffmanngroup.
- value="8" id="Reference_Titile_Link">Arregui CA, Lenardón D, Sanchez Maitre MI,Scotta R,et al.(2003) Residues Monitoring in Transgenic Glyphosate GlyphosateSoyben. Pest Mang163-167.
- value="9" id="Reference_Titile_Link">Lorenzatti E,Maitre MY, Lenardón A, LajmanovichR,PeltzerP, et al.(2004 ) Pesticides Residues in Soybeans of Argentina Croplands Immature.Fresenius Environmental Bulletin 13: 675-678.
- value="10" id="Reference_Titile_Link">Bolognesi C, Bonatti S, Gallerani E, Peluso M, Rabboni R,et al.(1997)Genotoxic Activity of Glyphosate and Its Technical Formulation Roundup JAgri Food Chem 45:1957-1962.
- value="11" id="Reference_Titile_Link">Robert M,Zablotowicz, Krishna N. R (2004)Nitrogenase activity, nitrogen content, and yield responses to glyphosate in glyphosate-resistant soybean. Crop Protection 26:370-376.
- value="12" id="Reference_Titile_Link">Hutchinson GL, Livingston GP, Healy RW, Striegl RG (1995) Chamber-induced disturbances of soil-atmosphere trace gas exchange: Evaluation by a numerical gas diffusion model. Eos 76:308-309.
- value="13" id="Reference_Titile_Link">Forlani G, Mantelli M, Branzoni M, Nielsen EY, Favilli F (1995)Differential sensitivity of plant-associated bacteria to sulfonylurea and imidazonline herbicides. Plant and Soil 176:243-253.
- value="14" id="Reference_Titile_Link">Bezbaruah B, Saikia NY, BoraT (1995)Effect of pesticidas on most probable number of soil microbes from tea (Camellia sinensis) plantations and uncultivated land enumerated in enrichment media. India J of Agric Sciences 65:578-583.
- value="15" id="Reference_Titile_Link">Eberbach PL,Douglas LA (1983)Persistente of glyphosate in sandy loam. Soil Biol. Biochem 15:485-487.
- value="16" id="Reference_Titile_Link">Omar MNA, Berge O,Hassanein EE,Shalan SN (1992) In vitro and in situ effects of herbicide thiobencarb on rice-Azospirillum association. Symbiosis 13:55-63.
- value="17" id="Reference_Titile_Link">VenkateswarluK, Sethunathan N (1984) Fate of 14C carbofuran in a flooded acid sulphate saline soil. Current Science 53:925-927.
- value="18" id="Reference_Titile_Link">Blum K, Noble EP, Sheridan PJ, Finely O, Montgomery A, et al. (1991) Association of the A I allele of the D., dopamine receptor gone with severe alcoholism. Alcohol 8:409-416.
- value="19" id="Reference_Titile_Link">HaahtelaK, Laakso T, Nurmiaho-Lassila E, Korhonen T (1988) Effects of inoculation Poapratensis and Triticumaestivum with roots associated, N2-fixyng Klebsiella, Enterobacter and Azospirillum. Plant Soil 106:239-248.
- value="20" id="Reference_Titile_Link">Dobereiner J (1997) Biological nitrogen fixation in the: social and economic contributions. Soil Biol Biochem 29:771-774.
- value="21" id="Reference_Titile_Link">Schilling B, De-Medina T, Syken J, Vidal M, Munger K (1998) A novel human Dnaj protein, hTid-1, a homolog of the Drosophiala tumor Suppression protein Tid56, can interact with the human papillomavirus type 16 E7 oncoprotein. Virology 247:74-85.
- value="22" id="Reference_Titile_Link">Reis VM, Olivares FL, Dobereiner J(1994)Improved methodology for isolation of Acetobacterdiazotrophicus and confirmation of its endophytic habitat. World J MicrobiolBiotechnol 10:401-405.
- value="23" id="Reference_Titile_Link">Zahir ZA, ArshadM,Frankenberger WT(2004) Plant growth promoting rhizobacteria: Applications and perspectives in agriculture. AdvAgron 81:97-168.
- value="24" id="Reference_Titile_Link">Masciarelli O (2010) new criteria to interpret the role of AIA produced in vitro by Azospirillumbrasilense Az39. Its importance in the formulation and quality control of inoculants. Master's thesis in biotechnology, Faculty of Exact, physicochemical and Natural Sciences, National university of Rio curato.
- value="25" id="Reference_Titile_Link">Oscar M, Lucia U, HermindaR, Virginia L (2013) Alternative mechanism for the evaluation of indole-3-acetic acid (iaa) production by azospirillumbrasilensestrains and its effects on the germination and growth of maize seedlings. The Journal of Microbiology 51:590-597.
- value="26" id="Reference_Titile_Link">Perrig D, Boiero M L, Masciarelli OA, Penna C,Ruiz OA, et al. (2007)Plant growth regulators production by two agronomical used strains of Azospirillumbrasilense, and possible implications to inoculants formulation. Applied Microbiology and Biotech 75:1143-1150.
- value="27" id="Reference_Titile_Link">Esitken A,Ercisli S, Karlidag H, Sahin F(2005) Potential use of plant growth promoting rhizobacteria (PGPR) in organic apricot production.Proceedings of the International Scientiï¬c Conference of Environmentally Friendly Fruit Growing90-97.
- value="28" id="Reference_Titile_Link">Esitken A, Pirlak L, Turan M, Sahin F (2006) Effects of �?oral and foliar application of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrition of sweet cherry. Sci Hortic 110:324-327.
- value="29" id="Reference_Titile_Link">Peter JD (2004) Plant Hormones, Biosynthesis, Signal the Transduction and Action. Kluwer Academic Publishers 3 rd edition.
- value="30" id="Reference_Titile_Link">Travaglia C, Reinoso H, Bottini R (2009) Application of abscisic acid promotes yield in field-cultured soybean by enhancing production of carbohydrates and their allocation in seed. Crop & Pasture Science, 60:1131-1136.
- value="31" id="Reference_Titile_Link">Rodríguez-Cáceres E (1982) Improved medium for isolation of Azospirillum spp. Appl Environ Microbiol 44:990-991.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
 |
 |
 |
 |
| Figure 6 | Figure 7 | Figure 8 | Figure 9 |
Relevant Topics
- Agricultural science
- Agronomy
- Climate impact on crops
- Crop Productivity
- Crop Sciences
- Crop Technology
- Field Crops Research
- Hybrid Seed Technology
- Irrigation Technology
- Organic Cover Crops
- Organic Crops
- Pest Management
- Plant Genetics
- Plant Breeding
- Plant Nutrition
- Seed Production
- Seed Science and Technology
- Soil Fertility
- Weed Control
Recommended Journals
Article Tools
Article Usage
- Total views: 15097
- [From(publication date):
August-2015 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10583
- PDF downloads : 4514
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
