The Natural Product Dehydroepiandrosterone Depletes Brain and Cardiac Adenosine
Received: 03-May-2018 / Accepted Date: 22-Jun-2018 / Published Date: 29-Jun-2018 DOI: 10.4172/2573-4555.1000274
Keywords: Anabolic androgenic steroids; Adenosine; Aggression; Dehydroepiandrosterone; DHEA; Testosterone; Methyl-testosterone; Glucose-6-phosphate dehydrogenase; Folate pathway; Folinic acid
Introduction
According to the United States Department of Justice, 0.5% of the adult population in the United States (approximately 1,084,000 people) self-administer anabolic androgenic steroids (AASs) in order to improve male-specific traits [1]. The National Institute on Drug Abuse estimates that a half million junior high school-aged children in the United States use AAS for this same purpose (Table 1). Worldwide, a significant number of amateur and professional athletes use AASs to increase physical size, strength and athletic performance [2]. The adverse health effects of AAS abuse has recently prompted the Endocrine Society to re-publish a formal position paper detailing their impacting not only on users, but also on society [3]. AAS abuse has had far reaching effects in professional athletics, with highly publicized cases of athletes attempting to obtain a competitive advantage by their use. In recent news, the use of steroids to increase competitive edge has even caused the expulsion or threat of expulsion of national teams at the Rio Olympics. Certainly, there can be a political cost to misusing steroid hormones in the quest for performance enhancement. But what are the physiological and behavioural costs of AAS abuse?
| Drug | Time Period | 8th Graders | 10th Graders | 12th Graders |
|---|---|---|---|---|
| Steroids | Lifetime | 1.00 | 1.20 | 2.30 |
| Past year | 0.50 | 0.70 | 1.70 | |
| Past month | 0.30 | 0.40 | 1.00 |
Table 1: Trends in prevalence of steroids for 8th Graders, 10th Graders, and 12th Graders; 2015 (in percent)*. Reprinted with permission https://www.drugabuse.gov/drugs-abuse/steroids-anabolic Cite: Johnston LD, O'Malley PM, Miech RA, Bachman JG, Schulenberg JE (2016) Monitoring the Future national survey results on drug use, 1975-2015: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan, pp: 98.
AAS have been shown to affect the CNS, including the mesolimbic reward system, and up to 30% of admitted AAS users report becoming dependent upon them [4]. Long term steroid use leads to a variety of pathological conditions, including neuropsychiatric effects such as rage, hostility, and cognitive impairment [5], and cardiovascular anomalies [6]. Pope and Katz have described a series of men with benign premorbid psychiatric histories, no evidence of antisocial personality disorder, and no history of violence who have impulsively committed violent crimes, including murder, while taking AASs [7,8]. Choi and Pope have reported that women familialy associated with male athletes who abuse steroids are at a significantly increased risk of spousal violence [9], and placebo-controlled studies demonstrating neuropsychiatric effects of AASs have been published by Su et al. [10], and Pope et al. [11]. In the Pope study, testosterone cypionate was administered to 56 men ages 20–50 years for 6 weeks in doses up to 600 mg/week, followed by 6 weeks of no treatment and then placebo for 6 weeks. Testosterone was observed to significantly increase mania; to be liked and sought after; and to significantly increase aggression. These investigators noted that the response to the drug, however, was highly variable. Eighty-four percent of the subjects exhibited minimal to no psychiatric effects, 12% became mildly hypomanic, and 4% became markedly hypomanic. Several studies have controlled for variability in inter-individual personality disorder by using steroid abusers as their own controls. These studies have demonstrated significant differences in the degree of hostility, aggression, and severity of maniclike symptoms during periods of use and non-use [12,13,14]. In their reviews of the literature, Pope and Katz [15,16] reported that studies in which steroid use is quantified and categorized on the basis of total weekly dosing show that psychiatric symptoms become more common and severe as the dose increases. A correlation was demonstrated to exist between dose of AASs used, with medium steroid use classified as between 300 and 1000 mg/week and high dose use being more than 1000 mg/week of any AAS. Thus, 23% of subjects using either of these doses of steroids met the DSM-III-R criteria for a major mood syndrome (mania, hypomania, and major depression), and 3.4%–12% developed psychotic symptoms [17,18].
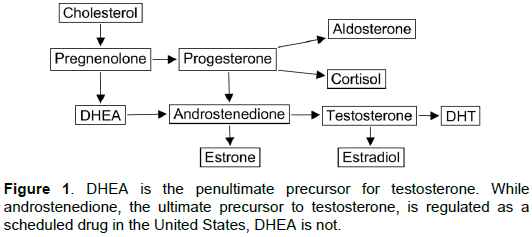
There are a number of AASs that are in use for the purpose of enhancing male-specific traits and/or competitive edge, one of the most common of these being 1-delta-methyltestosterone (MT). All testosterone and testosterone analogue preparations are regulated by the Food and Drug Administration (FDA) in the United States, and by comparable agencies in most other countries of the world. In 2004, the U.S. Controlled Substances Act was amended to include androstenedione as an AAS because it is the direct precursor of testosterone. In most respects, it is thus becoming increasingly difficult for persons wishing to use AASs to increase athletic performance to obtain such drugs, with one glaring exception. Dehydroepiandrosterone (DHEA), the direct precursor to androstenedione, and therefore the penultimate precursor of testosterone, has been specifically excluded from regulation under the U.S. Controlled Substances Act, and is freely available as an OTC (over the counter) supplement in the United States. This is in stark contrast to the regulatory status of DHEA in other western nations, where it is regulated as an AAS requiring a physicianoriginated prescription for its use. It is also at odds with the fact that the National Collegiate Athletic Association (NCAA), the dominant sports regulatory authority for college athletics in the United States, the National Football League, the International Olympic Committee and the World Anti-Doping Agency have all specifically banned the use of DHEA by athletes competing under their auspices. Why DHEA remains unregulated in the United States is probably accounted for by the fact that virtually all studies of it in man have been performed using exceedingly small doses (25-100 mg per day), which are orders of magnitude lower than would be used to improve male-specific traits and/or athletic performance [4].
Steroids and G6PD
A variety of steroids are known to inhibit Glucose-6-phosphate dehydrogenase (G6PD), the rate limiting enzyme of the hexose monophosphate shunt, and a major source of cellular NADPH [19,20,21]. One possible mechanism by which high dose AAS abuse might lead to physiological abnormalities therefore could be by reducing cellular pools of NADPH and inhibiting NADPH-dependent processes. It has been demonstrated that enzymes requiring two mols of NADPH for every mol of product formed, such as HMG CoA reductase, are potently inhibited by DHEA [22,23]. Another such enzyme that requires two mols of NADPH for every mol of product formed is Dihydrofolate Reductase (DHFR), the rate limiting enzyme of the one carbon, or folate, pathway. Thus, to create one mol of tetrahydrofolate from one mol of dietary folate, DHFR requires two mols of NADPH. The two and eight carbon atoms of purines derive from N10-formyltetrahydrofolate, and N5, N10-methenyltetrahydrofolate, respectively, which are downstream products of DHFR. Thus, if steroid-mediated inhibition of G6PD reduces the NADPH compartment required for function of the folate pathway, the synthesis of purines would be expected to be inhibited, and adenosine (and guanosine) pools will diminish. Furthermore, if inhibition of DHFR underlies AAS-mediated adenosine depletion, then folinic acid treatment should rescue folate interconversion just as it does with other inhibitors of DHFR [24], restoring adenosine pools.
Adenosine is a ubiquitous autocoid that is involved in myriad cellular processes. It acts through four known receptors, A1, A2a, A2b and A3. It has been called a retaliatory autocoid because its levels are rapidly increased when cells are faced with a variety of cellular stresses, including hypoxia [25], and ischemia [26,27]. It also acts as a neuromodulator and homeostatic effector in the brain [28], is involved in sleep and arousal [29], and in the mediation of the effects of ethanol [30]. In the CNS, adenosine is known to inhibit the release of a variety of neurotransmitters including noradrenalin, serotonin, GABA, acetylcholine, dopamine, and glutamate. Thus, it mainly acts in a depressant fashion, inhibiting neurotransmission and depressing neuronal firing, accounting for the stimulation induced by adenosine receptor antagonists like caffeine. Clearly, any exogenous agent capable of significantly reducing physiological levels of adenosine in the CNS could have profound health implications for users of that agent.
Materials and Methods
Animal care and handling
Male Fisher 344 rats (Charles River, Wilmington, MA), weighing 120 grams, were housed in 70 × 45 × 45 cm stainless steel cages with natural bedding. Food and water were made available ad libitum. Cages were maintained in a temperature (20°C) and humidity (40- 50%) controlled vivarium with a reversed light cycle (lights on between 8:00 PM and 8:00 AM). All procedures followed the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were reviewed and approved by the Institutional Animal Care Committee.
Administration of AAS and folinic acid
DHEA and MT were taken up in carboxymethylcellulose and administered daily by gavage. DHEA was administered at doses of 300 mg/kg and 100 mg/kg, and MT at doses of 120 mg/kg and 40 mg/kg. These doses were selected in preliminary experiments based upon their ability to inhibit Glucose-6-phosphate Dehydrogenase (G6PD) activity in brain tissue from animals treated with AAS for 48 hours (data not shown). To measure G6PD activity, rats were killed by decapitation, their brains rapidly removed and rinsed in ice cold 1.15% (w/v) KCl. Whole brains were flash frozen in liquid nitrogen, then were stored in a −80°C freezer until analysis. Each sample was homogenized in 500 μL of 50 mM Tris-HCl pH 7.4, and the homogenate was sonicated for 10 seconds. The homogenates were centrifuged for 30 min at 15,000g in a refrigerated microcentrifuge. The pellet was discarded, and the protein content was determined in the supernatant in preparation for G6PD quantitation. To quantitate G6PD activity, the method of Glock and McLean was used [31]. In brief, two reactions are run side by side. In the first, the NADPH produced by both G6PD and 6-Phosphogluconate Dehydrogenase (6PGD) in the presence of saturating G6P and 6PG is measured spectrophotometrically. In the second, the NADPH produced by 6PGD in the presence of saturating 6PG is measured spectrophotometrically. G6PD activity is then calculated as the difference in rate between the assays with both substrates and only 6-PGA.
Where folinic acid was used, it was taken up in physiological saline and administered by intraperitoneal injection at a dose of 50 mg/kg.
Quantitation of Adenosine
The half-life of adenosine is 0.5 seconds in vivo. This is because adenosine kinase (AdoK) and adenosine deaminase (AdoD) are ubiquitous enzymes and instantaneously degrade adenosine to AMP (AdoK) or inosine (AdoD) if it is not bound immediately by one of its four receptors. We used the method of deGraff et al. [32] to instantaneously fix adenosine levels via focused brain microwave irradiation (1.33 kW, 2450 MHz) which instantaneously denatures AdoK and AdoD but retains brain NMR features in a close to normal condition. Adenosine was subsequently extracted from whole brain, derivitized to 1, N6- ethenoadenosine using 225 mM chloroacetaldehyde at pH 4.5 and 60°C for 60 min, and analyzed by HPLC with fluorometric detection as described by Zhang et al. [33]. With this method as little as 0.2 pmol of ethenoadenosine can be measured, and detection is linear up to 200 pmol.
Results
Table 2 shows that two weeks of continuous treatment with either DHEA or MT induced dose-dependent depletion of adenosine in the brains of Fisher 344 rats. Such depletion was dramatic in the case of high dose DHEA (0.19 ± 0.01 nmols/mg protein compared to 0.5 ±0.04 nmols/mg protein for control animals) and high dose MT (0.32 ± 0.03 nmols/mg protein). Treatment with folinic acid (50 mg/kg, i.p.) completely blocked both DHEA-induced and MT-induced adenosine depletion, strongly suggesting that such depletion was caused by inhibition of DHFR and the folate pathway.
| sadd | Control | DHEA HD | DHEA LD | MT HD | MT LD | DHEA HD+Fol | MT HD+Fol |
|---|---|---|---|---|---|---|---|
| BRAIN | 0.5 ± 0.04 (n=12) |
0.19 ± 0.01§ (n=12) |
0.35 ± 0.04§ (n=12) |
0.32 ± 0.04§ (n=6) |
0.42 ± 0.06 (n=6) |
0.55 ± 0.09ƥ (n=6) |
0.60 ± 0.06ƥ (n=6) |
CARDIAC |
10.6 ± 0.6 (n=12) |
6.7 ± 0.05§ (n=12) |
N.D. | 6.0 ± 0.4§ (n=6) |
8.3 ± 1.0§ (n=6) |
11.1 ± 0.6§ (n=5) |
9.1 ± 0.4§ (n=6) |
§ p<0.05 compared to control group, Student’s t-test.
ƥ p<0.05 compared to DHEA- or MT-treated groups, Student’s t-test.
Table 2: Effects of DHEA and MT on brain adenosine levels in Fisher 344 rats, with and without folinic acid rescue Adult male Fisher 344 rats (325-350 grams) were administered DHEA or MT in carboxymethylcellulose by gavage once daily for 14 consecutive days. Where indicated, folinic acid (50 mg/kg) was administered i.p. once daily during this same period of time. On the morning of the fifteenth day, animals were subjected to microwave pulse to the cranium which instantaneously denatures all protein and prevents further metabolism of adenosine. Brain tissue was dissected, and adenosine was extracted, derivitized to 1, N6-ethenoadenosine, and adenosine quantitated by HPLC with fluorometric detection as described above. Results are expressed as the mean ± S.E.M. Animal care and euthanasia were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Discussion
Because the half-life of adenosine precludes measurement of its levels in the brains of humans, are studies in rodents sufficient to provide information applicable to humans abusing AAS? We believe that the answer to this question is yes, because the adenosine system is evolutionarily ancient and underlies many of the most basic circuits of brain physiology. Our work shows that at high concentrations, steroids inhibit G6PD in vivo, which has the end result of blocking the biosynthesis of adenosine. This is simple stoichiometric chemistry and is likely to hold across species. We can therefore potentially identify neurobehavioral effects of AAS that can reasonably be attributed to adenosine depletion, either in humans or experimental animals.
Aggression
Adenosine exerts its actions via the receptors noted above. Thus, genetic knockout of one or more of these receptors simulates adenosine depletion, at least with respect to that specific receptor. Gimenez-Llort and colleagues demonstrated that adenosine A1 receptor knockout mice showed high levels of aggression [34]. Similarly, when mice were injected with the adenosine A1 receptor agonist N6-cyclohexyl adenosine, simulating supraphysiologic levels of adenosine with respect to this receptor, aggression measured via the Resident Intruder model was abolished [35].
Allopurinol is a drug which blocks xanthine oxidase, an enzyme involved in the degradation of purines such as adenosine. In humans exhibiting aggression secondary to dementia, allopurinol was shown to effectively reduce aggression [36,37], presumably by increasing the level of purines, including adenosine, in the brain.
Sleep
Adenosine is known to play a key role in sleep, with adenosine levels rising in the brain during wakefulness, creating a pressure to sleep [38]. The sleep degrading effects of caffeine, a non-specific adenosine receptor antagonist, are well known, and offer a theoretical simulation of an adenosine-depleted brain. AAS use sufficient to deplete brain adenosine might then be expected to cause insomnia. Bolding and colleagues studied a group of men using AAS in London gyms and found that almost half reported bouts of insomnia while using AAS [39].
Schizophrenia-like symptoms, mania, depression
Reports from physicians treating the acute and chronic effects of AAS use describe schizophrenia-like symptoms in abusers [40,41]. There is accumulating evidence that schizophrenia may involve a loss of adenosine activity [42]. This hypoadenosinergic hypothesis of schizophrenia proposes that reduced extracellular adenosine levels contribute to dopamine D2 receptor hyperactivity, which is supported by the observation that increasing brain adenosine levels ameliorates many of the psychotic and cognitive aspects of this disease. [43,44,45]. Acute ingestion of large amounts of the nonspecific adenosine receptor antagonist caffeine is associated with the induction or worsening of psychiatric illness [46,47]. Further support for the adenosine hypothesis of schizophrenia comes from the finding that the ectonucleotidase enzymes responsible for production of adenosine from its nucleotides are significantly depressed in the putamen of schizophrenics as compared to age and sex matched controls. Brunstein and colleagues showed that adenosine deaminase activity, which degrades adenosine, was significantly elevated in patients with schizophrenia as compared to age and sex-matched controls [48]. Akhondzadeh et al. [49] performed a double blind, randomized, placebo-controlled study of the effects of allopurinol as an add-on in the treatment of refractory schizophrenia. Allopurinol, as stated above, inhibits purine degradation, thereby presumably raising brain adenosine levels. This group demonstrated that allopurinol significantly reduced psychotic symptoms in schizophrenics receiving drug as compared to placebo-treated controls [50]. Shen and colleagues demonstrated that augmentation of adenosine by pharmacologic inhibition of adenosine kinase, another key enzyme of adenosine clearance, exerted anti-psychotic-like activity in mice. An animal model mimicking many of the symptoms of schizophrenia was developed by Moscoso-Castro et al. by knocking out the adenosine A2a receptor in mice [51,52] Haloperidol, a drug with demonstrated anti-psychotic activity in schizophrenia, has recently been shown to inhibit adenosine deaminase in zebrafish brains [53]. Clearly, adenosine depletion appears to play a role in schizophrenia. It is thus reasonable to hypothesize that the schizophrenia-like symptoms observed by clinicians in their AAS abusing patients may be due to AAS-induced adenosine depletion.
Adenosine also appears to play a role in major depression (MD). As noted above, the levels of adenosine, in the brain are regulated by several enzymes, among them adenosine deaminase, which degrades adenosine to inosine; xanthine oxidase, which degrades inosine to uric acid; and a series of ectonucleases that dephosphorylate ATP, ADP and AMP to produce adenosine. Herken et al. showed that patients suffering from MD have elevated levels of adenosine deaminase and xanthine oxidase in their blood, suggesting that adenosine depletion may be involved in the pathogenesis of MD. Uric acid levels have been demonstrated to be increased in bipolar disorders, and several studies have hypothesised that this is caused by amplified purinergic metabolism and a reduction in brain adenosine [54,55]. Support for the idea that upregulation of adenosine is therapeutic in bipolar disorder is provided by studies showing that lithium, an effective drug for this disease, stimulates the activity of ATP and ADP ectonucleotidases in rat brain synaptosomes, which would raise adenosine levels [56].
Folinic acid in the treatment of neuropsychiatric disorders
Our discovery that folinic acid (FA) raises brain adenosine levels is novel and may help to explain recent findings that FA shows efficacy in the so-called cerebral folate deficiency (CFD) syndromes. In CFD, folate transport to the brain is impaired, leading to developmental and psychiatric disorders including infantile-onset CFD syndrome, infantile autism, spastic-ataxic syndrome and intractable epilepsy in young children, escalating to refractory schizophrenia in adolescents, and treatment-resistant major depression in adults [57]. High dose FA has been demonstrated to abolish acoustic hallucinations in a series of schizophrenic patients [58]. We propose that it does so by elevating brain adenosine levels beyond their normal, possibly hypoadenosinergic state in schizophrenia.
DHEA as an AAS
Studies of the effects of AAA abuse have not generally included DHEA even though it is the penultimate precursor to testosterone (Figure 1). However, DHEA is clearly an AAS as it produces identical changes in gene expression as compared to the AAS entities tetrahydrogestrinone (THG) and dihydrotestosterone (DHT). This was elegantly demonstrated by Labrie et al. in a series of microarray analyses showing a virtually identical epigenetic signature for all three substances [59]. Our discovery that DHEA and MT also share the capacity to deplete brain adenosine adds to this shared AAS epigenetic signature.
While there have been reports linking DHEA to aggression in animals, these have invariably focused upon endogenous DHEA [60]. Studies of administration of pharmacological doses of DHEA (≥ 25 mg/ kg) in animals are rare. Even if they did exist, they would be complicated by the fact that DHEA synthesis is profoundly different in primates as compared to non-primates. Thus, in primates, large amounts of DHEA and other C 19 steroids (e.g., androstenedione) are synthesized in the adrenals, and large amounts of DHEA sulfate (DHEAS) are present in the circulation [61]. In rodents, dogs and other non-primates that have been studied, DHEA is synthesized in gonadal tissue, not the adrenals, and, particularly in rodents, circulating levels of DHEAS are negligible [62-65]. The breeding cycles of non-tropical rodent species further complicates their use in studies of DHEA-induced aggression. Thus, in most rodent species, short winter photoperiods reduce testosterone concentrations, induce gonadal regression and reduce testosteronedependent behaviours such as mating and aggression [66,67]. Steroid synthesis and aggression in rodents thus appears to show little similarity to that observed in primates. For these reasons, studies utilizing DHEA in such paradigms as the Resident-Intruder model in rats may not be informative of DHEA’s effects in primates. Even in primates, the circulating levels of DHEA vary widely among species [68], and the diverse reproductive and steroid hormone cycles of non-human primates again complicate studies of DHEA and aggression. Thus, the proper study of pharmacological doses of DHEA— doses that would be used by persons attempting to increase male-specific traits, muscle mass or other aspects of athletic performance— are most reliably performed in humans. Several studies have used pharmacological doses of DHEA in humans over short periods of time (1600 mg/day; equivalent to 25- 26 mg/kg), but each focused on reduction in body fat, not aggression, as their studied endpoint [69-72].
Adenosine is also known to regulate myocardial and coronary circulatory functions. It dilates coronary vessels, and attenuates betaadrenergic receptor-mediated increases in myocardial contractility and depresses both sinoatrial and atrioventricular node activities. The cardiotoxic potential of AAS has been demonstrated in case reports [73]. Palpitaiton, tachycardia, precordial pain, hypertension, ventricular hypertrophy, cardiomyopathy and cardiomegaly have been reported in persons abusing anabolic steroids, in some cases progressing to death or the requirement for heart transplantation [74]. Depletion of adenosine may be the root cause of these toxic actions of AAS.
Conclusion
Our finding here that DHEA depletes brain and cardiac adenosine should be viewed with alarm since the adenosinergic pathways are highly conserved throughout species, unlike steroidal pathways, which vary widely among species. Thus, the mechanism of AAS-induced adenosine depletion is likely to hold across all animal species. In view of the facts that (1) millions of people worldwide are using high doses of AAS to improve male-specific traits and/or athletic performance; (2) that DHEA is not regulated as an anabolic steroid in the United States, and may therefore now be the AAS of choice in that country; and (3) that a rationale— adenosine depletion in the brain— now exists to explain the neurobehavioral symptoms reported to occur in abusers of AAS, priority should be given to performing toxicity studies in humans with DHEA at the pharmacologic doses (≥ 50 mg/kg) presumed to be used to achieve androgenic effects. If AAS induce adenosine depletion in the brains of humans, as is likely, then the OTC distribution of DHEA may be creating a burgeoning health problem in the United States and elsewhere. DHEA is now being advertised in the United States as a “fertility nutraceutical.” A large variety of nutraceuticals are available OTC in the United States and elsewhere. For an excellent review, see Scicchitano et al. (75). In our view, DHEA’s ability to deplete brain and cardiac adenosine sets it apart from all other such nutraceuticals in terms of potential to do harm.
References
- http://www.deadiversion.usdoj.gov/pubs/brochures/steroids/professionals/
- Kokkevi A, Fotiou A, Chileva A, Nociar A, Miller P (2008) Daily exercise and anabolic steroids use in adolescents: A cross-national european study. Subst Use Misuse 43: 2053-2065.
- Pope HG, Wood RI, Rogol A, Nyberg F, Bowers L, et al. (2014)Adverse health consequences of performance-enhancing drugs: An endocrine society scientific statement. Endocrine Reviews 35: 341-375.
- Grönbladh A, Nylander E, Hallberg M (2016) The neurobiology and addiction potential of anabolic androgenic steroids and the effects of growth hormone. Brain Research Bulletin 126: 127-137.
- Trenton AJ, Currier GW (2005) Behavioural manifestations of anabolic steroid use. CNS Drugs 19: 571-595.
- Payne JR (2004) Cardiac effects of anabolic steroids. Heart BMJ 90:473-475.
- Pope HG Jr, Katz DL Jr (1990) Homicide and near-homicide by anabolic steroid users. J Clin Psychiatry 51: 28-31.
- Pope HG (1994) Psychiatric and medical effects of anabolic-androgenic steroid use. Arch Gen Psychiatry. American Medical Association 51: 375.
- Choi P, Pope H (1994) Violence toward women and illicit androgenic-anabolic steroid use. Ann Clin Psychiatry 6:21-25.
- Su TP (1993) Neuropsychiatric effects of anabolic steroids in male normal volunteers. JAMA: The Journal of the American Medical Association 269: 2760.
- Pope HG, Kouri EM, Hudson JI (2000) Effects of supraphysiologic doses of testosterone on mood and aggression in normal men. Arch Gen Psychiatry. American Medical Association 57:1 33.
- Lefavi R, Reeve T, Newland MC (1990) Relationship between anabolic androgenic steroid use and selected psychological parameters in male body builders. J Sport Behav 13:157-166.
- Brower KJ (2009) Anabolic steroids: Addictive, psychiatric, and medical consequences. Am J Addict. Wiley 1:100-114.
- Pope HG Jr, Katz D (1992) Psychiatric effects of anabolic androgenic steroids. Psychiatr Ann 22: 24-29.
- Affective and psychotic symptoms associated with anabolic steroid use (2006) Am J Psychiatry. American Psychiatric Publishing 145: 487-490.
- Frati P, Busardo FP, Cipolloni L, Dominicis ED, Fineschi C (2015) Anabolic androgenic steroid (aas) related deaths: Autopic, histopathological and toxicological findings. Curr Neuropharmacol 13: 146-159.
- Uzych L (1992) Anabolic-androgenic steroids and psychiatric-related effects: A Review. Can J Psychiatry 37:23-28.
- Marks Pa, Banks j (1960 ) Inhibition of mammalian glucose-6-phosphate dehydrogenase by steroids. Proceedings of the National Academy of Sciences 46: 447-452.
- Oertel GW, Benes PJ (1972) The effects of steroids on glucose-6-phosphate dehydrogenase. Steroid Biochemistry 3:493-496.
- Raineri RR, Levy HR (1970) Specificity of steroid interaction with mammary glucose 6-phosphate dehydrogenase. Biochemistry American Chemical Society 9:2233-2243.
- Schulz S, Nyce JW (1991) Inhibition of protein isoprenylation and p21ras membrane association by dehydroepiandrosterone in human colonic adenocarcinoma cells in vitro. Cancer Res 51: 6563-6567.
- Schulz S, Klann RC, Schonfeld S, Nyce JW (1992) Mechanisms of cell growth inhibition and cell cycle arrest in human colonic adenocarcinoma cells by dehydroepiandrosterone: Role of isoprenoid biosynthesis. Cancer Res 52:1372-1376.
- Howard SC, McCormick J, Pui CH, Buddington RK, Harvey RD (2016) Preventing and managing toxicities of high-dose methotrexate. The Oncologist 21: 1471-1482.
- Gorlach A (2005) Control of adenosine transport by hypoxia. Circ Res 97: 1-3.
- Ely SW, Berne RM (1992) Protective effects of adenosine in myocardialz ischemia. Circulation 85: 893-904
- Rudolphi KA, Schubert P (1995) Adenosine and brain ischemia. Adenosine and Adenine Nucleotides: From Molecular Biology to Integrative Physiology 391-397.
- Cunha RA (2001) Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: Different roles, different sources and different receptors. Neurochemistry International 38: 107-125.
- Huang ZL, Urade Y, Hayaishi O (2011) The role of adenosine in the regulation of sleep. Curr Top Med Chem 11: 1047-1057.
- Phan TA, Gray AM, Nyce JW (1997) Intrastriatal adenosine A1 receptor antisense oligodeoxynucleotide blocks ethanol-induced motor incoordination. Eur J Pharmacol 323: R5-R7.
- Glock GE, McLean P (1953) Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J 55: 400-408.
- De Graaf RA, Chowdhury GMI, Brown PB, Rothman DL, Behar KL (2009) In situ3D magnetic resonance metabolic imaging of microwave-irradiated rodent brain: A new tool for metabolomics research. J Neurochem 109: 494-501.
- Zhang Y, Geiger JD, Lautt WW (1991) Improved high-pressure liquid chromatographic-fluorometric assay for measurement of adenosine in plasma. Am J Physiol Gastrointest Liver Physiol 260: G658-G664.
- Giménez-Llort L, Fernández-Teruel A, Escorihuela RM, Fredholm BB, Tobeña A, et al. (2002) Mice lacking the adenosine A1receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. Eur J Neurosci 16: 547-550.
- Navarro JF, Romero C, Maldonado E (2000) Effects of N6-cyclohexyl adenosine (CHA) on isolation-induced aggression in male mice. Methods and Findings in Experimental and Clinical Pharmacology 22:43.
- Lara DR, Belmonte-de-Abreu P, Souza DO(2000) Allopurinol for refractory aggression and self-inflicted behaviour. J Psychopharmacol 14: 81–83.
- Lara DR, Cruz MR, Xavier F, Souza DO, Moriguchi EH (2003) Allopurinol for the treatment of aggressive behaviour in patients with dementia. Int Clin Psychopharmacol 18: 53-55.
- Bjorness TE, Dale N, Mettlach G, Sonneborn A, Sahin B, Fienberg AA, et al. (2016) An adenosine-mediated glial-neuronal circuit for homeostatic sleep. Int J Neurosci 36: 3709-3721.
- Bolding G, Sherr L, Elford J (2002) Use of anabolic steroids and associated health risks among gay men attending london gyms. Addiction 97: 195-203.
- Piacentino D, Kotzalidis GD, de Casale A, Aromatoario MR, Pomara C, et al. (2015) Anabolic-androgenic steroid use and psychopathology in athletes. A systematic review. Curr Neuropharmacol 13: 101-121.
- Annitto WJ, Layman WA (1980) Anabolic steroids and acute schizophrenic episode. J Clin Psychiatry 41: 143-144.
- Boison D, Singer P, Shen HY, Feldon J, Yee BK (2012) Adenosine hypothesis of schizophrenia--opportunities for pharmacotherapy. Neuropharmacology 62: 1527-1543.
- Shen HY, Singer P, Lytle N, Wei CJ, Lan Q, et al. (2012) Adenosine augmentation ameliorates psychotic and cognitive endophenotypes of schizophrenia. J Clin Invest 122: 2567-2577.
- Hirota T, Kishi T (2013) Adenosine hypothesis in schizophrenia and bipolar disorder: A systematic review and meta-analysis of randomized controlled trial of adjuvant purinergic modulators. Schizophr Res 149: 88-95.
- Rial D, Lara DR, Cunha RA (2014) The adenosine neuromodulation system in schizophrenia. Int Rev Neurobiol 119: 395-449.
- Wang HR, Woo YS, Bahk WM (2015) Caffeine-induced psychiatric manifestations: A review. Int Clin Psychopharmacol 30: 179-182.
- Broderick P, Benjamin AB (2004) Caffeine and psychiatric symptoms: A review. J Okla State Med Assoc 97: 538-542.
- Aliagas E,Villar-Menéndez I, Sévigny J, Roca M, Romeu M, et al. (2013) Reduced striatal ecto-nucleotidase activity in schizophrenia patients supports the “adenosine hypothesisâ€. Purinergic Signal 9: 599-608.
- Brunstein MG, Silveira EM Jr, Chaves LS, Machado H, Schenkel O, et al. (2007) Increased serum adenosine deaminase activity in schizophrenics receiving antipsychotic treatment. Neurosci Lett 414: 61-64.
- Akhondzadeh S, Safarcherati A, Amini H (2005) Beneficial antipsychotic effects of allopurinol as add-on therapy for schizophrenia: Adouble blind, randomized and placebo controlled trial. Prog Neuropsychopharmacol Biol Psychiatry 29: 253-259.
- Moscoso-Castro M, Gracia-Rubio I, Ciruela F, Valverde O (2016) Genetic blockade of adenosine A2A receptors induces cognitive impairments and anatomical changes related to psychotic symptoms in mice. Eur Neuropsychopharmaco 26: 1227-1240.
- Seibt KJ, Oliveira Rda L, Bogo MR, Senger MR, Bonan CD (2015) Investigation into effects of antipsychotics on ectonucleotidase and adenosine deaminase in zebrafish brain. Fish Physiol Biochem 41: 1383-1392.
- Herken H, Gurel A, Selek S, Armutcu F, Ozen ME, et al. (2007) Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: Impact of antidepressant treatment. Arch Med Res 38: 247-252.
- Bartoli F, Crocamo C, Mazza MG, Clerici M, Carrà G (2016) Uric acid levels in subjects with bipolar disorder: A comparative meta-analysis. J Psychiatr Res 81: 133-139.
- Wilot LC, Da Silva RS, Ferreira OJ, Bonan CD, Sarkis JJ, et al. (2004) Chronic treatment with lithium increases the ecto-nucleotidase activities in rat hippocampal synaptosomes. See comment in PubMed Commons belowNeurosci Lett 368: 167-170.
- Ramaekers VT, Sequeira JM, Quadros EV (2016) The basis for folinic acid treatment in neuro-psychiatric disorders. See comment in PubMed Commons belowBiochimie 126: 79-90.
- Ramaekers VT, Thöny B, Sequeira JM, Ansseau M, Philippe P, et al. (2014) Folinic acid treatment for schizophrenia associated with folate receptor autoantibodies. See comment in PubMed Commons below Mol Genet Metab 113: 307-314.
- Labrie F, Luu-The V, Martel C, Chernomoretz A, Calvo E, et al. (2006) Dehydroepiandrosterone (DHEA) is an anabolic androgenic steroid like dihydrotestosterone (DHT), the most potent natural androgen and tetrahydrogestrinone (THG). J Steroid Biochem Mol Biol 100: 52-58.
- Soma KK, Rendon NM, Boonstra R, Albers HE, Demas GE (2015) DHEA effects on brain and behavior: insights from comparative studies of aggression. J Steroid Biochem Mol Biol 145: 261-272.
- Abbott DH, Bird IM (2009) Nonhuman primates as models for human adrenal androgen production: Function and dysfunction. Rev Endocr Metab Disord 10: 33-42.
- Tchernof1 A, Labrie F (2004) Dehydroepiandrosterone, obesity and cardiovascular disease risk: A review of human studies. Eur J Endocrinol 151: 1-14.
- Van Weerden WM, Bierings HG, van Steenbrugge GJ, de Jong FH, Schröder FH (1992) Adrenal glands of mouse and rat do not synthesize androgens. Life Sci 50: 857-861.
- Mongillo P, Prana E, Gabaio G, Bertotto D, Marinelli L (2014) Effect of age and sex on plasma cortisol and dehydroepiandrosterone concentrations in the dog (Canis familiaris). Res Vet Sci 96: 33-38.
- Cutler GB Jr, Glenn M, Bush M, Hodgen GD, Graham CE, et al. (1978) Adrenarche: A survey of rodents, domestic animals and primates. Endocrinology 2112-2118.
- Ahlem CN, White SK, Page TM, Frincke JM (2011) Differential metabolism of androst-5-ene-3β,17β-diol between rats, canines, monkeys and humans.Steroids 76: 669-674.
- Bedrosian TA, Fonken LK, Demas GE, Nelson RJ (2012) Photoperiod- dependent effects of neuronal nitric oxide synthase inhibition on aggression in siberian hamsters. Horm Behav 61: 176-180.
- Bernstein RM, Sterner KN, Wildman DE (2012) Adrenal androgen production in catarrhine primates and the evolution of adrenarche. Am J Phys Anthropol 389-400.
- Nestler J (1994) Insulin and Adrenal Androgens. Semin Reprod Med 12: 1-5.
- Mortola JF, Yen SC (1990) The effects of oral dehydroepiandrosterone on endocrine-metabolic parameters in postmenopausal women. J Clin Endocrinol Metab 71: 696-704.
- Welle S, Jozefowicz R, Statt M (1990) Failure of dehydroepiandrosterone to inflluence energy and protein metabolism in humans. J Clin Endocrinol Metab 1259-1264.
- Usiskin KS, Butterworth S, Clore JN, Arad Y, Ginsberg HN, et al. (1990) Lack of effect of dehydroepiandrosterone in obese men. Int J Obes 14: 457-463.
- Rockhold RW (1993) Cardiovascular toxicology of anabolic steroids. Annu Rev Pharmacol Toxicol 33: 497-520.
- Sondergaard EB, Thune JJ, Gustafsson F (2014) Characteristics and outcomes of patients with heart failure due to anabolic androgenic steroids. Scand Cardiovasc J 48: 339-342.
- Hori M, Kitakaze M (1991) Adenosine, the heart and coronary circulation. Hypertension 18: 565-574.
- Scicchitano P, Cameli M, Maiello M, Modesti PA, Muisesan MK, et al. (2014) Nutraceuticals and dyslipidemia: Beyond the common therapeutics. J Funct Foods 6: 11-32.
Citation: Nyce AT, Nyce JW (2018) The Natural Product Dehydroepiandrosterone Depletes Brain and Cardiac Adenosine. J Tradit Med Clin Natur 7:275. DOI: 10.4172/2573-4555.1000274
Copyright: © 2018 Nyce AT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6288
- [From(publication date): 0-2018 - Nov 13, 2025]
- Breakdown by view type
- HTML page views: 5328
- PDF downloads: 960