The Microorganism Community in Subcutaneous Abscess of Goat
Received: 04-Mar-2022 / Manuscript No. JIDT-22-56065 / Editor assigned: 07-Mar-2022 / PreQC No. JIDT-22-56065 / Reviewed: 21-Mar-2022 / QC No. JIDT-22-56065 / Revised: 20-May-2022 / Manuscript No. JIDT-22-56065 / Published Date: 27-May-2022
Abstract
Background: Subcutaneous abscess is a common disease, which seriously affects the quality and yield of goat breeding. The main pathogens that because abscess is well understood, but the microorganism community yet remains relatively unexplored.
Methods: To determine the population and diversity of the of microorganisms in subcutaneous abscess of goat, in this work, 5 pus samples collected from different goat farms (Jiangsu Province, China) were subjected to metagenomics sequencing and bioinformatics analysis.
Results: The microorganism communities of each sample contain about 79-86 microbial species. Interestingly, each sample contained similar microbial species, including 53-59 species of bacteria, 5-6 fungi, 3 viruses and 16-18 parasites. The top 5 dominant bacteria are Staphylococcus aureus, Lactococcus garvieae, Helicobacter pylori, Streptococcus pneumoniae and Klebsiella pneumoniae, with average abundance value 29.88%, 8.2%, 6.16%, 3.5%, and 3.26%, respectively. The remaining microbial abundances ranging from 0.01% to 3%. Although each of these frequent microorganisms is a tiny part of the total community, they constitute a major portion of individual reads (-1/3). It is very noteworthy that some zoonotic bacteria, such as Lactococcus garvieae, Helicobacter pylori and Shigella, etc. are detected in goats, and this leads us to wonder if goats are the reservoirs of these microbes and pose a risk to human public health.
Conclusion: The microorganism community in subcutaneous abscess of goat was proved to be highly diverse, Staphylococcus aureus, Lactococcus garvieae, Helicobacter pylori, Streptococcus pneumoniae and Klebsiella pneumoniae are the dominant bacteria. It remains difficult to predict which species of bacteria might be found on a particular goat, but predicting which species are most frequent (or rare) seems more straightforward, at least for those species living in subcutaneous abscess.
Keywords
Goat; Subcutaneous abscess; Microorganism; Diversity
Introduction
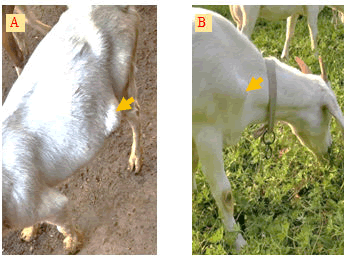
In recent years, sheep and goat raising in China has developed rapidly [1,2], but the breeding benefits are still affected by many digestive tract diseases, respiratory diseases, obstetric diseases, and various puzzling and incurable diseases [3]. Subcutaneous abscess is a common disease in goat, especially in the house feeding conditions. The incidence of the disease is 1% to 5% in many goat farms, and often more than 15% in some farms with poor sanitation. This disease is more found in goats, while sheep have a lower incidence. The main symptoms are subcutaneous swelling and maturation in the neck, chest, and abdomen (Figure 1), but generally do not show significant systemic symptoms. Early swelling is like egg size and then gradually larger, round or oval, larger than the fist. In the late stages, the skin can rupture and oozing pus. The pus is thinner and light yellow-green in early phase, and later becomes sticky like bean residue. The ruptured region often can form scab and clears without treatment, but some cases can form a fistula because of the long flow of pus. This disease generally does not lead to the death of goat, but will affect its sales. To do a good job in the prevention and treatment, the pathogens of the disease should be clearly revealed. Therefore, this work performed metagenomic analysis of microorganism community on 5 samples from different goat farms in Jiangsu Province, China.
- The abscess like egg size; 2) The abscess larger than the first; 3) The scabby abscess after rupture.
Materials and Methods
Sample collectionIn August 2021, five pus samples were collected from 5 different goat farms (4 farms are house feeding and another one is grazing locally) in Jiangsu Province, China. The number of goats in each farm is about 2000, and the incidence of subcutaneous abscess is 1% to 5%. The diseased goats with large swelling (Figure 2) were picked out and the swollen area were disinfected with iodophor diluent and alcohol (75%) after fixation. Following the abscess was cut open with a sterilized scalpel (Figure 3); about 2 ml of pus was sampled with a sterilized pipette and store in a 5 ml EP tube.
- A) An abscess located in the ventral side; B) An abscess located in the front of the forelimb.
Following the EP tubes which contained pus were stored in the biosafety transport box (UN3373), all the samples were sent to Suzhou Genomics Biotech Co., Ltd. (China) for metagenomics sequencing and bioinformatics analysis.
To investigate into the microbial species contained in the samples, all the tested sequences were blasted to the NCBI's nucleic acid database, including bacteria, fungi, archaea, viruses and parasites. The microbial abundance information in the samples was calculated based on the unigene. The samples for this research were analyzed without removal of the host genome, so some genes could be annotated to the host animal or closely related species.
Results
Validity of samplesThe quality of the sequencing is not only affected by the sequencer itself and the sequencing reagent, etc., but also by the amount of the sample. So each pus sample in this research was provided more than 2 g, which was referred to the requirements for feces samples. The DNA extraction of the samples carried out by the company is valid for testing. The extracted DNA has obvious main band, no degradation, no impurities such as RNA and protein (OD260/280 ≥ 1.5, OD260/230 ≥ 1.0).
Diversity of microorganism in subcutaneous abscess of goatThe unigene sequences were blasted to the NCBI's database and analyzed by using the LCA method. By analyzing the species classification information corresponding to each sequence, the taxonomic level before the first branch is used as the species classification of the sequence. No removal of the host genome was performed in test at this time, so some genes were annotated to host or closely related species, such as Bovidae, Cervidae, Gekkonidae, Bos mutus, Bos taurus, Capra hircus, Bos indicus, Bubalus bubalis, and so on.
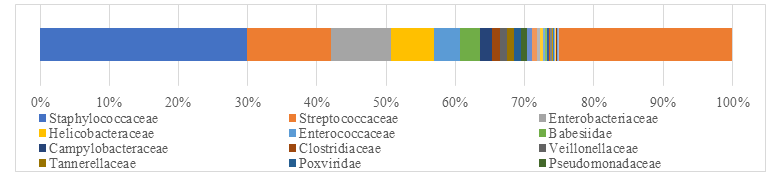
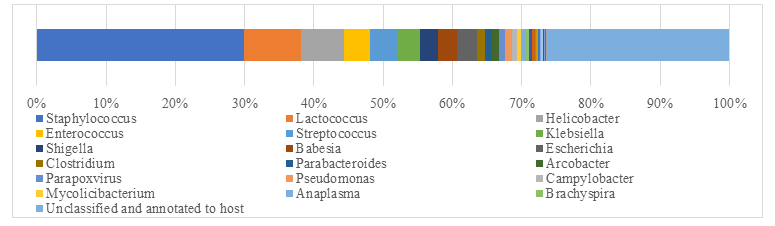
After remove the host animal and closely related species, a total of 79-86 kinds of organisms were identified, which suggested that the microorganism community in subcutaneous abscess of goat is highly diverse. The microorganisms are mainly distributed in 12 families (Figure 4) and 18 genera (Figure 5).
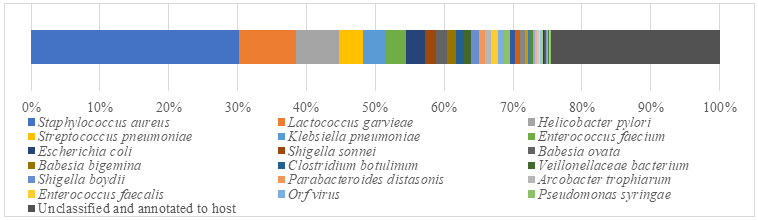
Interestingly, each sample contained similar microbial species, including 53-59 species of bacteria, 5-6 fungi, 3 viruses and 16-18 parasites. The top 5 dominant bacteria are Staphylococcus aureus, Lactococcus garvieae, Helicobacter pylori, Streptococcus pneumoniae and Klebsiella pneumoniae (Figure 6).
Abundance of microorganism in subcutaneous abscess of goatIdentification of microbial species is key to disease diagnosis. Among the 5 samples tested, the microorganism communities were almost identical. There are 14 species of bacteria with abundance more than 1%, and 24 species with an abundance of 0.05% to 1%, whiles the remaining ones with an abundance of less than 0.05% respectively. Thereinto, Staphylococcus aureus is the most dominant bacterium (29.88% in average), followed by Lactococcus garvieae (8.20%), Helicobacter pylori (6.16%), Streptococcus pneumoniae (3.50%) and Klebsiella pneumoniae (3.26%). Some viruses, fungi and parasites have also been detected, but their abundance is low and their involvement in pathogenicity is debatable. More detailed data are shown in (Table 1).
| Taxon | Goat 1 | Goat 2 | Goat 3 | Goat 4 | Goat 5 |
|---|---|---|---|---|---|
| 1. Staphylococcus aureus | 29.18 | 29.2 | 29.88 | 29.7 | 31.42 |
| 2.Lactococcus garvieae | 9.03 | 8.81 | 8.01 | 8.34 | 6.79 |
| 3.Helicobacter pylori | 6.78 | 6.58 | 6 | 6.37 | 5.08 |
| 4. Streptococcus pneumoniae | 3.45 | 3.44 | 3.5 | 3.46 | 3.66 |
| 5. Klebsiella pneumoniae | 3.16 | 3.2 | 3.27 | 3.27 | 3.42 |
| 6. Enterococcus faecium | 3.14 | 3.13 | 2.75 | 2.97 | 2.4 |
| 7. Escherichia coli | 2.62 | 2.72 | 2.96 | 2.73 | 2.95 |
| 8. Shigella sonnei | 1.71 | 1.7 | 1.55 | 1.59 | 1.25 |
| 9. Babesia ovata | 1.51 | 1.49 | 1.55 | 1.52 | 1.59 |
| 10. Babesia bigemina | 1.26 | 1.25 | 1.31 | 1.26 | 1.35 |
| 11. Clostridium botulinum | 1.19 | 1.19 | 1.23 | 1.21 | 1.29 |
| 12. Veillonellaceae bacterium DNF00626 | 1.13 | 1.15 | 1.04 | 1.05 | 0.85 |
| 13. Shigella boydii | 1.11 | 1.13 | 1.02 | 1.05 | 0.82 |
| 14. Parabacteroides distasonis | 1.09 | 1.05 | 0.95 | 1.03 | 0.81 |
| 15. Arcobacter trophiarum | 0.94 | 0.93 | 0.83 | 0.88 | 0.71 |
| 16. Enterococcus faecalis | 0.92 | 1 | 0.98 | 0.73 | 0.84 |
| 17. Orf virus | 0.92 | 0.92 | 0.97 | 0.95 | 0.99 |
| 18. Pseudomonas syringae | 0.85 | 0.85 | 0.88 | 0.86 | 0.93 |
| 19. Campylobacter coli | 0.7 | 0.7 | 0.74 | 0.73 | 0.77 |
| 20. Mycolicibacterium malmesburyense | 0.68 | 0.68 | 0.7 | 0.67 | 0.73 |
| 21. Anaplasma phagocytophilum | 0.63 | 0.65 | 0.65 | 0.67 | 0.67 |
| 22. Acinetobacter baumannii | 0.4 | 0.4 | 0.43 | 0.42 | 0.44 |
| 23. Oenococcus oeni | 0.39 | 0.39 | 0.36 | 0.36 | 0.3 |
| 24. Streptococcus agalactiae | 0.36 | 0.38 | 0.42 | 0.38 | 0.47 |
| 25. Brachyspira hampsonii | 0.32 | 0.32 | 0.33 | 0.32 | 0.34 |
| 26. Eggerthia catenaformis | 0.28 | 0.28 | 0.3 | 0.29 | 0.31 |
| 27.Dictyocaulus viviparus | 0.21 | 0.25 | 0.26 | 0.25 | 0.23 |
| 28. Clostridioides difficile | 0.17 | 0.18 | 0.18 | 0.19 | 0.19 |
| 29.Corynebacterium diphtheriae | 0.17 | 0.17 | 0.18 | 0.18 | 0.18 |
| 30. Neisseria meningitidis | 0.16 | 0.17 | 0.17 | 0.17 | 0.16 |
| 31.Brachyspira murdochii | 0.15 | 0.15 | 0.14 | 0.15 | 0.11 |
| 32. Haemonchus contortus | 0.13 | 0.14 | 0.13 | 0.14 | 0.15 |
| 33. Arcobacter defluvii | 0.1 | 0.1 | 0.11 | 0.11 | 0.11 |
| 34.Helicobacter equorum | 0.09 | 0.1 | 0.09 | 0.09 | 0.08 |
| 35. Penicillium italicum | 0.04 | 0.05 | 0.05 | 0.05 | 0.04 |
| 36.Candida intermedia | 0.04 | 0.04 | 0.03 | 0.04 | 0.03 |
| 37. Chlamydia trachomatis | 0.03 | 0.04 | 0.04 | 0.03 | 0.04 |
| 38. Enzootic nasal tumour virus of goats | 0.03 | 0.03 | 0.04 | 0.04 | 0.04 |
| 39. Mycobacterium tuberculosis | 0.03 | 0.03 | 0.03 | 0.04 | 0.04 |
| 40. Pseudomonas plecoglossicida | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| 41. Microbacterium esteraromaticum | 0.03 | 0.03 | 0.02 | 0.03 | 0.03 |
| 42. Monosiga brevicollis | 0.02 | 0.03 | 0.03 | 0.03 | 0.04 |
| 43. Staphylococcus pseudintermedius | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| 44. Bacillus sp VT-16-64 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| 45. Onchocerca flexuosa | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| 46. Neisseria polysaccharea | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| 47. Lodderomyces elongisporus | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| 48. Trypanosoma congolense | 0.01 | 0.02 | 0.02 | 0.02 | 0.03 |
| 49. Human betaherpesvirus 6A | 0.01 | 0.02 | 0.02 | 0.02 | 0.03 |
| 50. Burkholderia pseudomallei | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 |
| 51. Schizophyllum commune | 0.01 | 0.01 | 0.02 | 0.02 | 0.03 |
| 52. Lysobacter antibioticus | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 |
| 53. Bacillus anthracis | 0.01 | 0.01 | 0.02 | 0.01 | 0.02 |
| 54. Clostridium symbiosum | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 |
| 55. Campylobacter jejuni | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 |
| 56. Salmonella enterica | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 57. Vibrio parahaemolyticus | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 58. Bacteroides sp An51A | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 59. Haemophilus influenzae | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 60. Naegleria gruberi | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 61. Chlamydia abortus | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 62. Mucor circinelloides | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 63. Burkholderia multivorans | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 64. Symbiodinium microadriaticum | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 65. Enterobacter roggenkampii | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 66. Brugia malayi | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 67. Trichinella britovi | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 68. Trichinella patagoniensis | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 69. Trypanosoma brucei | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 70. Salpingoeca rosetta | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 71. Coniophora puteana | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 72. Herbaspirillum sp VT-16-41 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 73. Trichinella sp T9 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 74. Besnoitia besnoiti | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 75. Trichinella pseudospiralis | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 76. Enterobacter mori | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 77. Ciceribacter sp F8825 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 78. Clohesyomyces aquaticus | 0.01 | 0.01 | - | 0.01 | - |
| 79. Mycolicibacterium fortuitum | 0.01 | - | 0.01 | 0.01 | |
| 80. Enterobacter hormaechei | 0.01 | - | 0.01 | - | 0.01 |
| 81. Butyricicoccus pullicaecorum | 0.01 | - | - | - | - |
| 82. Trypanosoma cruzi | - | 0.01 | 0.01 | 0.01 | 0.01 |
| 83. Bacillus cereus | - | - | - | 0.01 | - |
| 84. Neisseria subflava | - | - | - | 0.01 | - |
| 85. Trichuris suis | - | - | - | - | 0.01 |
| 86. Pyricularia oryzae | - | - | - | - | 0.01 |
Table 1: The abundance of microorganisms in each sample (%).
Discussion
The aim of this research was to reveal the microorganism community in subcutaneous abscess of goat on the basis of analyzing the abundance of various microorganisms. The tests identified Staphylococcus aureus as the main pathogen of this disease, but did not exclude the involvement of other pathogens, such as Lactococcus garvieae, Helicobacter pylori, Streptococcus pneumoniae, Klebsiella pneumoniae, Enterococcus faecium, Escherichia coli, etc. Among the remaining microorganisms and parasites, some may play a role in causing disease, but many members are more likely to be animal skin flora or environmental microbes and take advantage of the opportunity of suppuration to invade tissues.
Clinically, this disease is often misdiagnosed as Pseudotuberculosis (superficial form) by veterinarians, but Corynebacterium Pseudotuberculosis was not found in the 5 samples. The main symptom of pseudotuberculosis is the enlarged and firm lymph nodes with purulent lymphadenitis [4-6], while the subcutaneous abscess in the study were usually free of lymph node lesions. Lactococcus garvieae was first found in bovine mastitis [7], and can also be isolated from diseased fish [8,9]. More importantly, it can also cause various infections in humans, such as sepsis, cardiometritis and osteomyelitis, septic hip arthritis and liver abscess [10], but no infections have been reported in goat. Helicobacter pylori are the only microbial species known to survive in the human stomach and are a class of carcinogens published by the World Health Organization's International Agency for Research on Cancer. Shigella sonnei and Shigella boydii are the most common pathogens of bacterial dysentery in humans and primates [11]. Some zoonotic bacteria (such as Lactococcus garvieae, Helicobacter pylori and Shigella) are harbored in goat infection, but what role they play is unknown. Remarkably, this leads us to wonder if goats are the reservoirs of these microbes and pose a risk to humans.
Conclusion
This small pilot study suggested that S. aureus is the most dominant but nonunique bacterium responsible for goat abscess, and the microorganism community in subcutaneous abscess is highly diverse. Although some microorganisms are a tiny part of the total community, they constitute a major portion of individual reads. Co-infection of other bacteria should also play an important role. It remains difficult to predict which species of bacteria might be found on a particular goat, but predicting which species are most frequent (or rare) seems more straightforward, at least for those species living in subcutaneous abscess. Demonstrating the impact of bacteria on this disease may be of value in demonstrating the need to implement preventive healthcare strategies, and subsequently improve animal health and welfare.
Acknowledgements
The authors would like to thank the four farms that allowed samples collection for use in this study.
Authors’ contributions
Cheng D conceived and designed the study, and jointly executed the experiments with Cheng C, Chen X and Zhu S. Cheng D and Tao J co-wrote the paper. All authors read and approved the final manuscript.
Funding
This project was supported by the earmarked fund for Jiangsu Agricultural Industry Technology System (JATS (2021) 492), and partial funded by the Advantageous Discipline Construction Project of Universities in Jiangsu Province (2018).
Data availability statement
The further data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statement
The samples collection in these farms took place under the direction of the farms’ usual vet as part of their routine veterinary herd health programme; therefore, no changes in animal treatment or handling occurred as a result of the samples collection required for this study.
Consent for publication
All the authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
References
- Baird GJ, Fontaine MC (2007) Corynebacterium pseudotuberculosis and its role in ovine caseous lymphadenitis. J Comp Pathol 137:179-210.
[Crossref] [Google Scholar] [Pubmed]
- Almeida S, Sousa C, Abreu V, Diniz C, Dorneles E, et al (2017) Exploration of nitrate Reductase metabolic pathway in Corynebacterium pseudotuberculosis. Int J Genomics
[Crossref] [Google Scholar] [Pubmed]
- Ruiz H, Ferrer LM, Ramos JJ, Baselga C, Alzuguren O, et al (2020) The relevance of caseous lymphadenitis as a cause of culling in adult sheep. Anim 10:1962.
[Crossref] [Google Scholar] [Pubmed]
- Rodrigues MX, Lima SF, Higgins CH, Canniatti-Brazaca SG, Bicalho RC (2016) The Lactococcus genus as a potential emerging mastitis pathogen group: A report on an outbreak investigation. Int J Dairy Sci 99:9864-9874.
[Crossref] [Google Scholar] [Pubmed]
- Vendrell D, Balcázar JL, Ruiz-Zarzuela I, De Blas I, Gironés O, et al (2006) Lactococcus garvieae in fish: A review. Comp Immunol Microbiol Infect Dis 29:177-198.
[Crossref] [Google Scholar] [Pubmed]
- Meyburgh CM, Bragg RR, Boucher CE (2017) Lactococcus garvieae: an emerging bacterial pathogen of fish. Dis Aquat Org. 123:67-79.
[Crossref] [Google Scholar] [Pubmed]
- Gibello A, Galán-Sánchez F, Blanco MM, Rodríguez-Iglesias M, Domínguez L (2016) The zoonotic potential of Lactococcus garvieae: an overview on microbiology, epidemiology, virulence factors and relationship with its presence in foods. Vet Sci Res J. 109:59-70.
[Crossref] [Google Scholar] [Pubmed]
- Van Zanten SV, Sherman PM (1994) Helicobacter pylori infection as a cause of gastritis, duodenal ulcer, gastric cancer and nonulcer dyspepsia: a systematic overview. Can Med Assoc J.150:177.
[Google Scholar] [Pubmed]
- Arif M, Syed S (2007) Association of Helicobacter pylori with carcinoma of stomach. JPMA.
- Torraca V, Holt K, Mostowy S. Shigella sonnei (2020) Trends Microbiol.28:696-697.
[Crossref] [Google Scholar] [Pubmed]
- Peng J, Yang J, Jin Q. Research progress in Shigella in the postgenomic era (2010) Sci China Life Sci. 53:1284-1290.
[Crossref] [Google Scholar] [Pubmed]
Citation: Cheng D, Chen X, Zhu S, Cheng C, Tao J (2022) The Microorganism Community in Subcutaneous Abscess of Goat. J Infect Dis Ther 10: 501.
Copyright: © 2022 Cheng D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2071
- [From(publication date): 0-2022 - Nov 23, 2024]
- Breakdown by view type
- HTML page views: 1810
- PDF downloads: 261