The Mechanisms and Functions of Transforming Growth Factors-β1 in Tendon Injury
Received: 21-Sep-2021 / Accepted Date: 22-Oct-2021 / Published Date: 29-Oct-2021 DOI: 10.4172/1165-158X.1000217
Abstract
Tendon injury is a common injury with slow healing and is usually treated surgically and non-surgically. However, the clinical results are not always satisfactory. Growth factors play an important role in tendon healing, and people increasingly believe that biological intervention can accelerate and improve healing. In recent years, it has been discovered that therapies using bioaugmentation technology have potential benefits and injuries during the healing process of tendon. Transforming growth factors-β1 GF-β1 has been identified as a promising therapeutic target in tendon healing. A lot of evidence shows that exogenous TGF-β1 can accelerate the process of tendon repair. TGF-β1 plays an important role in tendon repair and is a very effective therapeutic agent for tendon diseases. This review summarizes the mechanism and function of TGF-β1 in the repair of tendon injuries.
Introduction
As a highly specialized structure, tendon plays an essential role in the transmission of force between muscles and bones. However, tendon injury is the most common musculoskeletal disease, especially Achilles tendon, patellar, and rotator cuff tendon. These tendon injuries account for more than 30% of all musculoskeletal diseases that people have in major healthcare systems, and there are 30 million tendon surgeries worldwide each year. Tendon disorders bring a great personal burden to individual patients by reducing the quality of life, and together it brings a huge economic burden to society. Tendon disorders bring an extremely high personal burden to the individual patient by reducing quality of life, and collectively place enormous economic burden on society. The hypocellular and the hypovascular of the tendon determine its poor self-healing ability after injury [1]. Due to poor healing ability, it is almost impossible to completely repair the tendon, which often leads to persistent symptoms and re-injury. At present, the tendon is usually treated by conservative treatment and surgical treatment, but the treatment result is usually unsatisfactory [2,3]. Over the years, biological therapies have made great progress under the impetus of scientists. Growth factor therapy is one of them [1]. Transforming growth factor-β1 has been identified as a promising therapeutic target in tendon healing. It has been proven to accelerate the healing process of tendons [4-6]. To clarify the mechanism and role of TGF-β1 in tendon healing, it is inevitable to accelerate the process of tendon healing.
TGF-β1 Promotes Inflammation
Inflammation is a physiological response to injury and is part of tendon healing. The initial inflammation phase begins shortly after the injury. Inflammatory cells such as neutrophils, monocytes, and macrophages are attracted to the injury site by pro-inflammatory cytokines and produce cytokines and growth factors [7]. The expression level of TGF-β1 increases immediately after tendon injury, released from degranulated platelets, and secreted by all major cell types involved in the repair process, including lymphocytes, macrophages, endothelial cells, and smooth muscle cells, Epithelial cells and fibroblasts [8,9]. TGF-β1 attracts inflammatory cells such as white blood cells and macrophages. These cells will release more TGF-b1 and induce a more pronounced inflammatory response [10]. In vitro experiments have shown that the addition of exogenous TGF-β1 (2 ng/ml) to mast cells enhances the expression of COX-2 in tendon cells and reaches a peak in 6 hours. The TGF-β1 receptor ALK5 kinase inhibitor A83-01 significantly blocked COX-2 mRNA and COX-2 protein expression [11]. COX-2 is one of the main enzymes that contribute to the production of PGE2, which is the main inflammatory mediator in tendons [12]. Therefore, Mast cell-derived TGF-β1 plays an important role in enhancing the production of inflammatory mediator PGE2 by human tendon cells. In contrast, neutralizing TGF-β1 with anti-TGF-β1 antibodies can reduce inflammation and angiogenesis. In addition, no inflammatory cells were found at the site of sheep fetal tendon injury. In contrast, there are inflammatory cells in adult tendons. And comparing the fetal tendon with the injured tendon specimen, the injured fetal tendon did not show an increase in TGF-β1 expression, and there was strong TGF-β1 staining around the injured adult tendon and the temporary wound area. The decrease of TGF-β1 in the fetal tendon may be the reason for the lack of inflammatory infiltration in the tendon injury site [13]. It can be concluded from the above that TGF-β1 is a growth factor, but it plays a pro-inflammatory role in the healing of tendon injuries.
Whether inflammation is beneficial to tendon healing is difficult to say. On the one hand, inflammation can promote tendon healing and closure through fibrosis and scar formation, and can prevent tendon infection; on the other hand, scar healing caused by inflammation can hinder tissue regeneration and may further reduce the function of tissues or organs [14]. However, inflammation is an inevitable process of adult tendon injury healing. In most cases, the inflammatory process will gradually subside, but in some cases it will enter the chronic inflammation stage. This chronic tendon inflammation is thought to be a long-term imbalance and maladaptive response to injury [15]. Therefore, inflammation should not be prevented as a necessary step for healing, but inflammation should be regulated and controlled. However, the molecular and cellular mechanisms involved in inflammation are poorly understood and need to be further understood.
TGF-β1 Affects Tenocytes
Tendons are hypocellular tissues mainly composed of tenocyte, and a stem and progenitor cell population [16]. Tenocytes make up the majority of the cellular content of the tendon and are integral in indispensable the healing process following injury [17]. Many previous literatures have studied the role of TGF-β1 after tendon injury to promote the healing of tendon injury. The following summarizes the effect of TGF-β1 on tenocytes.
Proliferation
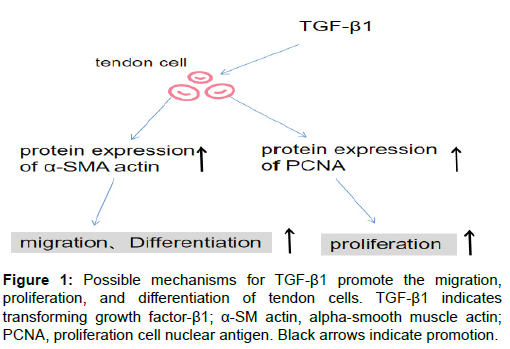
After the tendon tissue is injured, its natural healing is extremely slow and inefficient when it is severely damaged due to its intrinsic hypocellularity [18,19]. TGF-β1 increases the proliferation of tenocytes. In a recent in vitro study, acellular amnion released TGF-β1 to cause the rapid proliferation in tenocytes of adult Leghorn chickens with relatively static properties [20]. Identically,after 48 hours of exposure to 1ng/ml TGF-β1 in human tenocytes, there was a significant increase in cell proliferation, as shown with crystal violet method, as compared to control [21]. In addition, TGF-β1 promotes the proliferation of tendon stem cells (TSC, and when TGF-β receptors are blocked by TGF-β receptor inhibitor (ITD-1), the proliferation of TSC will be significantly reduced [22]. The activation of TGF-β/BMP signaling pathway by TGF-β1 may promote proliferation during tendon injury. TGF-β1 may promote the proliferation during tendon injury by activating the TGF-β/BMP signaling pathway [23]. The molecular mechanism of the proliferation of tendon cells may be through the up-regulation of proliferating cell nuclear antigen(PCNA) gene and protein expression through TGF-β1[24] (Figure 1).
Migration
The migration of tenocytes is a fundamental biological behavior and is a significant factor to promote tendon repair [25]. In the process of tendon healing, tenocytes migrate into the injured site, secreting collagen and reorganizing the extracellular matrix (ECM) to repair the tendon [16]. In vitro experiment showed that treatment of TGF-β1 promoted cell migration. However, inhibition of TGF-β1 by transfection of sh-TGF-β1 or treatment of TGFR I/II inhibitor partially reversed the migration of tenocytes [26]. In a wound healing experiment, the migration effect of TGF-β1 carried by the exosomes of TSC on TSC, compared with the control group, the migration ability of TSC was significantly enhanced. In addition, TGF-β receptor inhibitors can significantly reduce TSC migration [22]. However, the molecular mechanisms controlling the migration of TSC during tendon repair are not well understood.
Differentiation
It is generally accepted that cellular activation stimulates the conversion of fibroblasts and tenocytes to myofibroblasts represents a key event during wound healing and tissue repair [27]. Because myofibroblasts induce the expression of alpha-smooth muscle actin (α-SMA), they gain contractility and contribute to the repair of physiological tissues. It is well established that tendon fibroblasts can increase the expression of α-SMA and activate its transformation into myofibroblasts after treatment with TGF-β1 [28,29]. In addition, the contraction of myofibroblasts also results in the release of TGF-β1 from these cells, which may promote the further trans-differentiation of fibroblasts through a feed-forward mechanism [30,31]. Activation of TGF-β1 causes phosphorylation of smad2/3 to up-regulate the expression of α-SMA [32]. In addition, in vitro experimental studies have shown that TGF-β1 can induce tendon stem cells to differentiate into tendon cells. During the differentiation process, the levels of phosphorylated Smad1/ 5/8 and Smad2/3 were increased [23]. Further studies have shown that the inhibitor-Smad seems to be related to tendon Stem cell differentiation is negatively correlated, meaning that TGF-β/BMP signaling is involved in the differentiation process of TSC [33]. Further research will be required to determine the extent to which TGF-β1 promotes fibroblast and TSC trans-differentiation, as this may represent an important mechanism through which TGF-β1 may provide benefit to tendon repair.
TGF-β1 Affects of the Metabolism Extracellular Matrix
TGF-β1 is known for its effect on the production of extracellular matrix in tendons. TGF-β1 can modulate production of collagen. Collagen is an essential ECM component of tendons, and their formation and configuration are important factors that determine tendon repair. Although there are numerous collagen types, Type I collagen and Type Ⅲ collagen seem to play the most important role in tendon healing [34]. A number of in vitro experiments showed that compared with control tendon fibroblasts, TGF-β treatment of tendon fibroblasts resulted in increased expression of type I collagen and type III collagen [28,35,36]. Eduardo Anitua, et al. [37] showed that regardless of the concentration of TGF-β1, the production of type I collagen is similar. It is suggested that higher TGF-β1 concentration has nothing to do with the further increase of type I collagen. In another experiment, it was shown that TGF-β1 inhibited gene expression of procollagen Type III with fibronectin anchorage only at a low concentration (0.1 ng/mL) [38]. It is suggested that in the later stage of tendon healing, the reduction of TGF-β1 levels can inhibit the accumulation of type III collagen. TGFβ1 has been shown to mediate the expression of collagen through Smad and ERK pathways [39]. In a rat Achilles tendon rupture repair model, one week after the injection of exogenous TGF-β1, it was found that the expression of type I and type III procollagen mRNA increased in a dose-dependent manner and increased the mechanical strength of the rat tendon repair [40]. Collagen cross-linking is an important step to impart strength to collagen fibers [7]. This process is mainly promoted by lysyl oxidase, and the synthesis of lysyl oxidase can be up-regulated by TGF-β1. And type III collagen is believed to be able to quickly form cross-links, thereby stabilizing the repair site. TGF-β1 can regulate the synthesis of type III collagen and participate in matrix remodeling [5]. So, TGF-β1 can regulate the synthesis and cross-linking of collagen to increase the strength of collagen fibers.
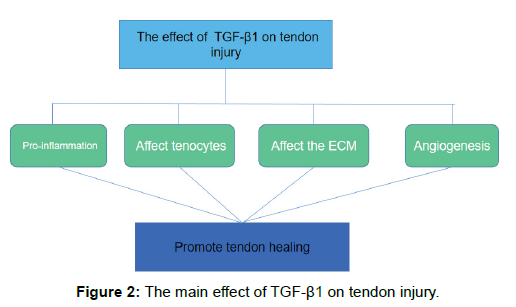
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases that can degrade various ECM components, including collagen, fibronectin and proteoglycans [41]. Tissue inhibitors of metalloproteinases (TIMPs) are a class of proteins that bind to the active site of the MMP catalytic domain to exert an endogenous inhibitory effect on MMPs [42]. MMPs and TIMPs interact to make ECM in dynamic equilibrium. TGF-β1 can regulate their expression. The cultured flexor tendon cells were treated with TGF-β1, and the effect of TGF-β1 on the expression of MMP and MMP inhibitor was studied. The results showed that TGF-β1 had little effect on the expression of MMP-2, MMP-3 and MMP-14, but it resulted in a significant dose-dependent decrease in MMP-16 gene expression and did not affect TIMP-2 expression [35]. In the rat rotator cuff repair model, the expression of MMP-9 and MMP-13 in the TGF-β1 treatment group was significantly lower than that of the control group at 2 weeks postoperatively [43]. Farhat, et al. [44] proposed that TGF-β1 produces plasminogen activator inhibitor 1 (PAI-1) that inhibits the production of plasmin and MMP-2. These experiments show that the effect of TGF-β1 on the extracellular matrix is inclined to favor the production of ECM. However, the regulation of TGF-β1 on the extracellular matrix is complex and diverse and requires in-depth study (Figure 2).
TGF-β1 Promotes Angiogenesis
A well-known feature of TGF-β1 in tendon tissue is its ability to stimulate collagen synthesis. Less attention is paid to the fact that TGF-β1 can indirectly promote angiogenesis. Healthy tendon tissue is an area lacking blood supply. After tendon injury, blood vessels are destroyed, and new blood vessels form new vascular network and granulation tissue to transport nutrients necessary for tendon repair [45]. The formation of new blood vessels is mainly mediated by vascular endothelial cell growth factor (VEGF). The up-regulation of VEGF expression in endogenous tendon cells plays an important role in the process of neovascularization and tendon repair [46]. TGF-β has been shown to up-regulate the expression of VEGF in many connective tissues [47-49]. In the rotator cuff tear model, TGF-β1 slows the expression of VEGF in the extracellular matrix of fibroblasts [50]. In vitro, experiments have shown that TGF-β1 secreted by platelets leads to an increase in VEGF of tendon cells and induces a potential angiogenic response [37]. The above suggests that TGF-β1 promotes angiogenesis by up-regulating VEGF, which may be one of the mechanisms by which TGF-β1 promotes tendon repair.
Conclusion
TGF-β1 is an important regulator of tendon repair. Accelerate the healing process of tendons by promoting cell proliferation, angiogenesis, collagen remodeling, etc. However, while exogenous TGF-β1 promotes tendon repair, it also promotes inflammation, collagen synthesis, and extracellular matrix deposition, and at the same time causes tendon adhesion and scar formation. The formation of adhesions causes the tendons to bond with each other and with surrounding structures, thereby preventing normal sliding motion. Scarring causes the tendon tissue to weaken. Studies have shown that anti-TGF-β1 neutralization reduces tendon adhesions and scars, but reduces the mechanical strength of tendons. In the future, it is possible to in-depth study of the mechanism of TGF-β1 on tendon repair, so that the tendon can heal under the condition of intact function, and can reduce the formation of minimal adhesion.
Acknowledgments
YJL, XYL designed the present study. YJL and RZ prepared and wrote the manuscript. BZ, XLL, SG and CLW performed a literature search and selected the studies to be included. YJL, DXW and SL revised the manuscript. All authors approved the final version of the article.
Conflict of Interest
The authors report no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Sheng, R., Jiang, Y., Backman, L. J., Zhang, W., & Chen, J. The application of mechanical stimulations in tendon tissue engineering. Stem Cells Int., 2020;2020: 8824783.
- Majewski, M., Widmer, K. H., & Steinbrück, K. Achilles tendon ruptures: 25 year's experience in sport-orthopedic treatment. Sportverletz Sportschaden., 2002;16(4): 167-173.
- Nourissat, G., Berenbaum, F., & Duprez, D. Tendon injury: From biology to tendon repair. Nat Rev Rheumatol., 2015;11(4): 223-233.
- Heisterbach, P. E., Todorov, A., Flückiger, R., Evans, C. H., & Majewski, M. Effect of BMP-12, TGF-β1 and autologous conditioned serum on growth factor expression in achilles tendon healing. Knee Surg Sports Traumatol Arthrosc., 2012;20(10): 1907-1914.
- Hou, Y., Mao, Z., Wei, X., Lin, L., Chen, L., Wang, H., et al. The roles of TGF-beta1 gene transfer on collagen formation during Achilles tendon healing. Biochem Biophys Res Commun., 2009;383(2): 235-239.
- Speed, C. Inflammation in tendon disorders. Adv Exp Med Biol., 2016;920: 209-220.
- Docheva, D., Müller, S. A., Majewski, M., & Evans, C. H. Biologics for tendon repair. Adv Drug Deliv Rev., 2015;84: 222-239.
- Pakyari, M., Farrokhi, A., Maharlooei, M. K., & Ghahary, A. Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care (New Rochelle)., 2013;2(5): 215-224.
- Zhang, A. Y., Pham, H., Ho, F., Teng, K., Longaker, M. T., Chang, J., et al. Inhibition of TGF-beta-induced collagen production in rabbit flexor tendons. J Hand Surg Am., 2004;29(2): 230-235.
- Crispim, J., Fernandes, H. A. M., Fu, S. C., Lee, Y. W., Jonkheijm, P., & Saris, D. B. F. TGF-β1 activation in human hamstring cells through growth factor binding peptides on polycaprolactone surfaces. Acta Biomater., 2017;53: 165-178.
- Behzad, H., Sharma, A., Mousavizadeh, R., Lu, A., & Scott, A. Mast cells exert pro-inflammatory effects of relevance to the pathophyisology of tendinopathy. Arthritis Res Ther., 2013;15(6): R184.
- Ciardulli, M. C., Marino, L., Lamparelli, E. P., Guida, M., Forsyth, N. R., Selleri, C., et al. Dose-response tendon-specific markers induction by growth differentiation factor-5 in human bone marrow and umbilical cord mesenchymal stem cells. Â Int J Mol Sci., 2020;21(16): 5905.
- Beredjiklian, P. K., Favata, M., Cartmell, J. S., Flanagan, C. L., Crombleholme, T. M., & Soslowsky, L. J. Regenerative vs. reparative healing in tendon: A study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng., 2003;31(10): 1143-1152.
- Wang, Y., He, G., Tang, H., Shi, Y., Kang, X., Lyu, J., et al. Aspirin inhibits inflammation and scar formation in the injury tendon healing through regulating JNK/STAT-3 signalling pathway. Cell Prolif., 2019;52(4): e12650.
- Alim, M. A., Peterson, M., & Pejler, G. Do mast cells have a role in tendon healing and inflammation? Cells., 2020;9(5): 1134.
- Vinhas, A., Rodrigues, M. T., & Gomes, M. E. Exploring stem cells and inflammation in tendon repair and regeneration. Adv Exp Med Biol., 2018;1089: 37-46.
- Andarawis-Puri, N., Flatow, E. L., & Soslowsky, L. J. Tendon basic science: Development, repair, regeneration, and healing. J Orthop Res., 2015;33(6): 780-784.
- Chen, J. L., Zhang, W., Liu, Z. Y., Heng, B. C., Ouyang, H. W., & Dai, X. S. Physical regulation of stem cells differentiation into teno-lineage: Current strategies and future direction. Cell Tissue Res., 2015;360(2): 195-207.
- Walden, G., Liao, X., Donell, S., Raxworthy, M. J., Riley, G. P., & Saeed, A. A clinical, biological, and biomaterials perspective into tendon injuries and regeneration. Tissue Eng Part B Rev., 2017;23(1): 44-58.
- Sang, R., Liu, Y., Kong, L., Qian, L., & Liu, C. Effect of acellular amnion with increased tgf-β and bfgf levels on the biological behavior of tenocytes. Front Bioeng Biotechnol., 2020;8: 446.
- Fong, G., Backman, L. J., Alfredson, H., Scott, A., & Danielson, P. The effects of substance P and acetylcholine on human tenocyte proliferation converge mechanistically via TGF-β1. PLoS One., 2017;12: e0174101.
- Li, M., Jia, J., Li, S., Cui, B., Huang, J., Guo, Z., et al. Exosomes derived from tendon stem cells promote cell proliferation and migration through the TGF β signal pathway. Biochem Biophys Res Commun., 2021;536: 88-94.
- Han, P., Cui, Q., Yang, S., Wang, H., Gao, P., & Li, Z. Tumor necrosis factor-α and transforming growth factor-β1 facilitate differentiation and proliferation of tendon-derived stem cells in vitro. Biotechnol Lett., 2017;39(5): 711-719.
- Tsai, W. C., Tang, S. T., & Liang, F. C. Effect of therapeutic ultrasound on tendons. Am J Phys Med Rehabil., 2011;90(12): 1068-1073.
- Yao, Z., Wang, X., Zhang, W., Liu, Y., & Ni, T. Photochemical tissue bonding promotes the proliferation and migration of injured tenocytes through ROS/RhoA/NF-κB/Dynamin 2 signaling pathway. J Cell Physiol., 2018;233(10): 7047-7056.
- Li, J., Liu, Z. P., Xu, C., & Guo, A. TGF-β1-containing exosomes derived from bone marrow mesenchymal stem cells promote proliferation, migration and fibrotic activity in rotator cuff tenocytes. Regen Ther., 2020;15: 70-76.
- Best, K. T., Nichols, A. E. C., Knapp, E., Hammert, W. C., Ketonis, C., Jonason, J. H., et al. NF-κB activation persists into the remodeling phase of tendon healing and promotes myofibroblast survival. Sci Signal., 2020;13(658): eabb7209.
- Cui, Q., Wang, Z., Jiang, D., Qu, L., Guo, J., & Li, Z. HGF inhibits TGF-β1-induced myofibroblast differentiation and ECM deposition via MMP-2 in Achilles tendon in rat. Eur J Appl Physiol., 2011;111(7): 1457-1463.
- Thampatty, B. P., & Wang, J. H. A new approach to study fibroblast migration. Cell Motil Cytoskeleton., 2007;64(1): 1-5.
- Hinz, B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol., 2007;127(3): 526-537.
- Lan, H. Y. Tubular epithelial-myofibroblast transdifferentiation mechanisms in proximal tubule cells. Curr Opin Nephrol Hypertens., 2003;12(1): 25-29.
- Feng, X. H., & Derynck, R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol., 2005;21: 659-693.
- Wang, D., Pun, C. C. M., Huang, S., Tang, T. C. M., Ho, K. K. W., Rothrauff, B. B., et al. Tendon-derived extracellular matrix induces mesenchymal stem cell tenogenesis via an integrin/transforming growth factor-β crosstalk-mediated mechanism. Faseb J., 2020;34(6): 8172-8186.
- Klass, B. R., Rolfe, K. J., & Grobbelaar, A. O. In vitro flexor tendon cell response to TGF-beta1: A gene expression study. J Hand Surg Am., 2009;34:495-503.
- Farhat, Y. M., Al-Maliki, A. A., Chen, T., Juneja, S. C., Schwarz, E. M., O'Keefe, R. J., et al. Gene expression analysis of the pleiotropic effects of TGF-β1 in an in vitro model of flexor tendon healing. PLoS One., 2012;7(12): e51411.
- Mendias, C. L., Gumucio, J. P., & Lynch, E. B. Mechanical loading and TGF-β change the expression of multiple miRNAs in tendon fibroblasts. J Appl Physiol., 2012;113(1): 56-62.
- Anitua, E., Sanchez, M., Nurden, A. T., Zalduendo, M., de la Fuente, M., Azofra, J., et al. Reciprocal actions of platelet-secreted TGF-beta1 on the production of VEGF and HGF by human tendon cells. Plast Reconstr Surg., 2007;119(3): 950-959.
- Fu, S. C., Wong, Y. P., Cheuk, Y. C., Lee, K. M., & Chan, K. M. TGF-beta1 reverses the effects of matrix anchorage on the gene expression of decorin and procollagen type I in tendon fibroblasts. Clin Orthop Relat Res., 2005;(431): 226-232.
- Ruan, H., Liu, S., Li, F., Li, X., & Fan, C. Prevention of tendon adhesions by erk2 small interfering RNAs. Int J Mol Sci., 2013;14(2): 4361-4371.
- Kashiwagi, K., Mochizuki, Y., Yasunaga, Y., Ishida, O., Deie, M., & Ochi, M. Effects of transforming growth factor-beta 1 on the early stages of healing of the Achilles tendon in a rat model. Scand J Plast Reconstr Surg Hand Surg., 2004;38(4): 193-197.
- Davis, M. E., Gumucio, J. P., Sugg, K. B., Bedi, A., & Mendias, C. L. MMP inhibition as a potential method to augment the healing of skeletal muscle and tendon extracellular matrix. J Appl Physiol., 2013;115(6): 884-891.
- Alameddine, H. S. Matrix metalloproteinases in skeletal muscles: Friends or foes? Neurobiol Dis., 2012;48(3): 508-518.
- Arimura, H., Shukunami, C., Tokunaga, T., Karasugi, T., Okamoto, N., Taniwaki, T., et al. TGF-β1 improves biomechanical strength by extracellular matrix accumulation without increasing the number of tenogenic lineage cells in a rat rotator cuff repair model. Am J Sports Med., 2017;45(10): 2394-2404.
- Farhat, Y. M., Al-Maliki, A. A., Easa, A., O'Keefe, R. J., Schwarz, E. M., & Awad, H. A. TGF-β1 suppresses plasmin and mmp activity in flexor tendon cells via pai-1: Implications for scarless flexor tendon repair. J Cell Physiol., 2015;230(2): 318-326.
- Ackermann, P. W., Li, J., Lundeberg, T., & Kreicbergs, A. Neuronal plasticity in relation to nociception and healing of rat Achilles tendon. J Orthop Res., 2003;21(3): 432-441.
- Petersen, W., Pufe, T., Unterhauser, F., Zantop, T., Mentlein, R., & Weiler, A. The splice variants 120 and 164 of the angiogenic peptide Vascular Endothelial Cell Growth Factor (VEGF) are expressed during Achilles tendon healing. Arch Orthop Trauma Surg., 2003;123(9): 475-480.
- Frank, S., Hübner, G., Breier, G., Longaker, M. T., Greenhalgh, D. G., & Werner, S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem., 1995;270(21): 12607-12613.
- Nakagawa, T., Li, J. H., Garcia, G., Mu, W., Piek, E., Böttinger, E.P., et al. TGF-beta induces proangiogenic and antiangiogenic factors via parallel but distinct Smad pathways. Kidney Int., 2004;66(2): 605-613.
- Riedel, K., Riedel, F., Goessler, U. R., Germann, G., & Sauerbier, M. Tgf-beta antisense therapy increases angiogenic potential in human keratinocytes in vitro. Arch Med Res., 2007;38(1): 45-51.
- Zhang, C., & Liu, Y. J. Biomechanic and histologic analysis of fibroblastic effects of tendon-to-bone healing by Transforming Growth Factor β1 (TGF-β1) in rotator cuff tears. Acta Cir Bras., 2017;32(12): 1045-1055.
Citation: Li Y, Qiu Y, Zhu B, Liu X, Liu X, et al. (2021) The Mechanisms and Functions of Transforming Growth Factors-β1 in Tendon Injury. Cell Mol Biol 67: 217. DOI: 10.4172/1165-158X.1000217
Copyright: © 2021 Li Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1955
- [From(publication date): 0-2021 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1335
- PDF downloads: 620