The Management of a Discordant Toxoplasmosis Infection in a Dizygotic Twin Pregnancy
Received: 12-Aug-2020 / Accepted Date: 20-Aug-2020 / Published Date: 27-Aug-2020 DOI: 10.4172/2376-127X.1000435
Abstract
In twin pregnancy the clinical course of congenital toxoplasmosis is concordant for monozygotic twins and is generally discordant between dizygotic ones, where one twin is symptomatic and the other has a subclinical infection [1,2]. It is unknown how the parasite infects the placenta and the fetus, but the severity of the disease is correlated with the extent of placental damage [3]. Case presentation. We present the case of a pregnant patient, known with primary infertility who undergoes an in-vitro fertilization procedure and becomes pregnant with a twin pregnancy, dichorionic, diamniotic. An acute toxoplasmosis infection suspicion occurs in the second trimester and immunoglobulin M (IgM) and immunoglobulin G (IgG) toxoplasmosis are tested and a high value is found. One of the fetuses is affected, shown by ultrasound, magnetic resonance imaging (MRI) and polymerase chain reaction (PCR) from amnion, the other one healthy. The patient opts for feticide, that is legal in Austria, for proven malformations. Conclusions. This case report wants to highlight the fact that the management of twin pregnancy with discordant affliction is a challenge therefore selective feticide is a sensitive issue not accepted in many countries.
Introduction
Toxoplasma gondii is an intracellular parasite that is responsible for both toxoplasmosis, as an illness, and severe congenital birth defects. If the infection occurs during pregnancy, the parasite is transmitted across the placenta to the fetus. It can result in congenital toxoplasmosis which can cause abortion, stillbirth, microcephaly, retinochoroiditis, blindness, serious mental retardation [4, 5].Some countries such as Germany, South Chorea and China opted to test the maximum of people and seem to control better the epidemy.
The infection in pregnancy is severe if it occurs within the first trimester and the risk of transplacental transmission varies from 20 to 70% [6,7]. The further in pregnancy that the infection occurs, the more likely it is that the fetus will be infected but with a less severe form of illness [8].
In twin pregnancy the clinical course of congenital toxoplasmosis is concordant for monozygotic twins and can be discordant for dizygotic ones, where one twin is symptomatic and the other has a subclinical infection [1,2].
In all cases, the preferred diagnosis test is serum testing for IgG and IgM, even with current limitations [9,10]. Confirmation of congenital toxoplasmosis is done using PCR from the amniotic fluid, from both fetuses.
The management of twin pregnancies with congenital toxoplasmosis acquired in the 2nd trimester, with the severe affliction of only one fetus remains a challenge and the perspective of selective feticide raises ethical issues and requires precise guidelines. The obstetrical attitude is different from country to country as seen in the case report below.
Case report
We present the case of a pregnant patient, age 32, Caucasian, Ms. Twin, known with primary infertility that underwent an in-vitro fertilization procedure and became pregnant with a twin pregnancy that is dichorionic, diamniotic. The first trimester scan’s morphology presents with no signs of trisomy, and the pregnancy is developed according to the date of the embryo transfer.
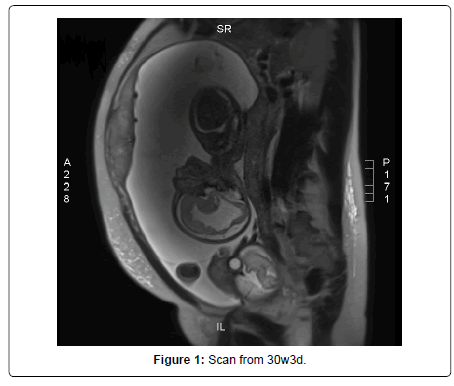
At 17 weeks of pregnancy, she presented general malaise and laterocervical adenopathy, therefore, an acute toxoplasmosis infection was suspected, and IgM and IgG toxoplasmosis were tested and the results showed a high value. The ultrasound had shown normal aspects for the moment, but at 19 weeks of gestation fetus B was smaller, with moderate ascites and ventriculomegaly and periventricular hyperechogenicity. All of these findings were due to Toxoplasma infection, as the seroconversion was proven two weeks before. She was prescribed a treatment: Pyrimethamin 25mg 1-0-0 Sulfadiazin 500mg 1-0-1, Spyramicin 375mg 2-2-2 and Folic Acid 15mg 1-0-0 (Figure 1).
Amniocentesis was performed, together with the testing for toxoplasma, and the results show that deoxyribonucleic acid (DNA) toxoplasma was positive and the karyotype was normal for fetus B. The karyotype was also normal for fetus A and DNA Toxoplasma from the amniotic fluid was negative. The next ultrasound scans had shown the progression of the ventriculomegaly, the ascites and the polyhydramnios. The parents were informed that feticide is legal in Austria until labour starts if there is a serious malformation. The patient was evaluated in Austria Centrum fur Fetalmedizin, Privatklinik Dobling, at 30w3d of gestation, and the findings were as follows: Fetus B had polyhydramnios and growth restriction, there was severe ventriculomegaly with irregular delineation of the brain ventricles and a very thin corpus callosum, mild ascites, the fetus moved very little, and the hands and feet seemed to be in unusual position: the fingers were stretched and the feet were in plantarflexion.
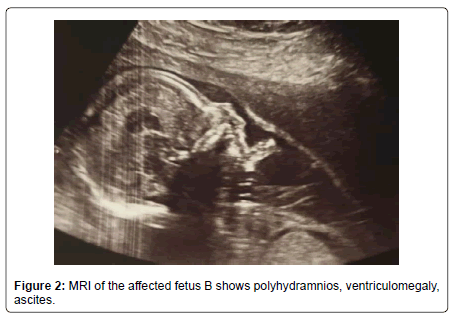
The MRI showed that fetus A had no abnormalities, but fetus B showed intracranial ventriculomegaly up to 20 mm with irregular ventricle walls, the parenchyma was thin, the cortical folding developed similar to fetus A, but in some regions with irregular gyration. The transversal cerebellar diameter is 36mm. The lungs showed identical signal intensity to fetus A, the size of the lungs normal in relation to the body size. In the abdomen small ascites lamella around the liver (5mm), polyhydramnios and irregular signals in the placenta. All the findings pointed to an infection in keeping with toxoplasmosis in fetus B (Figure 2 and 3).
An ethical board was issued and selective feticide was decided at 32w5d: first amniocentesis and evacuation of 3150 ml of amniotic fluid from fetus B was done. The feticide was performed with potassium chlorite (KCL) 15% injected directly into the left ventricle with a 20G spinal needle using ultrasound guidance to induce asystole. After 10 minutes the confirmation of the absence of cardiac activity in fetus B by Doppler scan proved the success of the procedure. The patient was discharged the same day of the procedure and was advised to follow the treatment with Progesterone 200mg, 1-1-1; Spiramycine 500mg 1-0-0 and Nifedipin 10mg 1/2-0-1/2.
In the following weeks, the development of fetus A was normal, the pregnancy was supervised in our clinic. At 34w3d she had a premature rupture of membranes and delivered by C-section, an infant girl, 2600g, Apgar score 7/9, and a dead infant girl, 1800g, with signs of being soaked in 2/3 degree. The placental histopathological findings from fetus A showed signs of chorioamniotitis, and the placenta of fetus B autolytic lesions.
The premature newborn was resuscitated at birth with tactile stimulation and a positive-pressure ventilation (PPV) device with the oxygen flow and rate as per neonatal resuscitation program (NRP) recommendations [11]. After birth, the prevention of hemorrhagic disease in newborns was done with Phytomenadione 0.2 ml intramuscularly and another dose after one week. In the second day of life, the newborn had an apnea episode with 70% desaturation, that was interpreted as part of the congenital infection. Because of the history of mother’s infection, a treatment with Ceftriaxone and Clindamycin was given for 10 consecutive days, including oxygen therapy for the first 3 days. Laboratory studies revealed normal blood cell count, easily elevated liver enzymes, IgM and immunoglobulin A (IgA) toxoplasma negative and IgG positive, due to placental transfer. She was discharged after 10 days, with no signs of congenital toxoplasmosis. The infant was retested at 12 months of age, which revealed an absence of anything indicative of congenital toxoplasmosis, such as retinochoroiditis or mental retardation.
Discussions
Toxoplasmosis is a parasitic disease that requires pre and post birth management. The occurrence in dizygotic twins with discordant findings has not been frequently reported. This is because the manifestation depends on the coincidence of two independent and rare events, fetal toxoplasmic infection and twin births [2].
Prevalence of congenital infection ranges from 0.1 to 0.3 per 1000 live births. Among all women who were first infected by Toxoplasma gondii during pregnancy, 61% will not transmit the illness to the fetus, 26% of the conceptions will present subclinical infection and in 13% there will be a clinical infection [12].
The findings in twin dichorial pregnancies are similar to monochorial pregnancies. The placental lesions are important in the assessment of fetal involvement. Usually as a pattern, in dizygotic twins, both fetuses will be affected. In our situation only one fetus was infected as was proven by PCR from the amniotic fluid. Other studies have shown that there is a discordance between dizygotic twins [1, 12]. Early diagnosis and adequate anti-parasitary treatment of pregnant women can reduce the rate of transmission to the fetus and the severity of sequels in cases where intra-uterine infection has already occurred, and this can be the explanation why in our case one fetus was normal and why discordant affliction occurs [13].
Toxoplasmosis is usually diagnosed by antibody detection. In acute infections, increased levels of IgG and IgM antibodies usually appear within the first or second week of infection [14]. In our case the diagnosis was easily made because seroconversion was proven.
Pre-natal diagnosis of congenital toxoplasmosis using PCR in amniotic liquid was initially suggested by Grover et al. [15]. This is the golden standard of congenital toxoplasmosis diagnosis. This technique uses the amplification of a gene sequence of Toxoplasma gondii; among the cloned genes, the most frequently used is gene B1, due to its greater specificity, being also found in different species of this parasite. Fast and simple, this technique can be used on the amniotic liquid starting at 18 weeks of pregnancy. The overall diagnosis sensitivity of PCR on amniotic fluid specimen was 54% and it`s specificity was 100% [16, 17]. This is why in our case amniocentesis was done from both fetuses, and infection was proven by PCR only in one fetus.
A study have showed that the most common ultrasonographic signs for computerised tomography (CT) are: ventricular dilatation (hydrocephaly), which occurs due to necrosis in the region of the Sylvius duct, intracranial calcifications, hyperechogenic foci, thickening of the placenta, fetal hepatomegaly, ascites and pleural and pericardial effusion [17]. In our case we observed ascites, ventriculomegaly and periventricular hyperechogenicity and we confirmed this finding with MRI.
Toxoplasma gondii can be detected in placenta by mouse inoculation and/or PCR but publications focusing on the results of placental analysis are rare, but studies on congenital toxoplasmosis occurring in twins confirm the role of the placenta in the modalities and mechanism of fetal affliction [18]. The differential rates of placental vascular supply may explain the discordance in infection in twins [19]. On the other hand, failure to detect Toxoplasma in the placenta does not rule out the diagnosis of congenital toxoplasm osis, as illustrated by the 38 cases of congenital toxoplasmosis in which the placenta was negative showed by a study made by Denis Filisetti et al [20]. We could not identify the presence of toxoplasma due to the autolytic lesions in our case.
Once it has been established that the mother’s serological test results are consistent with a recently acquired infection and that acquisition of the infection during the first 18 weeks of gestation or shortly before conception cannot be excluded, a treatment with Spiramycin should be administered in order to prevent vertical transmission of the parasite [21]. We used an association of drugs: Spiramycin – a macrolide antibiotic, with an antibacterial spectrum comparable to that of erythromycin, Pyrimethamine – a substituted phenylpyrimidine antimalarial drug; and Sulfadiazine – a sulfonamide antibiotic. Moreover, it may reduce the severity of infection in a fetus because it delays transmission to a later time in gestation when transmission is associated with less severe manifestations of infection. The combination of pyrimethamine and sulfadiazine is highly active against Toxoplasma gondii and is widely used as a treatment to reduce the risk of clinical manifestation in infected children [21]. In our case the treatment started early, may have sterilized the other twin and prevented further extent of the infection, and this could explain the discordance between the affliction.
Selective feticide in twin gestations is safe and efficient and yields a favorable outcome of the surviving fetus. Postponing the procedure to the beginning of the third trimester and treating the healthy infant for enhancement of lung maturity resulted in the birth of healthy newborns beyond 36 weeks’ gestation with good chances for survival [22].
The ethical controversies surrounding feticide are globally concerning, and disparate policies exist between and within nations creating ethical difficulties. Whether a fetus has a right to life often depends on its gestation, the severity of its abnormalities, the country`s policy and even the center in which its mother is receiving antenatal care. Only 20% of pregnancies end in abortion. An even smaller proportion in feticide. However, when the request is made, it is fraught with ethical concern. It is imperative to have evidence- based protocols and policies in place [23].
Conclusion
The management of twin pregnancies with congenital toxoplasmosis acquired in the 2nd trimester, with the severe affliction of only one fetuses is a challenge and the perspective of selective feticide raises ethical issues and requires precise guidelines.
Statements
Statement of Ethics: The patient, Ms. Twin (name changed), gave her informed written consent to the study and the preparation and publication of the case report.
Disclosure Statement: The authors have no conflicts of interest to declare.
Funding: There is no funding for this research to be declared
Authors’ contributions: Dr. Huniadi Anca contributed to this manuscript by participating in the initial diagnosis of the patient, researching the background and collecting information for the case report. All authors contributed by researching other cases and drafting the discussion, collecting clinical data and obtaining clinical images, formatting of the manuscript and overall supervision of the process. All authors reviewed and approved the final manuscript.
References
- Falavigna DLM, Roncada EV, Nakazora D, Pelloso MC, Falavigna LFM, et al. (2017) Congenital toxoplasmosis in dizygotic twins, Parana, Brazil. Rev Inst Med Trop S Paulo 49: 117-118.
- Peyron F, Ateba AB, Wallon M, Kodjikian L, Binquet C et al. (2003) Congenital toxoplasmosis in twins: report of 14 consecutive cases in comparation with published data. Pediat Infect Dis J 22: 695-701.
- Benirschke K, Kaufmann P (2000) Pathology of the human placenta. Springer.
- Wong SY, Remington JS (1994) Toxoplasmosis in pregnancy. Clin Infect Dis 18: 853–861.
- McAuley J, Boyer KM, Patel D, Mets M, Swisher C, et al (1994) Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago Collaborative Treatment Trial. Â Clin Infect Dis 18: 38-72.
- Sever JL, Ellenberg JH, Ley AC, Madden DL, Fuccillo DA et al. (1988) Toxoplasmosis: maternal and pediatric findings in 23,000 pregnancies. Pediatrics 82: 181–92.
- Desmonts G, Couvreur J (1974) Congenital toxoplasmosis: a prospective study of 378 pregnancies. N Engl J Med 290: 1110–6.
- Nelson WE, Behrman RE, Kliegman R (1998) Nelson essentials of pediatrics. Philadelphia 415.
- Liesenfeld O, Press C, Ramirez R, Flanders R, Remington JS (1996) Study of Abbott Toxo Imx system for detection of immunoglobulin G and immunoglobulin M toxoplasma antibodies: value of confirmatory testing for diagnosis of acute toxoplasmosis. J Clin Microbiol 34: 2526-30.
- Suzuki LA, Rocha RJ, Rossi KL (2001) Evaluation of serological markers for the imunodiagnosis of acute acquired toxoplasmosis. J Med Microbiol 50: 62-70.
- Weiner GM, Zaichkin J (2016) Textbook of Neonatal Resuscitation. Â Am Acad Pediatr 328.
- Tjalma W, Vandreheyden T, Naessens A, Vanderheyden J, Foulon W (1998) Discordant prenatal diagnosis of congenital toxoplasmosis in a dizygotic pregnancy. Eur J Obstet Gynecol Reprod Biol 79: 107-108.
- Hohlfeld P, Daffos F, Thulliez P, Aufrant C, Couvreur J, et al. (1989) Fetal toxoplasmosis outcome of pregnancy and infant follow-up after in utero treatment. J Pediatr 95:11-20.
- Lindsay D.S., Blagburn B.L., Dubey J.P. Feline toxoplasmosis and the importance of the T.gondii oocyst. Compend Contin Education Pract Vet 1997;19:448-61.
- Grover CM, Thulliez P, Remington JS, Boothroyd JC (1990) Rapid prenatal diagnosis of congenital Toxoplasma infection by using polymerase chain reaction and amniotic fluid. J Clin Microbiol 28:2297-301.
- Filisetti D, Year H, Villard O, Escande B, Wafo E, et al. (2020) Contribution of Neonatal Amniotic Fluid Testing to Diagnosis of Congenital Toxoplasmosis. 53: 1719–1721.
- Hohlfeld P, MacAlesse J, Capella-Pavlovski M, Giovangrandi Y, Thulliez P, et al. (1991) Fetal Toxoplasmosis: ultrasonografhic signs. Ultrasound Obstet Gynecol 1: 241-244.Â
- Â Helene FH, Pierre BPM, Patrick SJ, Veronique E, Cecile BB et al. (2007) Value of Toxoplasma gondii detection in one hundred thirty-tree placentas for the diagnosis of congenital toxoplasmosis. 26: 845-846.
- Behrman RE, Kliegman RM, Jenson HB, Stanton B (2007) Nelson Textbook of Pediatrics 18th Edition. Saunders Elsevier 1486-1495.
- Denis F, Vanessa C, Alexander P, Odile V, Ermanno C (2010) Placental testing for toxoplasma gondii is not useful to diagnose congenital toxoplasmosis. Pediatr Infect Dis J 29: 665-667.Â
- Serranti D, Buonsenso D, Valentini P (2011) Congenital toxoplasmosis treatment. Eur Rev Med Pharmacol Sci 15: 193-198.Â
- J Shalev, I Meizner, D Rabinerson, R Mashiach, M Hod, et al. (1999) Improving pregnancy outcome in twin gestations with one malformed fetus by postponing selective feticide in the third trimester. Fertil Steril 72: 257-260.Â
- K Moodley (2008) Feticide and late termination of pregnancy: five levels of ethical conflict. O&G Forum 18: 93-95.
Citation: Anca H, Andrea S, Ioana Z, Liana A, Mihai B, et al. (2020) The Management of a Discordant Toxoplasmosis Infection in a Dizygotic Twin Pregnancy. J Preg Child Health 6: 435. DOI: 10.4172/2376-127X.1000435
Copyright: © 2020 Anca H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2234
- [From(publication date): 0-2020 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1521
- PDF downloads: 713