Mini Review Open Access
The LIPT-Study: On Risk Markers of Vascular Thrombosis in Polycystic Ovary Syndrome. A Randomized, Double-Blind, Placebo-Controlled Study of the Effect of Liraglutide
| Signe Frøssing1, Malin Nylander2, Caroline Kistorp1,3, Sven Skouby2,3 and Jens Faber1,3* | |
| 1Department of Endocrinology and Internal Medicine, Herlev Hospital, Copenhagen Denmark | |
| 2Department of Obstetrics and Gynecology, Reproductive Medicine, Herlev Hospital, Copenhagen, Denmark | |
| 3Faculty of Health and Medical Sciences, University of Copenhagen, Denmark | |
| Corresponding Author : | Signe Frøssing MD, Department of Endocrinology and Internal Medicine Herlev University Hospital, Copenhagen, Herlev Ringvej 75, Dk-2730 Herlev, Denmark Tel: 45 3868 2139 Fax: 45 3868 4101 E-mail: signe.froessing@regionh.dk |
| Received February 16, 2015; Accepted March 14, 2015; Published March 30, 2015 | |
| Citation: Frøssing S, Nylander M, Kistorp C, Skouby S, Faber J (2015) The LIPT-Study: On Risk Markers of Vascular Thrombosis in Polycystic Ovary Syndrome. A Randomized, Double-Blind, Placebo-Controlled Study of the Effect of Liraglutide. J Obes Weight Loss Ther 5:254. doi:10.4172/2165-7904.1000254 | |
| Copyright: © 2015 Frøssing S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Overweight and insulin resistance (IR) are central pathogenic features of the Polycystic Ovary Syndrome (PCOS), and weight loss is the main treatment option. PCOS is also associated with signs of a chronic inflammation, activation of the coagulation system, defect endothelial function and increased arterial stiffness, all regarded as risk factors or markers for the development of cardiovascular disease. These factors are not taken into account in the definition of the syndrome, which is based on the 3 Rotterdam criteria. An uncertainty of the clinical risk of cardiovascular disease (CVD) in these relatively young women has led to many studies on surrogate markers of CVD in PCOS, including the search for new markers with additional information of the arteriosclerotic burden in PCOS. GLP-1 analogues, originally developed for the treatment of diabetes, induce weight loss also in non-diabetic people. We therefore questioned whether treatment with the GLP-1 analogue Liraglutide to women with PCOS in doses used for diabetes could induce weight loss and improve IR and through this action, or independently, improve markers of vascular thrombosis in women with PCOS. Thus, 70 overweight and/or insulin resistant PCOS women were planned treated for 26 weeks in a placebo controlled randomized trial with the following effect parameters to be evaluated: Changes in Thrombin generation time, Adrenomedullin, Atrial natriuretic peptide, body fat composition (DEXA), liver fat content (MRI), BMI, IR, sex hormones and ovarian morphology. The protocol and the background for the study are brought in this report.
| Keywords |
| Overweight; GLP-1 analogue; Polycystic ovary syndrome; Cardiovascular biomarkers; Insulin resistance |
| Abbreviations |
| BMI: Body Mass Index; CVD: Cardiovascular Disease; ETP: Endogenous Thrombin Potential; GLP-1: Glucagon Like Peptide 1; GIP: Glucose-Dependent Insulinotropic Peptide; IR: Insulin Resistance; MS: The Metabolic Syndrome; PAI-1: Plasminogen Activator Inhibitor 1; PCOS: Polycystic Ovary Syndrome; RCT: Randomized Clinical Trial; SD: Standard Deviation; TGT: Thrombin Generation Test; T2D: Type 2 Diabetes; VAT: Visceral Adipose Tissue |
| Introduction |
| Polycystic Ovary Syndrome (PCOS) is the most common endocrine dysfunction in women in the fertile age with a prevalence of 5-10%. PCOS is a complex syndrome characterized by heterogeneity in phenotypic manifestations. The clinical phenotype of PCOS includes reproductive and hormonal aberrations, namely anovulation andhyperandrogenism, along with metabolic disturbances. The interference in the normal crosstalk between the reproductive system and metabolic tissues, affects not only the risk of obesitythrough deterioration of the metabolic profile but also aggravates ovulatory dysfunction and hyperandrogenism [1]. Although the pathogenesis of PCOS remains unclear, the syndrome appears to involve environmental and genetic components, and is diagnosed according to the presence of 2 out of 3 Rotterdam Criteria: Oligomenorrhea, polycystic ovaries and hyperandrogenism [2]. PCOS is associated with the elements of the Metabolic Syndrome (MS) (obesity, impaired glucose tolerance, hypertension and dyslipidaemia), and women with PCOS have approx. 10 times increased risk of developing MS [3], and 4 times increased risk of developing Type 2 Diabetes (T2D) as evaluated in BMI-matched studies [4]. Thus, Insulin Resistance (IR) is a prominent feature of the syndrome [5]. |
| Treatment of PCOS depends on the presenting phenotype and personal wishes from the patient, and includes life-style intervention with dietary counselling and increased physical activity, as well as treatment of IR with Metformin, of hirsutism with testosterone antagonists, and of oligo/amenorrhoea through intervention with antiandrogen oral contraceptives. Moreover, treatment of the other elements of the MS (hypertension, dyslipidaemia, diabetes) is often required [6]. PCOS is similar to MS associated with increased signs of achronic inflammation [7,8], activation of the coagulation system [9], defect endothelial function [10] and increased arterial stiffness [11,12], all regarded as risk factors or markers for the development of Cardiovascular Disease (CVD) [13]. Because of an increased accumulation of CVD risk factors, women suffering from PCOS are regarded as being in increased risk of developing CVD, although precise figures are not known [14]. This is due to lack of prospective studies with precise diagnostic criteria and hard endpoints as cardiovascular events. |
| There is an increasing recognition that the Rotterdam criteria do not take into account the accumulation of cardiovascular risk factors seen in PCOS [8]. Only hyperandrogenism seems to reflect this, although no definite association has been demonstrated [15]. |
| The uncertainty on the clinical CVD risk in these relatively young women has led to many studies on surrogate markers of CVD in PCOS including also the search for markers with additional information on the arteriosclerotic pathophysiology in PCOS. In line with this we recently studied the dynamic hemostatic marker Endogenous Thrombin Potential (ETP) [16], measured by a Thrombin Generation Test (TGT) as well as Plasminogen Activator Inhibitor 1 (PAI-1) in PCOS, and found increased levels of both [17]. ETP and PAI-1 were both driven by total fat mass and IR. |
| Another novel marker of CVD, Adrenomedullin, which signals endothelial dysfunction, seems independently associated with increased androgens as well as visceral fat in PCOS (Frøssing et al. unpublished). |
| Recent studies have suggested that reduced plasma levels of the natriuretic peptide ANP might be a central pathophysiological feature in the development of hypertension [18]. A single study report decreased ANP levels in PCOS compared with age and BMI matched controls [19], which is of interest due to the link between MS including hypertension, and PCOS. |
| GLP-1-analogues induce weight loss and reduce IR in T2D. In diabetes, which has been the main indication for using GLP-1 analogues, a weight loss of approximately 3-4 kg body weight is seen [20]. Since IR and obesity are associated with PCOS, GLP-1 analogues might be an attractive therapeutic option. Thus, in one study the GLP-1 analogue Exenatide was administered for 24 weeks compared with Metformin alone or Exenatide/Metformin combination in a randomized, open-label trial on 42 women with PCOS. The combination treatment was found to be superior in improving free androgen index, insulin sensitivity, weight, abdominal fat mass, and ovulatory rate [21]. |
| In the present study we hypothesize, that GLP-1 analogue treatment with Liraglutide in PCOS without diabetes will result in a beneficial reduction in risk markers of vascular thrombosis and cardiovascular disease. This might be due to loss of weight, changed fat distribution and/or reduced IR. Further, ovary morphology might improve. On this background we undertook a RCT. The protocol is presented in this report. |
| Materials and Methods |
| Design |
| The LIPT study is an investigator-initiated single centre, randomized, double blind, parallel, placebo controlled intervention trial conducted at Herlev University Hospital, Denmark. Women with PCOS are randomized to subcutaneous injection of either Liraglutide 1.8 mg once daily or matching placebo for 26 weeks. The randomization is list-generated, in a 2:1 ratio (Liraglutide:placebo). |
| Patient population |
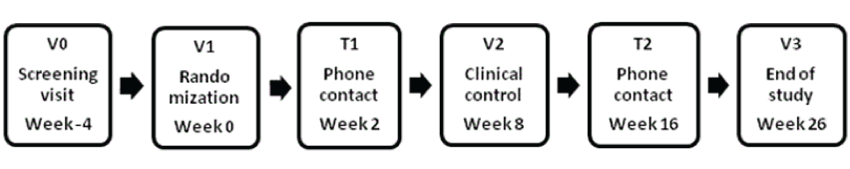
| Seventy patients with PCOS, according to the Rotterdam Criteria [2], and without T2D are scheduled for enrolment. Participants are recruited from an out-patient PCOS clinic at Department of Obstetrics and Gynaecology, Herlev University Hospital, from social media and from private specialists in Obstetrics and Gynaecology as well as General Practitioners. Women who seem likely to meet the in- and exclusion criteria are invited to screening (Table 1). |
| Screening-V0: Oral and written information is given and informed consent is obtained. Here after medical history is gathered, a general physical examination, a trans vaginal ultrasound and blood tests are performed. |
| Included participants will attend three planned visits: randomization (V1), clinical control (V2) and End of study (V3). The women will be contacted twice by phone and are all offered a one hour dietary consultation. |
| Randomization and End of study-V1 and V3: After overnight fasting a blood sample for the measurement of biomarkers is obtained. Prior to blood sampling, the participant rests for 20 minutes in sitting position, and when drawing the blood a stasis of 40 mm Hg is used. The blood is centrifuged and frozen at -80°C immediately, or sent to routine analysis. Morning urine sample is collected for albumin/ creatinine ratio analysis. A standardized oral glucose test with 75 g glucose is performed and blood samples are drawn at 0, 30, 60 and 120 minutes. A general physical examination, including blood pressure, hirsutisme score, BMI, waist, hip and thigh measurements is performed. MRI-scan, full body DEXA-scan and 2D/3D trans vaginal ultrasound are performed. Adequate contraception is mandatory in the study period, and a copper IUD is inserted at randomization. At randomization the participants will be instructed in the use of study medication and a bleeding diary. At end of study the injections pens will be returned in order to monitor compliance. |
| Safety contacts-T1, V2 and T2: Two and 16 weeks after randomization the participants will be contacted by phone in order to insure dose escalation and to monitor the tolerance to the drug. A clinical control and safety blood samples are performed eight weeks after randomization. |
| General: Pregnancy tests are obtained and concomitant medication and adverse effects/events are explored at every visit. The participants are instructed to call or text to the LIPT-study phone if they have questions or experience side effects. |
| Outcome measures |
| Primary objectives: To investigate change from randomization to end of study after 26 weeks of treatment with Liraglutide versus placebo in Thrombin Generation Time (TGT), measured as Endogenous Thrombin Potential (ETP). |
| Secondary objectives: To investigate change from randomization to end of study after 26 weeks of treatment with Liraglutide versus placebo in |
| • Cardiac biomarkers (MR-pro-Adrenomodullin, ANP, BNP, NTpro- BNP and Copeptin) |
| • Inflammatory and thrombotic biomarkers (hsCRP, PAI-1 and vWF) |
| • Male and female sex hormones |
| • Glucose metabolism (measured by HOMA-1, HOMA-2 and Matsuda Index) |
| • Liver-fat (measured by MRI-spectrometry) |
| • Body composition (measured by DEXA) |
| • Ovarian morphology (measured by 3D-ultrasonography and MRI) |
| • Anti Müllerian Hormone |
| • Bleeding pattern (bleeding diary) |
| • Hirsutism (measured by Ferriman-Gallway score). |
| Data management and statistics |
| Source data will be recorded in the patient record or on specific worksheets. A Case Report Form (CRF) is constructed for data capture. Data will be stored in coded form in 15 years according to recommendations from the Danish Data Protection Agency. |
| In the intention to treat population none of the randomized participants are excluded, and the patients are analysed according to the randomisation group. The per protocol population will consist of all patients who completed the study with a documented valid baseline and final week assessment of the primary objective ETP, measured by TGT, and without any major protocol violations. |
| The primary efficacy outcome is the difference in change from baseline to end of study in plasma levels of ETP between the treatment and the control group. The absolute values and mean change from baseline for each treatment group, and the difference in mean change between the Liraglutide group and the placebo group, and the 95% confidence interval will be presented. The primary analysis is based on the intention to treat population. Comparison of ETP levels between treatment groups will be performed after testing the parameter visually by histogram and using the Shapiro-Wilk test for normal distribution. Normally distributed variables will be presented as mean ± SD, nonparametric statistics and appropriate log-transformation will be performed if assumption of normality is not met. After log transformation the parameter will be further tested for normality distribution as indicated. A two-tailed p value of 0.05 or less is considered statistically significant. Comparisons between treatment groups will be performed by an unpaired two sample t-test, Mann- Whitney test, or χ2 test as appropriate. |
| Power analysis |
| Primary endpoint: Primary outcome parameter is change in ETP, measured by TGT, after 26 weeks of intervention. The study estimated to detect a difference (effect size) of 100 units in ETP (Cohen standardized mean difference). The power analysis was based on an estimated variance i.e. standard deviation (SD) of 130 as obtained inhouse from controls in the analysis. With 85% power and with a two sided significance level of 0.05, 63 patients should then be enrolled with a 2:1 randomisation (Liraglutide:placebo). |
| Secondary endpoints: A similar analysis was performed post-hoc using Adrenomedullin as primary end point, effect size 10% and experience with in-house analysis of Adrenomedullin. Being a more precise assay than TGT fewer patients are needed. The number of participants required to detect a difference in changes in liver fat is dependent of the SD of mean liver fat as measured by MRI spectroscopy. The estimated SD of liver fat content in moderately overweight women is 6.5% [22]. With a statistical power of 90% and a two-sided significance level of 5%, a clinical relevant difference in liver fat content of 6% can be detected with a sample size of 48:18 (Liraglutide:placebo). The anticipated clinical relevant difference of 6% is in accordance with a previous study reporting an absolute reduction in liver fat of 6% measured by MRI spectroscopy after 6 months treatment with GLP-1 analogue in 25 patients with [23]. |
| To allow for approximately 10% of randomized patients with major protocol deviations or withdrawal, 70 patients will be randomized. Cohen statistical power analysis, estimating the relationship between: significance level, effect size, power, estimated variance and sample size, was used for power calculations [24]. |
| Time schedule |
| The first patient was randomized in March 2014 and last patient, last visit is expected in January 2016. |
| Study drug |
| The study drugs Liraglutide or matching placebo (saline) are visually identical; a clear solution for subcutaneous injection in prefilled pen. The study drug will be introduced at a dose of 0.6 mg/ day, increased to 1.2 mg/day after one week and to 1.8 mg/day after two weeks according to recommendations for treatment in diabetes patients. A dose increase can be postponed based on the participant's tolerance to the study drug and the dose can be reduced at any time during the study if required. Common side effects (1-10%) are nausea, obstipation, vomiting, diarrhoea and headache. The dose of 1.8 mg is chosen for safety reasons as effects and tolerability is well documented as well as it enables us to test the effect in both lean insulin resistant and overweight PCOS-women, which is essential in determining whether Liraglutide-therapy is a treatment option for PCOS women. Log of supplied and returned study medication is kept on patient and site levels. All trial products are delivered, packed and labelled by Novo Nordisk A/S. |
| Ethics |
| The study is approved by the Danish Scientific Ethics Committee (H-2-2013-142), the Danish Medicines Agency (EudraCT-number 2013-003862-15), and the Danish Data Protection Agency. The study is conducted in accordance with the Helsinki Declaration and is monitored by the ICH-GCP-unit (good clinical practice) at Copenhagen University Hospital. Serious adverse events are reported according to GCP guidelines and Danish Medicines Agency rules. The trial is registered at www.clinicaltrials.org with ID: NCT02073929. |
| Intellectual properties are owned by the investigators only. Results will be published in international scientific journals. Novo Nordisk A/S will be given four weeks to comment on the manuscript prior to submission. |
| Discussion |
| Since the LIPT-study was initiated a few papers have been published on GLP-1 analogue treatment in PCOS. With focus on nonalcohol fatty liver disease, 19 PCOS and 17 age and BMI matched women were treated with 1.8 mg Liraglutide daily for 6 months in a case-control study [25]. On average they lost 3 kg and significantly improved HOMA-IR (5.1 to 4.3), hsCRP, triglycerides and the fibrosis marker Procollagen type 3-amino-terminal peptide compared to controls [25]. Another randomized, open-label study, including 36 PCOS patients, investigated the add-on effect of Liraglutide (1.2 mg daily) to Metformin (2 g daily) compared to either Metformin or Liraglutide as monotherapy for 12 weeks, on weight loss [26]. Combined treatment was the most effective with mean weight loss of 6.5 kg as compared to 3.8 kg in Liraglutide alone [26]. The same research group observed a reduction in visceral adipose tissue (VAT) measured by DEXA and improvement in uncontrolled and emotional eating behavior evaluated by questionnaires in 36 obese PCOS-women pretreated with Metformin and then switched to 12 weeks of Liraglutide (1.2 mg daily) mono-therapy [27]. Finally, a private gynecological clinic reported on 84 PCOS-patients with no weight loss on previous Metformin therapy and low carb diet. The patients were treated with 0.6-1.8 mg Liraglutide daily added to Metformin in an observational study without a control group for an average of 27.8 weeks (SD 19.2) and a self-reported mean weight loss of 9.0 kg (CI 7.8-10.1 kg) was found [28]. |
| The previous GLP-1 studies in PCOS have addressed weight issues, sex hormone levels, glucose metabolism, eating behavior and menstrual regularity, but the effect on markers of early cardiovascular disease has not been examined. ETP was chosen as the primary objective since it reflects the combined effect of pro- and antithrombotic pathways and not only a specific component. Increased thrombin generation is found independently predictive of CVD [29,30]. A cross-sectional study on 45 PCOS and 45 age and BMI matched controls showed the PCOS women to have faster thrombin generation suggesting a greater risk of hypercoagulation [31]. On this basis our study will add novel information as well as evaluate the previous mentioned parameters on a larger cohort. Including 48 PCOS women for Liraglutide treatment and 24 as blinded placebo controls makes this study the largest RCT on Liraglutide intervention in PCOS so far. |
| According to our in- and exclusion criteria, lean PCOS-women are included in the LIPT-study if they are insulin resistant (Table 1). This is done in order to investigate whether Liraglutide, by improving the glucose metabolism, has a role in the treatment of lean but insulin resistant PCOS-women. We also hope this will shed light on whether it is the weight loss or the improvement of the glucose metabolism from Liraglutide that improve the symptoms of the PCOS. |
| In december 2014 the American Food And Drug administration approved Liraglutide 3.0 mg daily for therapy in patients with BMI above 30, or 27 if there is weight related conditions such as hypertension, T2D or dyslipidemia. Many PCOS patients will fall into this category, and it opens a whole new treatment opportunity for women, who do not succeed in reducing weight or the PCOS symptoms on diet, excise and Metformin. Our responsibility as scientists is to insure that the treatment is beneficial for the women suffering from PCOS, and to determine the role of Liraglutide among the other available treatment modalities in this specific disease. |
| Expected results and outcome |
| From the above mentioned studies and studies in diabetes we expect an average weight loss from 3 to 9 kg during the treatment period, however we must keep in mind that we also include women with normal weight. We also expect a reduction in androgens [21], VAT and insulin resistance. We have previously reported that the primary end point ETP is increased in PCOS and mainly driven by high BMI as well as IR(17). Weight reduction intervention studies in obese children have demonstrated a reduction in ETP approximately similar to the expected reduction of 100 units ETP in this study [32]. Elevated ETP of similar magnitude as we have found in PCOS have been demonstrated to be associated with increased risk of first and recurrent venous thrombosis [33,34], and predictive of increased longterm risk of arterial cardiovascular disease [35]. With regard to liver fat we expect a reduction of about 6% due to weight loss, which is very similar to the effect of 6 months GLP-1 treatment in obese type 2 diabetes patients [23]. A 10% reduction in Adrenomedullin levels that we expect seems comparable to reductions found after gastric by-pass due to obesity [36]. |
| Contributors |
| Initiative and idea: Jens Faber, Caroline Kistorp and Sven Skouby. |
| Protocol: Signe Frøssing, Malin Nylander, Jens Faber, Caroline Kistorp and Sven Skouby. |
| Principal Investigator and sponsor: Jens Faber. |
| Conducting the study: Signe Frøssing and Malin Nylander. |
| Analysis and writing: Signe Frøssing, Malin Nylander, Jens Faber, Caroline Kistorp and Sven Skouby. |
| Acknowledgements |
| This study was supported by founding from: Copenhagen University - Faculty of Health and Medical Sciences, Novo Nordisk A/S, The Toyota Foundation Copenhagen, Research foundation of Herlev University Hospital, The dept. of Endocrinology and Internal Medicine Herlev Hospital Copenhagen Denmark, and The dept. of Obstetrics and Gynecology Reproductive Medicine Herlev Hospital Copenhagen Denmark. |
References
- Lim SS, Norman RJ, Davies MJ, Moran LJ (2013) The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev 14: 95-109.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81: 19-25.
- Vélez LM, Motta AB1 (2014) Association between polycystic ovary syndrome and metabolic syndrome. Curr Med Chem 21: 3999-4012.
- Moran LJ, Misso ML, Wild RA, Norman RJ (2010) Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 16: 347-363.
- Sprung VS, Jones H, Pugh CJ, Aziz NF, Daousi C, et al. (2014) Endothelial dysfunction in hyperandrogenic polycystic ovary syndrome is not explained by either obesity or ectopic fat deposition. Clin Sci (Lond) 126: 67-74.
- Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, et al. (2013) Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 98: 4565-4592.
- Lorenz LB, Wild RA (2007) Polycystic ovarian syndrome: an evidence-based approach to evaluation and management of diabetes and cardiovascular risks for today's clinician. Clin Obstet Gynecol 50: 226-243.
- Valle Gottlieb MG, da Cruz IBM, Duarte MMF, Moresco RN, Wiehe M, et al. (2010) Associations among metabolic syndrome, ischemia, inflammatory, oxidatives, and lipids biomarkers. J Clin Endocrinol Metab. 95: 586-91.
- Mannerås-Holm LBaghaei F, Holm G, Janson PO, Ohlsson C, et al. (2011) Coagulation and fibrinolytic disturbances in women with polycystic ovary syndrome. J Clin Endocrinol Metab 96: 1068-1076.
- Sprung VS, Atkinson G, Cuthbertson DJ, Pugh CJ, Aziz N, et al. (2013) Endothelial function measured using flow-mediated dilation in polycystic ovary syndrome: a meta-analysis of the observational studies. Clin Endocrinol (Oxf) 78: 438-446.
- Ketel IJ, Stehouwer CD, Henry RM, Serné EH, Hompes P, et al. (2010) Greater arterial stiffness in polycystic ovary syndrome (PCOS) is an obesity--but not a PCOS-associated phenomenon. J Clin Endocrinol Metab 95: 4566-4575.
- Agarwal N, Rice SPL, Bolusani H, Luzio SD, Dunseath G, et al (2010) Metformin reduces arterial stiffness and improves endothelial function in young women with polycystic ovary syndrome: a randomized, placebo-controlled, crossover trial. J Clin Endocrinol Metab. 95: 722-30.
- Madsbad S, Astrup AV (2004) [Obesity, the metabolic syndrome and cardiovascular disease]. Ugeskr Laeger 166: 1561-1564.
- Dokras A (2008) Cardiovascular disease risk factors in polycystic ovary syndrome. Semin Reprod Med 26: 39-44.
- Macut D, Antić IB, Bjekić-Macut J (2014) Cardiovascular risk factors and events in women with androgen excess. J Endocrinol Invest.
- Berntorp E, Salvagno GL (2008) Standardization and clinical utility of thrombin-generation assays. Semin Thromb Hemost 34: 670-682.
- Aziz M, Sidelmann J, Wissing ML, Faber J, Skouby S (2015). Endogenous thrombin potential in polycystic ovary syndrome: The association to body mass index, insulin resistance and inflammation. Gynecol Endocrinol. Accepted.
- Asferg CL, Nielsen SJ, Andersen UB, Linneberg A, Møller DV, et al. (2014). Metabolic rather than body composition measurements are associated with lower serum natriuretic peptide concentrations in normal weight and obese men. Am J Hypertens. 27: 620-7.
- Lauria PB, Del Puerto HL, Reis AM, Candido AL, Reis FM (2013) Low plasma atrial natriuretic peptide: a new piece in the puzzle of polycystic ovary syndrome. J Clin Endocrinol Metab 98: 4882-4889.
- Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL (2012) Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 344: d7771.
- Elkind-Hirsch K, Marrioneaux O, Bhushan M, Vernor D, Bhushan R (2008) Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab 93: 2670-2678.
- Bendsen NT, Chabanova E, Thomsen HS, Larsen TM, Newman JW, et al. (2011) Effect of trans fatty acid intake on abdominal and liver fat deposition and blood lipids: a randomized trial in overweight postmenopausal women. Nutr Diabetes 1: e4.
- Cuthbertson DJ, Irwin A, Gardner CJ, Daousi C, Purewal T, et al. (2012) Improved glycaemia correlates with liver fat reduction in obese, type 2 diabetes, patients given glucagon-like peptide-1 (GLP-1) receptor agonists. PLoS One 7: e50117.
- Parker RI, Hagan-Burke S (2007) Useful effect size interpretations for single case research. Behav Ther 38: 95-105.
- Kahal H, Abouda G, Rigby AS, Coady AM, Kilpatrick ES, et al (2014) Glucagon-like peptide-1 analogue, liraglutide, improves liver fibrosis markers in obese women with polycystic ovary syndrome and nonalcoholic fatty liver disease. Clin Endocrinol (Oxf). 81: 523-8.
- Jensterle Sever M, Kocjan T, Pfeifer M, Kravos NA, Janez A (2014) Short-term combined treatment with liraglutide and metformin leads to significant weight loss in obese women with polycystic ovary syndrome and previous poor response to metformin. Eur J Endocrinol 170: 451-459.
- Jensterle M, Kocjan T, Kravos NA, Pfeifer M, Janez A (2014) Short-term intervention with liraglutide improved eating behavior in obese women with polycystic ovary syndrome. Endocr Res. 20: 1-6.
- Rasmussen CBLindenberg S (2014) The effect of liraglutide on weight loss in women with polycystic ovary syndrome: an observational study. Front Endocrinol (Lausanne) 5: 140.
- Borissoff JI, Joosen IA, Versteylen MO, Spronk HM, ten Cate H, et al. (2012) Accelerated in vivo thrombin formation independently predicts the presence and severity of CT angiographic coronary atherosclerosis. JACC Cardiovasc Imaging 5: 1201-1210.
- Smid M, Dielis AW, Winkens M, Spronk HM, van Oerle R, et al. (2011) Thrombin generation in patients with a first acute myocardial infarction. J Thromb Haemost 9: 450-456.
- De Mendonça-Louzeiro MRMF, Annichino-Bizzacchi JM, Magna LA, Quaino SKP, Benetti-Pinto CL (2013) Faster thrombin generation in women with polycystic ovary syndrome compared with healthy controls matched for age and body mass index. Fertil Steril. 99: 1786-90.
- Fritsch P, Kleber M, Schlagenhauf A, Laschnik B, Fritsch M, et al. (2011) Normalization of haemostatic alterations in overweight children with weight loss due to lifestyle intervention. Atherosclerosis 216: 170-173.
- van Hylckama Vlieg A, Christiansen SC, Luddington R, Cannegieter SC, Rosendaal FR, et al. (2007) Elevated endogenous thrombin potential is associated with an increased risk of a first deep venous thrombosis but not with the risk of recurrence. Br J Haematol 138: 769-774.
- Besser M, Baglin C, Luddington R, van Hylckama Vlieg A, Baglin T (2008) High rate of unprovoked recurrent venous thrombosis is associated with high thrombin-generating potential in a prospective cohort study. J Thromb Haemost JTH. 6: 1720-5.
- Sørensen HT, Horvath-Puho E, Pedersen L, Baron JA, Prandoni P (2007) Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: a 20-year cohort study. Lancet. 370: 1773-9.
- Vila G, Riedl M, Maier C, Struck J, Morgenthaler NG, et al. (2009) Plasma MR-proADM correlates to BMI and decreases in relation to leptin after gastric bypass surgery. Obesity (Silver Spring) 17: 1184-1188.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 14431
- [From(publication date):
April-2015 - Apr 01, 2025] - Breakdown by view type
- HTML page views : 9866
- PDF downloads : 4565
