Research Article Open Access
The Intestinal Barrier in Air Pollution-Associated Neural Invol vement in Mexico City Residents: Mind the Gut, the Evolution of a Chan ging Paradigm Relevant to Parkinson Disease Risk
Lilian Calderón-Garcidueñas1,2*, Angélica Gónzalez-Maciel3, Aristo Vojdani4, Maricela Franco-Lira2, Rafael Reynoso-Robles3, Hortencia Montesinos-Correa3, Beatriz Pérez-Guillé3, Partha Sarathi Mukherjee5, Ricardo Torres-Jardón6, Ana Calderón-Garcidueñas7 and George Perry8
1The Center forStructural and FunctionalNeurosciences, The University of Montana, Missoula, USA
2Hospital Central Militar, Secretaria de la Defensa Nacional, Mexico City, Mexico 11649
3Instituto Nacional de Pediatría, Mexico City, Mexico
4Immunosciences Laboratory, Los Angeles, CA,USA
5Mathematics Department, Boise StateUniversity, Boise, Idaho, USA
6Centro de Ciencias de la Atmósfera, Universidad Nacional Autónoma de México, Mexico
7Instituto de Medicina Forense, Universidad Veracruzana, Boca del Río, México
8College of Sciences, University of Texas at San Antonio, USA
- Corresponding Author:
- Lilian Calderón-Garcidueñas
Center for Structural and Functional Neurosciences
The University of Montana, 287 Skaggs Building, 32
Campus Drive, Missoula, MT, USA
Tel: 406 243 4785
E-mail: lilian.calderongarciduenas@ umontana.edu
Received date: December 14, 2014; Accepted date: February 27, 2015; Published date: March 07, 2015
Citation: Calderón-Garcidueñas L, Gónzalez-Maciel A, Vojdani A, Franco-Lira M, Reynoso-Robles R, et al. (2015) The Intestinal Barrier in Air Pollution-Associated Neural Involvement in Mexico City Residents: Mind the Gut, the Evolution of a Changing Paradigm Relevant to Parkinson Disease Risk. J Alzheimers Dis Parkinsonism 5:179. doi:10.4172/2161-0460.1000179
Copyright: © 2015 Calderón-Garcidueñas L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Objective: Braak et al proposal in 2003 “a putative environmental pathogen capable of passing the gastric epithelial lining might induce α-synuclein misfolding and aggregation” could indeed be particulate matter gaining access through the most vulnerable section of the GI tract: the small bowel. This study is focused on the electron microscopy examination of tight junctions in duodenum of healthy dogs residing in one of the most polluted megacities in our continent, Mexico City Metropolitan Area (MCMA)with high concentrations of fine particulate matter (PM 2.5) and nanosize PM versus low-air pollution controls and to measure serum antibodies to tight junctions (TJ) and neural proteins in MCMA versus low air pollution exposed children.The small intestine would be a prime PM target: it has a single unattached mucus layer, particles have easy access to epithelial cells and Peyer’s patches, altering epithelial integrity and accessing the enteric nervous system.Autopsies in MCMA children v controls show extensive brainstem oxidative stress, microglial activation, and accumulation of α-synuclein, from the dorsal motor nucleus of the vagus to the substantianigrae.Air pollution targets the dorsal vagal complex in mice exposed to the polluted MCMA atmosphere. Methods: A pilot observational case-control dogs and children study of high versus low PM 2.5 exposures.We counted and evaluated the integrity of TJ’s in duodenal electron micrographs from 6 MCMA dogs (5.01 ± 1.36 years) and 4 control dogs (5.87 ± 1.50 years) and we measured by ELISA serum antibodies to tight junctions (TJ) and neural proteins in 95 MCMA versus controls (11.02 ± 3.6 years). Results: Disruption of epithelial integrity with TJ structural changes in MCMA v control dogs (p<0.0001), the major determinant of paracellular permeability characterized the MCMA dogs’ small bowel architecture. MCMA children had higher occludin-zonulin, actin, transglutaminase 3 and 6, and glutamic acid decarboxylase autoantibodies (p<0.01).Conclusion: The integrity of the gastrointestinal (GI) barrier is significantly compromised in MCMA dogs and could be altered in MCMA children as evidenced by the autoimmune response to TJ and neural proteins. The GI breakdown likely impacts neuronal enteric populations and PM could reach the vagus and the brainstem. In the setting of urban air pollution, the evolution of a changing paradigm favoring a pathogen penetrating an epithelial lining and via transsynaptic transmission reaching preganglionic parasympathetic motor neurons of the vagus nerve has to entertain particles as a potential culprit.Defining the linkage and the health consequences of the brain/ gut/ immune system interactions in urban children showing already the early hallmarks of Parkinson’s disease ought to be of pressing importance for public health, may provide a fresh insight into Parkinson disease pathogenesis and open opportunities for pediatric neuroprotection.
Keywords
Autoimmunity; Children; Nanoparticles; Particulate matter; Swallow PM; GI barrier; Parkinson; Tight junction and neural autoantibodies
Introduction
Mexico City Metropolitan Area (MCMA) children with no known risk factors for neurological or cognitive disorders exhibit cognition deficits, brain structural and volumetric changes and the neuropathological hallmarks of Alzheimer and Parkinson’s diseases i.e., tau hyperphosphorylation with pre-tangles, amyloid beta42 (Aβ42) plaques, and misfolded α-synuclein olfactory bulb and brainstem accumulation [1-8]. Lewy neurites and/or punctuate �?-synuclein deposits in the olfactory bulb, trigeminal thalamic tract, mesencephalic V, reticular and raphe nuclei, the glossopharyngeal-vagus complexes and lung and heart autonomic ganglia are seen in MCMA v control children as young as 11 years old [7]. We have also shown significant upregulation of COX2 in the right vagus and of CD14 in both right and left vagus of teens and young adults residing in MCMA v controls, suggesting the vagus nerve plays a role in the brainstem inflammation and neurodegeneration process [7,8]. Balb-c mice directly exposed to the polluted MCMA environment for 16 months v clear air controls developed significant inflammation involving the dorsal vagal complex (DVC), similar to mice given intraperitoneal endotoxins [9]. Moreover, MCMA-exposed mice displayed significant DVC imbalance in genes for antioxidant defenses, apoptosis, and neurodegeneration [9].
Extensive data in the literature support human and animal breakdown of the nasal/olfactory, blood-brain-barrier (BBB) and alveolar-capillary barriers and the expression of detrimental genes associated to urban air pollution [10-13]. Significant membrane structural changes to tight junction protein complexes and cilia and increased permeability of the lung/blood barrier are described in association with tobacco smog containing a combination of particulate matter (PM) and gases [14-15]. Moreover, recent research links inflammatory bowel diseases, changes in gut microbiome, and abdominal pain with air pollution [16-20]. The work by Kish and colleagues is of particular interest to us given the increased gut permeability in mice exposed to particulate matter with a diameter of <10μm (PM10)[18]. Braak et al. proposal [21] “a putative environmental pathogen capable of passing the gastric epithelial lining might induce α-synuclein misfolding and aggregation” and the dual-hit hypothesis of Hawkes et al. [22] obligate us to think about the possibility swallowed particulate matter is gaining access to the brain through the most vulnerable section of the GI tract: the small bowel [23].
Very little is known regarding the ultrastructural features of the tight junction (TJs) in small bowel epithelium of young healthy animals with a lifetime exposure to air pollution. Likewise, the presence of immunological cascades in pediatric megacity residents with negative autoimmune histories, including celiac disease has not been explored. Given that MCMA children have lifetime exposures to high concentrations of PM and well documented breakdown of epithelial and endothelial barriers [2,7,10] and having the experience of studying healthy dogs exposed to the same environment as the children [1,24], we hypothesized that healthy MCMA animal facility dogs will have a breakdown of their small bowel cell junction integrity and children living in the same area will have significant higher levels of antibodies to host proteins involved in cell adhesion [25].
There were two primary aims of this study: 1. To explore by electron microscopy the integrity of the small bowel epithelium in healthy young dogs residents in MCMA and a control,low air pollution city, 2. To measure the serum concentrations of autoantibodies against barrier proteins from cohorts of matched age, gender, and socioeconomic status (SES) Mexican children with exposures to MCMA pollution versus low air pollution controls. Concomitantly, given the association between PM, nanoparticle toxicity and the gastrointestinal route [18,20],we also explored transglutaminase 3 and 6, glutamic acid decarboxylase, and cerebellar antibodies.
Our results identify statistical significant abnormalities in the apical junctional complexes, desmosomal and gap junctions resulting in light and electron microscopy interepithelial gaps in the small bowel of MCMA dogs along with increased endocytic activity in the lamina propia endothelial cells loaded with nanosized PM. MCMA children versus controls showed significantly higher serum autoantibodies against occludin/zonulin associated with the integrity of tight junctions (TJs) and actin antibodies, a reliable marker of intestinal damage severity in celiac disease and an indirect marker of histological and biochemical activity of autoimmune hepatitis [26-28]. MCMA children had higher titers of transglutaminase 3 and 6, and glutamic acid decarboxylase (GAD65)antibodies v controls. The presence of autoantibodies against key nervous system components carries a large impact for a brain in development, raising questions about their role in neuroinflammatory and neurodegenerative disease mechanisms [29]. Our study suggests that the integrity of the GI barrier could be compromised in highly exposed urbanites and short and long-term neural and extraneural health consequences including higher risk for Parkinson’s disease have to be contemplated. Our ultimate intention is make the reader aware that the small bowel, a highly vulnerable section of the GI tract [23] could be key to explain our PD-like pathology and neuroinflammatory findings in highly exposed MCMA children, dogs and mice [4,6-9].
Materials and Methods
Study cities and air quality
Children’s cohorts were selected from the Mexico City Metropolitan Area (MCMA) and small locations and cities in Mexico (Polotitlán, State of México; Zacatlán and Huachinango, Puebla; Zitácuaro, Michoacán; Puerto Escondido, Oaxaca; Chalma, Veracruz; Tlaxcala, Tlaxcala). The control cities have <75,000 inhabitants and because of their small size it is expected that their levels for the main criteria air pollutants (ozone, particulate matter, sulfur dioxide, nitrogen oxides and carbon monoxide) could be lower than the current US EPA standards. Air quality monitoring in these locations is not common because they do not meet the minimum criteria of population and emissions for setting monitoring stations according the respective Mexican standard [30]. Our largest source of control children (40/47) was Polotitlán, in the Mexico State, 121 km north-northwest of Mexico City and at 7500 ft above sea level. Polotitlán has 13,000 inhabitants, mostly dedicated to agricultural and bovine milk production. There is a very restricted industrial production, including one concrete plant and one candle small factory. Polotitlán is included within the lower air pollutant emitters in the State of Mexico [31]. We have done extensive clinical studies in Polotitlán’s heathy pediatric populations [1-5,10,29,32]. Because of the lack of historical continuous air pollution data in this location, we have followed the trend of its air quality based on the review of reports or unpublished data of both, emission inventories and measurements in the nearby region.
Mexico City Metropolitan Area is an example of extreme urban growth and accompanying environmental pollution [33-35]. The metropolitan area of over 2,000 km2 lies in an elevated basin 2,200 m above sea level surrounded on three sides by mountain ridges. MCMA nearly 24 million inhabitants, over 50,000 industries, and 5.5 million vehicles consume more than 50 million liters of petroleum fuels per day, producing an estimated annual emission of 2.6 thousand tons of particulate and gaseous air pollutants [36]. MCMA motor vehicles produce abundant amounts of primary fine particulate matter (PM2.5). The high altitude and tropical climate where the MCMA is settled facilitate ozone production all year and contribute to the formation of PM2.5. High ozone levels are typical of the warmer months (April to May) and PM higher levels are worse in the winter, when rain is scanty and thermal inversions are frequent. Children from MCMA were residents in the northern-industrialized and southern-residential zones. Northern children have been exposed to higher concentrations of volatile and toxic organic compounds, PM10, and PM2.5 including high levels of its constituents: organic and elemental carbon, nitro- and polycyclic aromatic hydrocarbons and metals (Zn, Cu, Pb, Ti, Mn, Sn, V, Ba), while southern children have been exposed continuously to significant and prolonged concentrations of ozone, secondary aerosols (NO3�?) and particulate matter associated with lipopolysaccharidePMLPS [33-36]. Studies on the composition of PM2.5 with regards to sites and samples collected in 1997 show that composition has not changed during the last decade [33].
On the other hand, historical monitoring data in Polotitlán as well as mathematical modeling of air pollutants covering the central region of Mexico indicate that air quality in this part of the country has been typically below the equivalent US EPA air quality standards [37].
Participants
This research was approved by the research ethics committee at the University of Montana and the Hospital Central Militar. Children gave active assent and their parents gave written informed consent to participation in the study. This work includes data from 95 children 49F, 46M (Mean age=11.02 y, SD=3.6). Inclusion criteria for all participating children were: negative smoking history and environmental tobacco exposure, lifelong residency in MCMA or a control city, residency within 5 miles of the city monitoring stations, full term birth, and unremarkable clinical histories. These children had a history of vaginal delivery, breast feeding for a minimum of 6 months and were introduced to solid foods after age 4 months. Mothers had unremarkable, full term pregnancies with uncomplicated vaginal deliveries and took no drugs, including alcohol. Children were examined by the attending pediatrician and considered clinically healthy. A pediatric nutritionist interviewed the child and asked the mother to keep a detail seven day food intake written record, including a weekend. Participants were from middle class families living in single-family homes with no indoor pets, used natural gas for cooking and kitchens were separated from the living and sleeping areas. All included children were actively engaged in outdoor activities and outdoor daily exposures in hours per day were recorded by the mother and child for 7 days, including the transit time to and from school, the time spent in recess and PE during school, the outdoor time while playing and engaging in other activities. Low (n: 47) and high (n:48) air pollution exposed children were matched by age, gender, socioeconomic status, and diets.
Peripheral blood samples
Blood was collected between 7 am and 9.00 am from an antecubital vein using a 21-G needle. After centrifugation at 3,000 rpm for 10 min, aliquots of 1.5 ml serum were transferred to CryoTubes and samples were frozen at −20°C and then transferred to −80°C and stored until further analysis.
Reagents and methodology
Proteins and Peptides: Actin and tTG3 were purchased from Sigma/Aldrich St. Louis, MO. Various peptides HPLC grade with purity greater than 90% were synthesized by EZ Biolab Carmel, IN, including Glutamic Acid Decarboxylase (GAD-65),Transglutaminase-6, Occludin, Zonulin and Cerebellar peptide[38].The procedure for the detection of antibodies by ELISA is described in detail in a previous paper [29].
Dog small bowel samples
Previously harvested dog small bowel tissues for electron microscopy were used for this study. MCMA and control mixed beagles were whelped and housed in an outdoor-indoor kennel; husbandry was in compliance with the American Association of Laboratory Animal Certification Standards. Dogs were under daily veterinarian observation during their entire life, and at no time there was any evidence of respiratory, cardiovascular, gastrointestinal or neurological diseases. Dogs had all applicable vaccines and were treated with antihelmintics regularly. Dogs from both cohorts had the same diets. We selected to use small bowel optimally fixed electron microscopy tissues from 6 dogs (5.01 ± 1.36 years) from an independent longitudinal study involving the use of Nimesulide® in mixed beagle dogs. The 6 selected dogs for this study were in the non-treated Mexico City dog group exposed 24/7 to the Southwest MCMA atmosphere from birth. Four dogs average age 5.87 ± 1.50 years from a low pollution control city were also studied. Procedures used were in accordance with the guidelines of the Use and Care of Laboratory Animals (NIH Pub No.86-23).
Light microscopy
Sections 1 μm thick were cut and stained with toluidine blue. Boardcertified pathologists(LCG, ACG) without access to the identification codes reviewed the sections.
Examination of small bowel samples by Transmission Electron Microscopy (TEM)
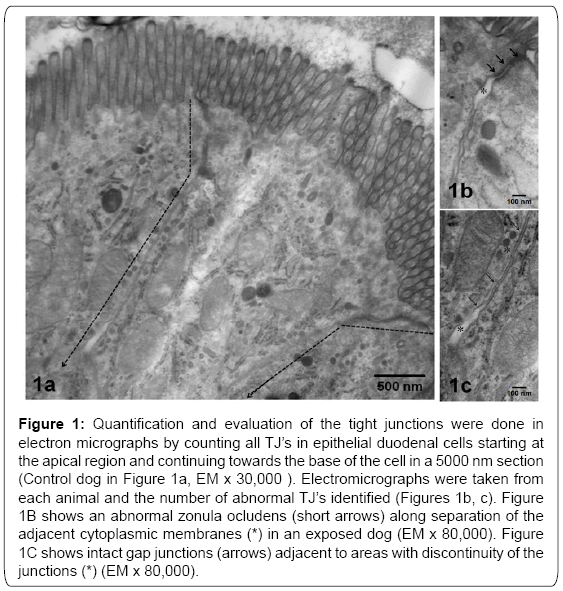
Tissues were post-fixed in 1% osmium tetraoxide and embedded in Epon. Semi-thin sections (0.5 to 1μm) were cut and stained with toluidine blue for light microscopic examination. Ultra-thin sections (60-90 nm) were cut and collected on slot grids previously covered with formvar membrane. Sections were stained with uranyl acetate and lead citrate, and examined with a JEM-1011 (Japan) microscope. Each electron micrograph was evaluated separately, and then compared by group. We captured ultrastructural epithelial images including sites of TJ’s complexes.We evaluated 100 TJ’s in each cohort. Electromicrographs were taken from TJ’ complexes in epithelial cells starting at the apical region and continuing towards the base of the cell in a 5000 nm section (Figure 1). Electromicrographs were evaluated blindly and the number of abnormal TJ’s counted (Figure 1).
Figure 1: Quantification and evaluation of the tight junctions were done in electron micrographs by counting all TJ’s in epithelial duodenal cells starting at the apical region and continuing towards the base of the cell in a 5000 nm section (Control dog in Figure 1a, EM x 30,000 ). Electromicrographs were taken from each animal and the number of abnormal TJ’s identified (Figures 1b, c). Figure 1B shows an abnormal zonula ocludens (short arrows) along separation of the adjacent cytoplasmic membranes (*) in an exposed dog (EM x 80,000). Figure 1C shows intact gap junctions (arrows) adjacent to areas with discontinuity of the junctions (*) (EM x 80,000).
Data analysis
For the antibody data, we first calculated the sample mean and sample standard deviation of each of the characteristic variables including the measurements of the antibodies in control and the Mexico City groups. Next, we calculated the p-values of the twosample t-tests to investigate whether the sample means of the variables are significantly different between the groups. We concluded that the sample means of a variable in the two groups are significantly different only if the corresponding p-value is smaller than 0.05. Next, we separately calculated the percentages of the Mexico City children that showed measurements higher than the control mean and the control median for some selected variables. We also calculated the p-values of those percentages for testing how different they are from 50%. We calculated Pearson’s correlation coefficients (PCC) among each pair of the variables in each group and in the pooled data irrespective of groups. PCC measures how well the relationship between two variables can be described by a linear function. Abnormal TJ’s identified by EM were counted for exposed and control dogs and p values calculated.We carried out the above mentioned statistical analyses in the statistical software ‘R’ (http://www.r-project.org/).
Results
Air quality data
Mexico City residents are exposed year-round to PM2.5 concentrations above United States National Air Ambient Quality Standards (NAAQS). The PM2.5 annual air quality standard of 12 μg/m3 has been historically exceeded across the metropolitan area (Table 1). For this work we focused on particulate matter (PM), broadly defined by the diameter of the aerodynamic particles, and classified into coarse particles (<10 to >2.5 μm; PM10), fine particles (<2.5 μm, PM2.5) and ultrafine PM (UFPM<100 nm). Fine and ultrafine PM are of particular interest given their capability to reach the brain [39]. MCMA children in this study have been exposed to significant concentrations of PM 2.5 during their entire life, including the prenatal period. The high concentrations of PM 2.5 coincide with the time children play outdoors and/or stay in schools with broken windows and doors. All other criteria pollutants for MCMA, including nitrogen dioxide, sulfur dioxide and lead were at or below the current EPA standards (data not shown). Control children have been lifelong residents in low pollution cities with all criteria air pollutants below the US EPA NAAQS standards.
| Year | Pedregal | Xalostoc | ||
| Mean | SD | Mean | SD | |
| 1997 | 21.6 | 16.6 | 71.3 | 34.1 |
| 1998 | 29.3 | 16.8 | 64.9 | 25.4 |
| 1999 | 24.4 | 9.2 | 71 | 26.6 |
| 2000 | 24.7 | 11.3 | 54.8 | 25.3 |
| 2001 | 23.6 | 10.1 | 41.1 | 17.2 |
| 2002 | 23.1 | 9.7 | 38 | 13.7 |
| 2003 | 23.4 | 11.3 | 41.8 | 14.4 |
| 2004 | 18.4 | 9.4 | 35.5 | 14.7 |
| 2005 | 20.9 | 11.5 | 30.4 | 17.1 |
| 2006 | 17.8 | 8.4 | 29.8 | 15.6 |
| 2007 | 16.2 | 8.5 | 25.3 | 11.3 |
| 2008 | 18 | 8.3 | 26.3 | 10 |
| 2009 | 18.4 | 8.7 | 26.4 | 10.7 |
| 2010 | 14.4 | 7.4 | 24.9 | 13.2 |
| 2011 | 16.7 | 8.3 | 24.7 | 11.5 |
| 2012 | 17 | 7.5 | 25.9 | 11.7 |
Table 1: PM2.5 annual concentrations in μg/m3 for North and South Metropolitan Mexico City area selected monitoring stations.
Electron Microscopic results
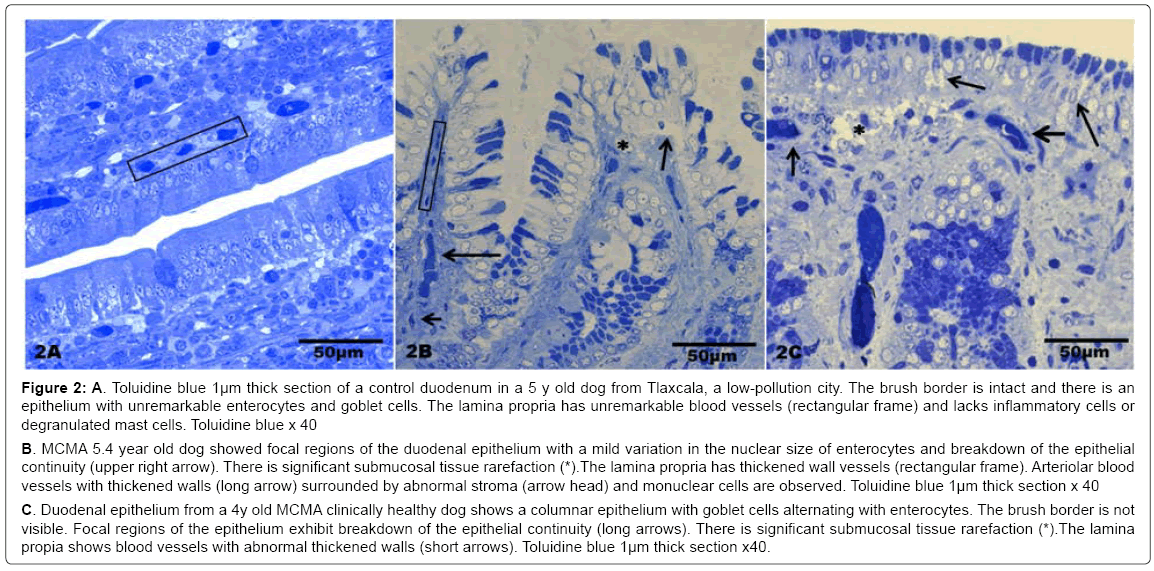
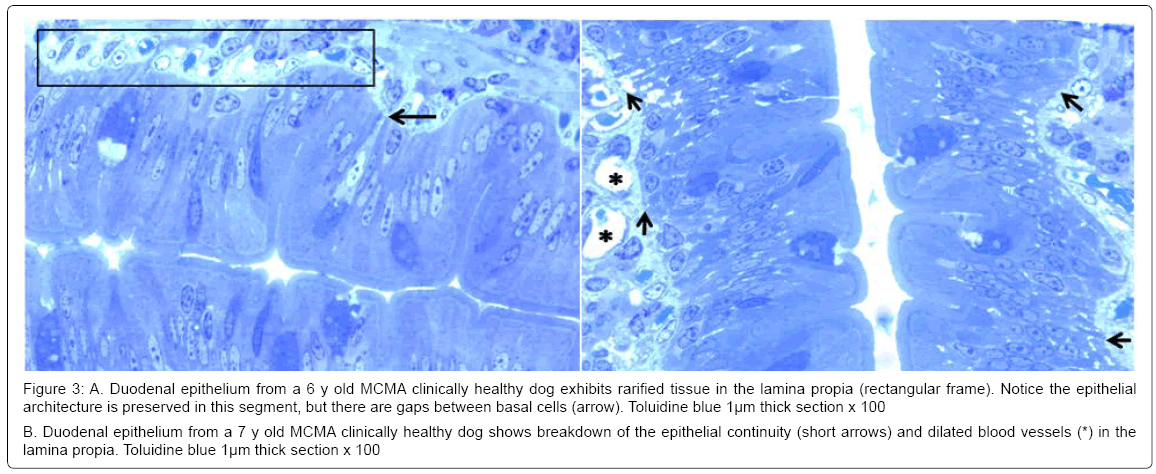
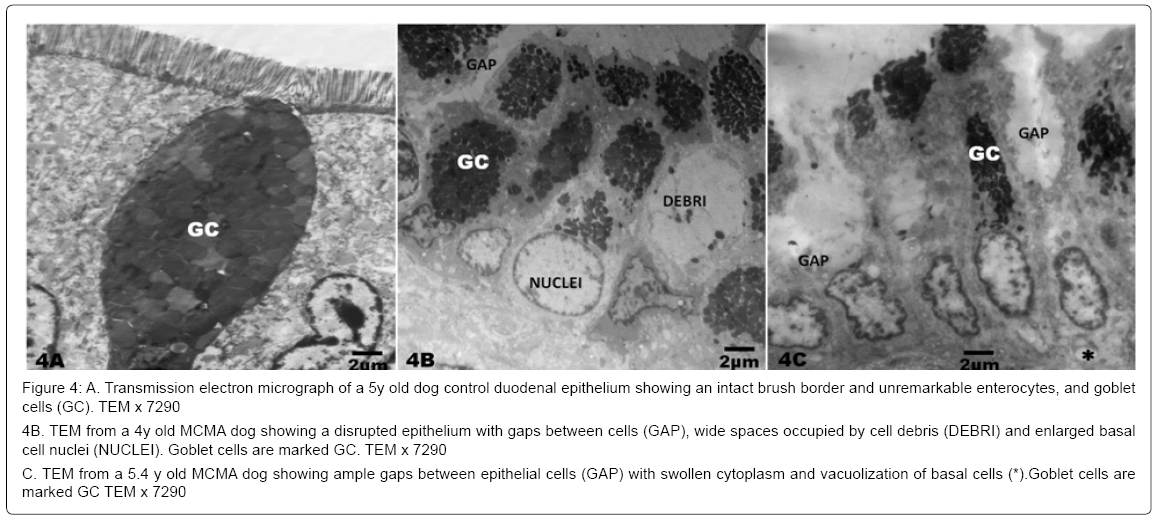
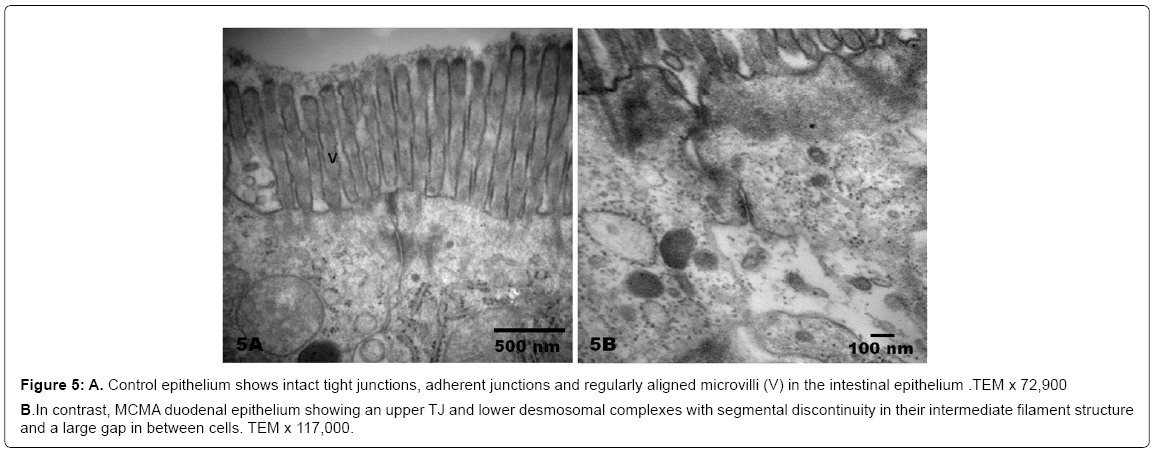
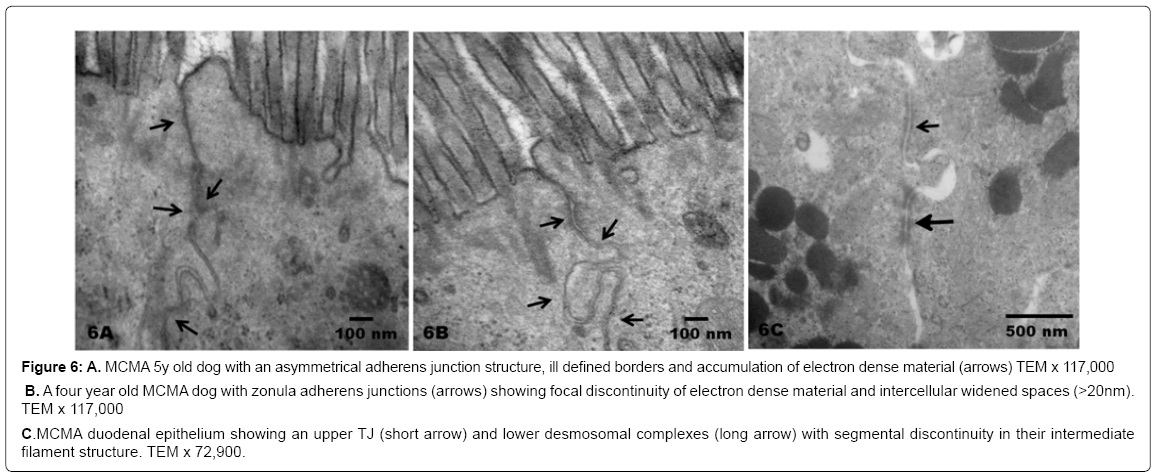
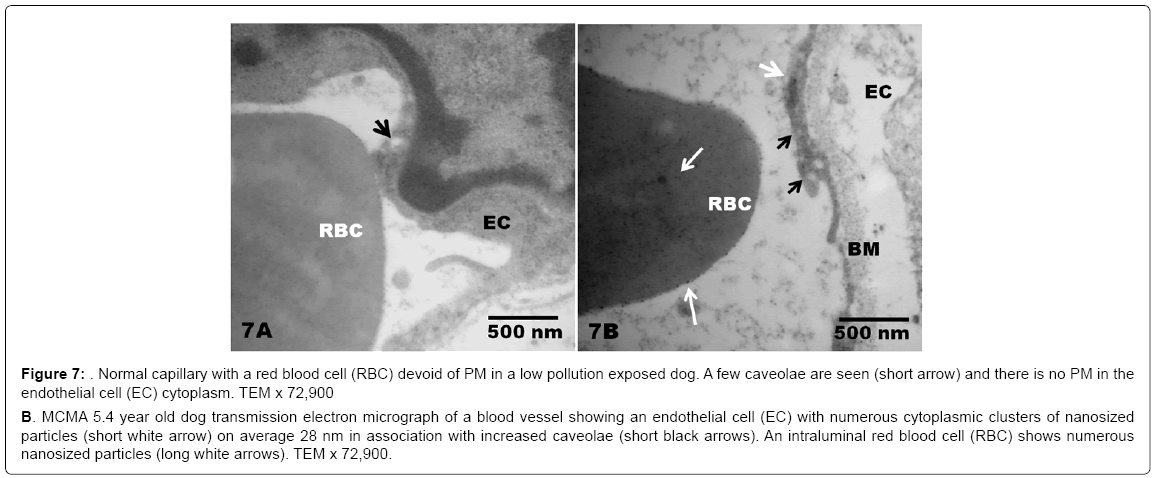
There was no statistical difference in the selected control v MCMA dogs’ ages (p=0.35). Quantification of abnormal TJ’s identified by EM yielded 14.2 ± 4.36 v 4.2 ± 1.3 in MCMA v controls (p<0.0001). Toluidine blue 1μm sections of normal duodenal epithelium with an intact brush zone and unremarkable enterocytes and goblet cells are characteristic of the control dogs (Figure 2A). In contrast, duodenum sections from Mexico City dogs exhibit segments of intestinal columnar epithelium with goblet cells and intercalated enterocytes with no visible brush border (Figure 2B). A few blood vessels in the lamina propria reveal thickened walls and focal disruption of the lamina propia (Figure 2B). Exposed dogs also display fibrosis at the level of the lamina propia (Figure 2C). Focal regions of the duodenal epithelium with a breakdown of the epithelial continuity, and submucosal tissue rarefaction with scattered mononuclear cells are observed in Figure 3A-3B. Low power transmission electron micrographs (TEM) reveal an intact epithelium and unremarkable brush border in a 5y old control dog (Figure 4A), while exposed dogs display a disrupted, fragmented epithelium with a few short villi in enterocytes, wide gaps separating epithelial cells, and intercellular spaces occupied by cellular debris (Figure 4B-4C). Control epithelium exhibits intact tight junctions and adherens junctions and regularly aligned microvilli (Figure 5A), while a wide range of abnormalities in TJ’s can be seen in exposed duodenum (Figure 5B). Desmosomal complexes exhibited abnormalities in their intermediate filaments (Figure 6A). TJ’s were significantly opened with electron dense material occupying the junctional complexes (Figure 6B-6C). In Figure 7A, a control capillary with minimal caveolar activity and a red blood cell devoid of PM in a low pollution dog are seen, while in Figure 7B exposed endothelial cells exhibit numerous cytoplasmic clusters of nanosized particles in association with increased caveolae. An intraluminal red blood cell (RBC) displays numerous nanosized particles.
Figure 2: A. Toluidine blue 1μm thick section of a control duodenum in a 5 y old dog from Tlaxcala, a low-pollution city. The brush border is intact and there is an epithelium with unremarkable enterocytes and goblet cells. The lamina propria has unremarkable blood vessels (rectangular frame) and lacks inflammatory cells or degranulated mast cells. Toluidine blue x 40
C. Duodenal epithelium from a 4y old MCMA clinically healthy dog shows a columnar epithelium with goblet cells alternating with enterocytes. The brush border is not visible. Focal regions of the epithelium exhibit breakdown of the epithelial continuity (long arrows). There is significant submucosal tissue rarefaction (*).The lamina propia shows blood vessels with abnormal thickened walls (short arrows). Toluidine blue 1μm thick section x40. MCMA 5.4 year old dog showed focal regions of the duodenal epithelium with a mild variation in the nuclear size of enterocytes and breakdown of the epithelial continuity (upper right arrow). There is significant submucosal tissue rarefaction (*).The lamina propria has thickened wall vessels (rectangular frame). Arteriolar blood vessels with thickened walls (long arrow) surrounded by abnormal stroma (arrow head) and monuclear cells are observed. Toluidine blue 1μm thick section x 40
C. Duodenal epithelium from a 4y old MCMA clinically healthy dog shows a columnar epithelium with goblet cells alternating with enterocytes. The brush border is not visible. Focal regions of the epithelium exhibit breakdown of the epithelial continuity (long arrows). There is significant submucosal tissue rarefaction (*).The lamina propia shows blood vessels with abnormal thickened walls (short arrows). Toluidine blue 1μm thick section x40.
Figure 3: A. Duodenal epithelium from a 6 y old MCMA clinically healthy dog exhibits rarified tissue in the lamina propia (rectangular frame). Notice the epithelial architecture is preserved in this segment, but there are gaps between basal cells (arrow). Toluidine blue 1μm thick section x 100
B. Duodenal epithelium from a 7 y old MCMA clinically healthy dog shows breakdown of the epithelial continuity (short arrows) and dilated blood vessels (*) in the lamina propia. Toluidine blue 1μm thick section x 100
Figure 4: A. Transmission electron micrograph of a 5y old dog control duodenal epithelium showing an intact brush border and unremarkable enterocytes, and goblet cells (GC). TEM x 7290
4B. TEM from a 4y old MCMA dog showing a disrupted epithelium with gaps between cells (GAP), wide spaces occupied by cell debris (DEBRI) and enlarged basal cell nuclei (NUCLEI). Goblet cells are marked GC. TEM x 7290
C. TEM from a 5.4 y old MCMA dog showing ample gaps between epithelial cells (GAP) with swollen cytoplasm and vacuolization of basal cells (*).Goblet cells are marked GC TEM x 7290
Figure 5: A. Control epithelium shows intact tight junctions, adherent junctions and regularly aligned microvilli (V) in the intestinal epithelium .TEM x 72,900
B. In contrast, MCMA duodenal epithelium showing an upper TJ and lower desmosomal complexes with segmental discontinuity in their intermediate filament structure and a large gap in between cells. TEM x 117,000.
Figure 6: A. MCMA 5y old dog with an asymmetrical adherens junction structure, ill defined borders and accumulation of electron dense material (arrows) TEM x 117,000
B. A four year old MCMA dog with zonula adherens junctions (arrows) showing focal discontinuity of electron dense material and intercellular widened spaces (>20nm). TEM x 117,000
C. MCMA duodenal epithelium showing an upper TJ (short arrow) and lower desmosomal complexes (long arrow) with segmental discontinuity in their intermediate filament structure. TEM x 72,900.
Figure 7: Normal capillary with a red blood cell (RBC) devoid of PM in a low pollution exposed dog. A few caveolae are seen (short arrow) and there is no PM in the endothelial cell (EC) cytoplasm. TEM x 72,900
B. MCMA 5.4 year old dog transmission electron micrograph of a blood vessel showing an endothelial cell (EC) with numerous cytoplasmic clusters of nanosized particles (short white arrow) on average 28 nm in association with increased caveolae (short black arrows). An intraluminal red blood cell (RBC) shows numerous nanosized particles (long white arrows). TEM x 72,900.
Children’s outdoor time, diet and serum results
The number of hours spent outdoors was significantly different between groups, control children spent an average of 5.19 ± 0.91 h v 3.69 ± 0.79 h in the MCMA cohort (p<0.0001).The nutritional intake based on a seven day recall showed no differences in control v Mexico City children. When the intake of corn, wheat and rice products was analyzed, the control children had a significantly higher intake of corn (p=0.009) v MCMA, while wheat and rice showed no significant differences. The results of the 5 selected antigens from 47 controls and 48 Mexico City children, age 11.02 ± 3.6 years are shown in Table 2. The two selected cell junction antibodies: occludin-zonulin and actin showed significant statistical differences among cohorts for the high affinity IgG isotypes, while IgM was significantly increased for actin in Mexico City children. Transglutaminases 3 and 6, GAD 65 and cerebellar antibodies exhibited high IgG but not IgA isotypes for MCMA children and were significantly higher in exposed children. Table 3 shows the Pearson’s correlation coefficients and p-values between the cell junction, and neural antibodies among Mexico City children. There was a striking correlation between cell junction and neural autoantibodies, particularly for actin and occludin-zonulin IgG isotypes. Age showed no significant correlations with any of the variables.
| Control | Mexico City | Comparison | |||||
| Mean | SD± | Mean | SD± | p value | % above the | % above the control median | |
| control mean | |||||||
| OZ IgA | 0.457 | 0.09 | 0.602 | 0.31 | 0.003 | 58.3 | 75 |
| OZ IgG | 0.482 | 0.15 | 0.581 | 0.16 | 0.003 | 62.5 | 68.8 |
| OZ IgM | 0.651 | 0.31 | 0.855 | 0.63 | 0.05 | 41.7 | 56.3 |
| Actin IgA | 0.596 | 0.19 | 0.679 | 0.26 | 0.08 | 54.2 | 62.5 |
| Actin IgG | 0.822 | 0.32 | 1.019 | 0.36 | 0.006 | 68.8 | 85.4 |
| Actin IgM | 0.724 | 0.22 | 0.873 | 0.35 | 0.016 | 68.8 | 68.8 |
| TGM3 IgA | 0.506 | 0.21 | 0.542 | 0.24 | 0.44 | 50 | 54.2 |
| TGM3 IgG | 0.659 | 0.22 | 0.825 | 0.3 | 0.002 | 68.8 | 70.8 |
| TGM6 IgA | 0.443 | 0.18 | 0.49 | 0.23 | 0.28 | 47.9 | 54.2 |
| TGM6 IgG | 0.589 | 0.17 | 0.736 | 0.37 | 0.016 | 58.3 | 66.7 |
| GAD 65 IgA | 0.406 | 0.14 | 0.452 | 0.17 | 0.15 | 50 | 66.7 |
| GAD 65 IgG | 0.578 | 0.17 | 0.671 | 0.2 | 0.019 | 66.7 | 72.9 |
| GAD 65 IgM | 0.58 | 0.18 | 0.674 | 0.28 | 0.05 | 62.5 | 62.5 |
| CEREB IgA | 0.511 | 0.17 | 0.617 | 0.34 | 0.06 | 41.7 | 54.2 |
| CEREB IgG | 0.545 | 0.23 | 0.845 | 0.44 | 0.0001 | 68.8 | 75 |
| CEREB IgM | 0.684 | 0.29 | 0.915 | 0.57 | 0.015 | 45.8 | 56.3 |
OZ=Occludin Zonulin, TGM3=Transglutaminase 3, TGM6=Transglutaminase 6, GAD-65=Glutamic Acid Decarboxylase, CEREB=Cerebellar peptide
Table 2. Serum autoantibodies in Controls v Mexico City Metropolitan Area children. Results are expressed in optical density units. Significant p-values are marked in bold. Percentages of the MCMA children that showed optical density values higher than the control mean and control median are also shown.
| Autoantibodies and isotypes | GAD65 IgA | GAD65 IgG | GAD65 IgM | TGM3 IgA | TGM3 IgG | TGM6 IgA | TGM6 IgG | CEREB IgA | CEREB IgG | CEREB IgM |
|---|---|---|---|---|---|---|---|---|---|---|
| OZ IgA | 0.22 (0.13) | 0.13 (0.39) | 0.28 (0.06) | 0.20 (0.18) | 0.28 (0.05) | 0.29 (0.04) | 0.43 (< 0.01) | 0.40 (< 0.01) | 0.26 (0.08) | 0.22 (0.13) |
| OZ IgG | 0.44 (< 0.01) | 0.35 (0.01) | 0.28 (0.05) | 0.31 (0.03) | 0.39 (< 0.01) | 0.43 (< 0.01) | 0.50 (< 0.01) | 0.43 (< 0.01) | 0.27 (0.07) | 0.16 (0.26) |
| OZ IgM | 0.01 (0.93) | -0.16 (0.29) | 0.31 (0.03) | 0.13 (0.39) | 0.15 (0.30) | 0.08 (0.61) | 0.11 (0.45) | -0.05 (0.76) | -0.08 (0.58) | 0.28 (0.06) |
| Actin IgA | 0.72 (< 0.01) | 0.38 (< 0.01) | 0.48 (< 0.01) | 0.67 (< 0.01) | 0.57 (< 0.01) | 0.76 (< 0.01) | 0.64 (< 0.01) | 0.52 (< 0.01) | 0.01 (0.95) | 0.31 (0.03) |
| Actin IgG | 0.43 (< 0.01) | 0.54 (< 0.01) | 0.34 (0.02) | 0.38 (< 0.01) | 0.38 (< 0.01) | 0.42 (< 0.010) | 0.43 (< 0.01) | 0.31 (0.03) | 0.38 (< 0.01) | 0.24 (0.11) |
| Actin IgM | 0.40 (< 0.01) | 0.19 (0.20) | 0.76 (< 0.01) | 0.24 (0.10) | 0.25 (0.09) | 0.35 (< 0.01) | 0.34 (0.02) | 0.13 (0.38) | -0.05 (0.74) | 0.58 (< 0.01) |
OZ=Occludin Zonulin, TGM3=Transglutaminase 3, TGM6= Transglutaminase 6, GAD-65=Glutamic Acid Decarboxylase, CEREB=Cerebellar peptide.
Table 3. Pearson’s correlation coefficients between the neural and cell junction autoantibodies within the group of Mexico City children. The numbers within the parenthesis are the p-values for testing the significance of the corresponding Pearson’s correlation coefficient. The significant ones are marked in bold.
Discussion
Disruption of epithelial integrity with structural changes in tight junctions (TJ), the major determinant of paracellular permeability, characterize the small bowel pathology in Metropolitan Mexico City healthy dogs. The integrity of the small bowel epithelial barrier is likely compromised as a result of the epithelial TJ disruption. These highly exposed dogs’ intestinal findings could be relevant to the increases in actin and occludin/zonulin proteins autoantibodies seen in seemingly healthy MCMA children. We fully expect that disrupted GI barriers will allow for major concentrations of swallowed PM entering the GI tract, impacting neuronal enteric populations and reaching the vagus nerve. In this regard, PM gaining access through the most vulnerable section of the GI tract: the small bowel, will be of deep interest to the possibility addressed by Braak et al. [21] and Hawkes et al. [22] about an environmental agent passing the GI epithelium and inducing abnormal changes in α-synuclein. The presence of abnormal TJ’s and brain autoantibodies in urban children with well documented systemic inflammation, neuroinflammation and the early brainstem and olfactory bulb hallmarks of Parkinson’s disease obligates us to carefully look at the importance of the GI tract as a direct pathway to vulnerable brain regions.
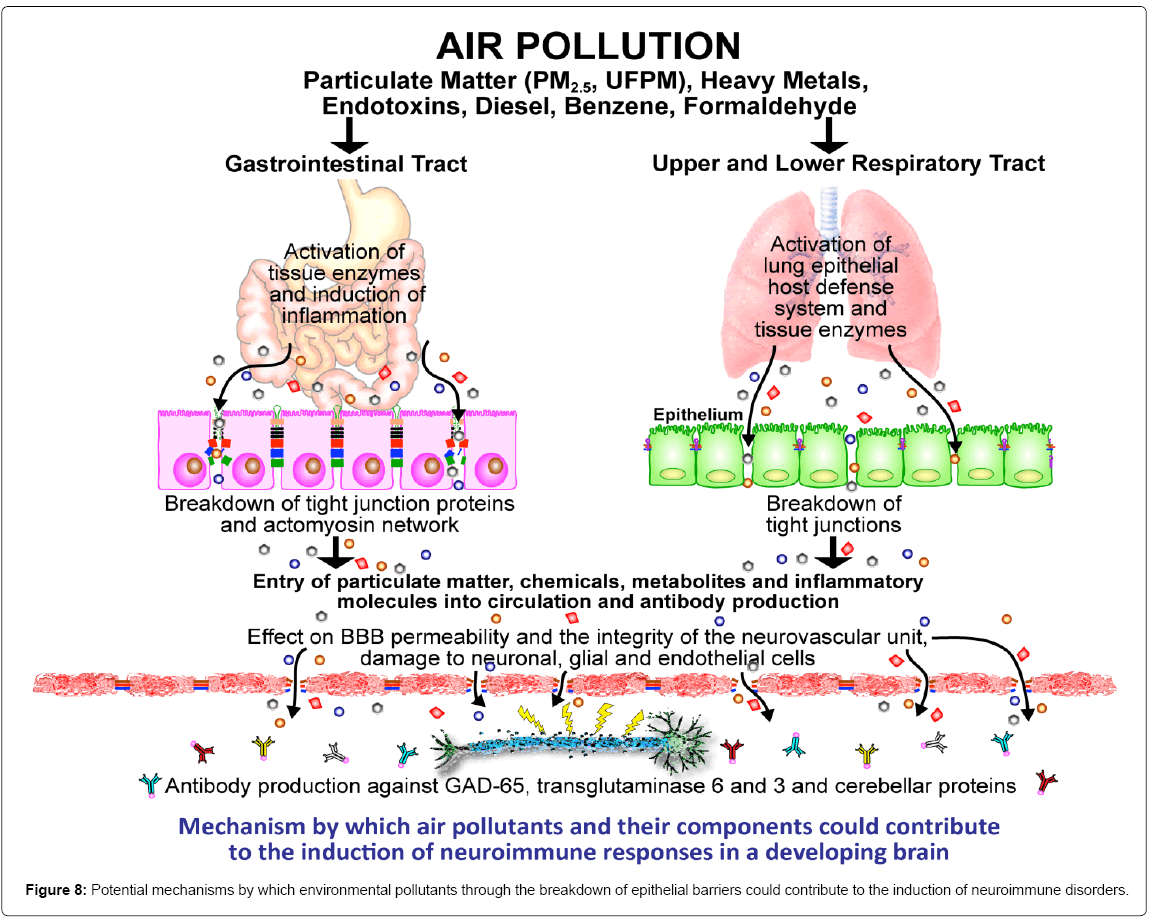
The issue of the gastrointestinal route as a direct target and a simultaneous pathway for the entrance of air pollutant components through a disturbed barrier illustrated in Figure 8 is at the core of our concerns for exposed children. An immune-reactive response against barrier forming proteins is key to understand air pollutant mechanistic pathways affecting epithelial and endothelial barriers, including the GI barrier [10,18,40-43]. Ultrafine and fine PM resulting from combustion products are rich in organic and inorganic components, including heavy metals, benzene, formaldehyde, and endotoxins, and because of their pro-oxidative potential and their inflammatory capacity they increase the risk for toxicity [41]. Nanosized particles are capable to enter cells by an endocytic pathway, can penetrate through cells and through tissue resulting in cellular inflammatory reactions and toxicity [41]. Thus, in the scenario of sustained lifelong exposures to high levels of PM <2.5 μm and <100 nm in diameter [33-35,44] and the capacity of PM to cause barrier damage, the presence of an impaired mucosal barrier, and autoantibodies against barrier forming proteins are not unexpected findings in urban children.
Although we and others, have shown the nasal/olfactory, alveolar, endothelial and the BBB are compromised upon exposure to air pollutants [6-7,10,15-18,41] the GI barrier has been a less explored target. The GI tract is very important for several reasons: i. direct ingestion of inhaled PM is common after being mobilized up the trachea via the mucociliary escalator and the particle size determines whether is cleared out by cough or swallowing [45], ii. Large increases in ventilation and GI intake of particles occur with increasing activity [46-48], a situation that is critical in children with outdoor physical activities in polluted environments.Once within the GI tract, PM enters in direct contact with luminal components, the mucus layer and the microbiome [20]. Very small particles in the nanosized range could gain direct access to the blood stream from the GI tract, while others damage the GI mucosa and alter the immune function. One critical issue to take into account is the vulnerability of the different GI anatomical compartments to PM. Indeed, in keeping with Johansson and colleagues [23] review of the GI mucus system; the small intestine would be the prime PM target: it has a single unattached mucus layer; nanoparticles can have easy access to epithelial cells and to Peyer’s patches, affecting immunosurveillance and altering epithelial integrity [49-52]. Compelling evidence shows that the small intestinal barrier function is immature in neonates, the anti-microbial peptide-dependent barrier function is weaker earlier in life [53] thus, the PM GI exposure-threat is present starting at birth for urban dwelling residents.
MCMA pre-adolescents and adolescents have a very low IgA but high levels of IgG against tTG-3, tTG-6, and GAD-65, which is an indication of autoimmune reactivity. The production of IgG isotype against various proteins (tTG-3, tTG-6, GAD-65) most likely relates to the detrimental impact of swallowed particulate matter upon the GI barrier, resulting in structural disruption and immune responses. These seemingly healthy children have not shown GI complaints and the increased burden of illness and use of health care pediatric services associated with celiac disease CD [54,55]. Our brain has very complex connections to the gut and there is bidirectional communication between GI cells and the CNS [56]. We agree with Hadjivassiliou et al. [57] that given TG6 is primarily expressed in the CNS, its presence obligates to question whether the intestine or the cerebellum primed the TG6 response in gluten ataxia. More importantly, TG6 is a protein associated with CNS development and motor control and an early brain insult and associated inflammation may predispose to future development of TG6 autoimmunity [58,59]. Thus, the critical role of TG6 in cortical and cerebellar neurons is very relevant in the context of air pollution particularly because we previously reported the presence of finger to nose dysmetria, gait deviation and positive Romberg in MCMA children of similar age as this cohort [4].
Complicating the neural autoimmune scenario, Mexico City children also have high titers of antibodies to the enzyme glutamic acid decarboxylase (GAD65) associated with the presence of gait abnormalities and the Stiff person syndrome, type 1 diabetes, and anxiety disorders [60-63]. Autoantibodies to GAD interfere in vitro with GABA production and in vivo have negative effects in the entire CNS GABAergic system resulting in unbalance of excitatory and inhibitory neurotransmission [64-66]. The potential development of non-celiac gluten sensitivity in MCMA children is an interesting clinical issue.A plausible immunogenic pathway resulting in non-celiac gluten sensitivity includes fine and ultrafine PM causing damage to the TJ’s at the most vulnerable follicle-associated epithelium protected by a single layer of epithelial cells and an easily removable mucus layer, [49] followed by an immune response and the production of TJ’s antibodies. The breakdown of the barrier could be followed by gluten sensitivity and the formation of transglutaminases 3 and 6, GAD 65 and cerebellar antibodies. The issue of non-celiac gluten sensitivity (NCGS) in the setting of severe PM air pollution has to be entertained even when these children do not have CD symptoms [67,68].
A critical issue for pediatricians and parents alike should be: what is the clinical impact of transglutaminases 3 and 6, GAD 65 and cerebellar antibodies in urban children? Neural reactive antibodies are present in approximately 2-3% of the general population and most researchers will agree they do not usually contribute to CNS or PNS pathology [69]. However, it is becoming clear that neural antibodies can penetrate brain tissue either early in development or under pathological conditions (i.e., BBB damage) [70]. The association of neural autoantibodies and pathogenicity with a leaky BBB could be very important for highly exposed children [1,7] and as Levin and coworkers suggested a “defective BBB allows access of autoantibodies to targets on the brain cells” [70]. The major factor determining the impact of the brain autoantibodies is the integrity of the BBB, which in turn is determining the extent and degree of reactivity and detrimental brain responses [69-72].
The presence of nanosized PM in small bowel endothelial cells deserves a special comment. The endothelial cells showed accumulation of single particles and conglomerates measuring 14 to 55 nm [73]. Endothelial cells have a higher expression of caveolae in comparison with epithelial cells, and the charge and lipophilic characteristics of a nanoparticle surface play a key role in their cellular uptake [74]. These are interesting observations because small molecular weight antigens such as dextran and bacterial LPS enter the lamina propria via goblet cell associated passage ways [75]. Internalization of particulate antigens i.e., bacterial cell debris and nano PM co-localized with the CD11c+ dendritic cells in the lamina propria [75]. Intestinal epithelial cells nano PM uptake depends on their size: 20-40 nm NPs are taken up readily, while NPs larger than 100 nm are taken up mainly by the epithelial cells overlying Peyer’s patches [75]. Thus, nano PM in GI endothelial cells in our study falls within the expected size range and we anticipate that the chemical composition of nano PM will have an impact on their cytotoxic effect.
Lastly, what is the impact of the GI tract as a portal of ultrafine (<100 nm) PM to the systemic circulation and the brain? Is this pathway key for the development of the early stages of Parkinson’s disease we are already documenting in children? [4,7,8].The distribution of misfolded α synuclein in MCMA children follows precisely the early stages I and II of Braak’s PD staging [76,21]. Exposed children have olfaction deficits and their olfactory bulbs show misfolded α synuclein [2]. Olfactory dysfunction precedes the onset of motor symptoms by years [77] and the intranasal administration of neurotoxicants in experimental animals supports the olfactory vector hypothesis of Parkinson’s disease [78]. We have empirically followed children with severe orthostatic hypotension and syncope that could represent the most vulnerable children to PD non-motor early effects [8]. The role of the intestinal barrier integrity, the intestinal microbiome, the experimental evidence showing that different α-synuclein forms can propagate from the gut to the brain, and the impact of molecular mimicry in the development of a disease for which there is no cure,can not be ignored [79-84].
Looking Forward and Limitations
While recognizing that the evaluation of the TJ’s was done in a small number of clinically healthy control and exposed dogs, and the serum antibodies in healthy control and exposed children, the results are nonetheless important to guide future investigations covering the gaps between the increase in TJ’s and neural antibodies and the development of Parkinson’s disease in highly exposed children. The significant damage to the TJ’s is a major factor for compromising paracellular permeability and thus allowing the entrance of bacteria, viruses, toxic chemicals and especially nanosized particulate matter [41]. A direct impact on the small bowel immunosurveillance capacity is expected [50] and the children’s autoimmune responses are critical given the formation of brain autoantibodies in the setting of a neurovascular unit that is equally compromised [69,85-86]. We fully agree with Levin and Diamond groups that in the presence of BBB compromise brain autoantibodies might contribute to initiation and/or pathogenesis of a wide spectrum of neurological diseases [69,70]. The issue is of critical importance in the developing brain because the damage may have short and long term detrimental consequences, including the development of Parkinson’s disease [7,8]. Since we strongly argue that early dysregulated neuroinflammation results in misfolded key brain proteins (i.e., α synuclein) as a protective initial response, chronic exposure to air pollutants could result in hyperphysiological induction of highly stable fibrillar aggregates and neurodegenerative progressive events [87]. We fully agreed with Brandenberger et al. [88] that although transmission electron microscopy (TEM) is an appropriate technique to use for visualizing NPs inside cells, elemental analysis is recommended to confirm the presence of NPs inside the cell.
Summary
Fine tuning of immune-to-brain communication is crucial to neural networks proper functioning, thus the evidence of GI barrier disruption associated with significant levels of cell junction and brain autoantibodies, is a subject of deep concern for urban children. There is increasing evidence linking diverse forms of air pollution to neuroinflammation and neuropathology seen in Parkinson’s disease, including natural exposures of mice to the MCMA environment [9], elevated �? synuclein in midbrain of mice exposed to high concentrations of diesel exhaust particles (DEP) [89] and nanosize DEP activating microglia through multiple mechanisms and resulting in DEP-induced microglial H2O2 production and loss of dopaminergic neuron function [90]. These animal studies and our neuropathology results in children and young adults strongly suggest that air pollution exposures may be associated with early Parkinson’s disease-like pathology.
A large body of work on the GI immunosurveillance role, effects of disrupted TJ’s in the small bowel, goblet cell responses, small bowel mucus properties, GI immunization, brain developmental programming, autoimmunity, brain-reactive antibodies and disease already exists [25,49-52,69-70,91-97], thus expanding this knowledge in the scenario of air pollution pediatric GI effects could greatly facilitate our understanding of the downstream mechanisms of the complex interaction of antigens and neural targets and to elucidate if the GI cell junction lack of integrity are the culprit of pediatric urban diseases and carry a future risk for neurodegenerative fatal diseases. Also of utmost relevance is the importance of the gut microbial ecology and the contributions of non-pathogenic commensal flora to the development of CNS-autoimmunity [56,71].
Defining the linkage and the health consequences of the brain/ gut/immune system interactions in children chronically exposed to air pollutants showing already the early hallmarks of Parkinson’s disease ought to be of pressing importance for public health, may provide a fresh insight into Parkinson disease pathogenesis and open opportunities for pediatric neuroprotection.
References
- Calderón-Garcidueñas L, Mora-Tiscareño A, Franco-Lira M, Zhu H, Lu Z et al. (2015)Decreases in short term memory, IQ and altered brain metabolic ratios in urban Apolipoprotein ε4 children exposed to air pollution. J Alzheimers
- Calderón-Garcidueñas L, Franco-Lira M, HenrÃquez-Roldán C, González-Maciel A, Reynoso-Robles R, et al. (2010)Urban air pollution: influences on olfactory function and pathology in exposed children and young adults. ExpToxicolPathol 62: 91-102.
- Calderón-Garcidueñas L, Engle R, Mora-Tiscareño A, StynerM, Gómez-Garza G, et al. (2011) Exposure to severe urban pollution influences cognitive outcomes, brain volume and systemic inflammation in clinically healthy children. Brain Cognition 77: 345-355.
- Calderón-Garcidueñas L, D'Angiulli A, Kulesza R J, Torres-Jardón R, Osnaya N, et al. (2011) Air pollution is associated with brainstem auditory nuclei pathology and delayed brainstem auditory evoked potentials. Int J DevNeurosci 29:365-375.
- Calderón-Garcidueñas L, Mora-Tiscareño A, Styner M, Gómez-Garza G, Zhu H, et al. (2012) White matter hyperintensities, systemic inflammation, brain growth, and cognitive functions in children exposed to air pollution. J Alzheimers Dis 31: 183-191.
- Calderón-Garcidueñas L, Kavanaugh M, Block ML, D'Angiulli A, Delgado-Chávez R, et al. (2012) Neuroinflammation, hyperphosphorilated tau, diffuse amyloid plaques and down- regulation of the cellular prion protein in air pollution exposed children and adults. J Alzheimer Dis 28: 93-107
- Calderón-Garcidueñas L, Solt AC, HenrÃquez-Roldán C, Torres-Jardón R, Nuse B, et al. (2008) Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain-barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. ToxicolPathol 36: 289-310.
- Calderón-Garcidueñas L, Franco-Lira M, Mora-Tiscareño A, Medina-Cortina H, Torres-Jardón R, et al. (2013) Early Alzheimer’s and Parkinson’s disease pathology in urban children: Friend versus Foe responses- It is time to face the evidence. Biomed Res Int23: 6
- Villarreal-Calderon R, Torres-Jardón R, Palacios-Moreno J, Osnaya N, Pérez-Guille B, et al. (2010) Urban air pollution targets the dorsal vagal complex and dark chocolate offers neuroprotection. Int J Toxicol 29:604-615
- Calderón-Garcidueñas L, Valencia-Salazar G, Rodriguez-Alcaraz A, Gambling TM, Garcia R, et al. (2001) Ultrastructural nasal pathology in children chronically and sequentially exposed to air pollutants. Am J Respir Cell MolBiol 24: 132-138.
- Harkema JR, Carey SA, Wagner JG (2006) The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. See comment in PubMed Commons below ToxicolPathol 34: 252-269.
- Guerra R, Vera-Aguilar E, Uribe-Ramirez M, Gookin G, Camacho J, et al. (2013) Exposure to inhaled particulate matter activates early markers of oxidative stress, inflammation and unfolded protein response in rat stratium. ToxicolLett222: 146-154.
- Ljubimova JY, Kleinman MT, Karabalin NM, Inoue S, Konda B, et al. (2013) Gene expression changes in rat brain after short and long exposures to particulate matter in Los Angeles basin air: Comparison with human brain tumors. ExpToxicolPathol 65: 1063-1071.
- Van Miert E, Dumont X, Bernard A (2005) CC16 as a marker of lung epithelial hyperpermeability in an acute model of rats exposed to mainstream cigarette smoke. See comment in PubMed Commons below ToxicolLett 159: 115-123.
- Carson JL, Brighton LE, Collier A M, Bromberg PA (2013) Correlative ultrastructural investigations of airway epithelium following experimental exposure to defined air pollutants and lifestyle exposure to tobacco smoke. InhalToxicol 25: 134-140.
- Kaplan GG, Hubbard J, Korzenik J, Sands BE, Panaccione R, et al. (2010) The inflammatory bowel diseases and ambient air pollution: A novel association. Am J Gastroenterol 105: 2412-2419.
- Kaplan GG, Szyszkowicz M, Fichna J, Rowe BH, Porada E, et al. (2012) Non-specific abdominal pain and air pollution: a novel association. PLoS One 7: e47669.
- Kish L, Hotte N, Kaplan GG, Vincent R, Tso R, et al. (2013) Environmental particulate matter induces murine intestinal inflammatory responses and alters the gut microbiome. PLoS One 8: e62220.
- Frolkis A, Dieleman LA, Barkema HW, Panaccione R, Ghosh S, et al. (2013) Environment and the inflammatory bowel diseases. See comment in PubMed Commons below Can J Gastroenterol 27: e18-24.
- Bergin IL, Witzmann FA (2013) Nanoparticle toxicity by the gastrointestinal route: evidence and knowledge gaps. See comment in PubMed Commons below Int J Biomed NanosciNanotechnol 3.
- Braak H, Rüb U, Gai WP, Del Tredici K (2003) Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. Neural Transm 110: 517-536.
- Hawkes CH, Del Tredici K, Braak H (2007) Parkinson's disease: a dual-hit hypothesis. See comment in PubMed Commons below NeuropatholApplNeurobiol 33: 599-614.
- Johansson ME, Sjövall H, Hansson GC (2013) The gastrointestinal mucus system in health and disease. See comment in PubMed Commons below Nat Rev GastroenterolHepatol 10: 352-361.
- Calderón-Garcidueñas L, Mora-Tiscareño A, Fordham LA, Chung CJ, GarcÃa R, et al. (2001) Canines as sentinel species for assessing chronic exposures to air pollutants: part 1. Respiratory pathology. See comment in PubMed Commons below ToxicolSci 61: 342-355.
- Bonazzi M, Cossart P (2011) Impenetrable barriers or entry portals? The role of cell-cell adhesion during infection. See comment in PubMed Commons below J Cell Biol 195: 349-358.
- Carroccio A, Brusca I, Iacono G, Alessio MG, Sonzogni A, et al. (2007) IgA anti-actin antibodies ELISA in coeliac disease: a multicentre study. See comment in PubMed Commons below Dig Liver Dis 39: 818-823.
- Porcelli B, Ferretti F, Vindigni C, Scapellato C, Terzuoli L (2013) Detection of autoantibodies against actin filaments in celiac disease. See comment in PubMed Commons below J Clin Lab Anal 27: 21-26.
- Couto C, Bittencourt P, Porta G, Abrantes-Lemos C, Carrilho F, et al. (2014) Anti-smooth muscle and anti-actin antibodies are indirect markers of histological and biochemical activity of autoimmune hepatitis. Hepatology 59: 592-600.
- Calderón-Garcidueñas L, Vojdani A, Blaurock-Busch E, Busch I, Friedle A, et al. (2014) Air pollution and children: neural and tight junction antibodies and combustion metals, the role of barrier breakdown and brain immunity in neurodegeneration. J Alzheimer’s Disease 43:1039-58
- NOM. (2012) Establecimiento y Operación de Sistemas de Monitoreo de la CalidaddelAire. NOM-156-SEMARNAT-2012. DiarioOficial de la Federación 16/07/2012. México.
- GEM (2012). Inventario de Emisiones a la Atmósferadel Estado de México, 2006. SecretarÃadelMedioAmbiente. Tlalnepantla de Baz, Estado de México.
- Calderón-Garcidueñas L, Macias-Parra M, Hoffmann HJ, Valencia-Salazar G, HenrÃquez-Roldán C, et al (2009) Immunotoxicity and Environment: Immunodysregulation and Systemic Inflammation in Children. ToxicolPathol 37:161-169.
- Molina LT, Madronich SJ, Gaffney JS,Apel E, de Foy B, et al (2010)An overview of the MILAGRO 2006 Campaign: Mexico City emissions and their transport and transformation. AtmosChemPhys 10: 8697–8760.
- Rosas Pérez I, Serrano J, Alfaro-Moreno E, Baumgardner D, GarcÃa-Cuellar C, et al. (2007) Relations between PM10 composition and cell toxicity: a multivariate and graphical approach. See comment in PubMed Commons below Chemosphere 67: 1218-1228.
- Querol X, Pey J, Minguillón MC, Pérez N, Alastuey A, et al. (2008) PM speciation and sources in Mexico during the MILAGRO-2006 Campaign. AtmosChemPhys 8:111–128.
- SecretarÃadelMedioAmbienteGobierno del Distrito Federal (SMA-GDF) (2012)Inventario de emisionesZonaMetropolitana del Valle de México 2010.Contaminantes Criterio 2010. http://www.sma.df.gob.mx/sma/links/download/biblioteca/inventarios_emisiones2010/IEcriterio10_.pdf
- CQRN Diagnóstico Integral de la Ciudad de San Juan del RÃo, Querétaro (2011). Centro Queretano de RecursosNaturales. Tomo XVII-ReporteTécnico. Consejo de Ciencia y TecnologÃa del estado de Querétaro. Santiago de Querétaro, Querétaro. México. Octubre.
- Vojdani A, Kharrazian D, Mukherjee PS3 (2013) The prevalence of antibodies against wheat and milk proteins in blood donors and their contribution to neuroimmunereactivities. See comment in PubMed Commons below Nutrients 6: 15-36.
- Karmakar A, Zhang Q, Zhang Y3 (2014) Neurotoxicity of nanoscale materials. See comment in PubMed Commons below J Food Drug Anal 22: 147-160.
- Klatt NR, Harris LD, Vinton CL, Sung H, Briant JA, et al. (2010) Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol 3: 387-398.
- Gehr P, Clift MJ, Brandenberger C, Lehmann A, Herzog F, et al. (2011) Endocytosis of environmental and engineered micro- and nanosized particles. See comment in PubMed Commons below ComprPhysiol 1: 1159-1174.
- Yu SH, Tang DW, Hsieh HY, Wu WS, Lin BX, et al (2013). Nanoparticle-induced tight-junction opening for the transport of an anti-angiogenicsulfated polysaccharide across Caco-2 cell monolayers. ActaBiomater 9: 7449-7459.
- Gumber S, Nusrat A, Villinger F3 (2014) Immunohistological characterization of intercellular junction proteins in rhesus macaque intestine. See comment in PubMed Commons below ExpToxicolPathol 66: 437-444.
- Vega E, Ruiz H, Escalona S, Cervantes A, Lopez–Veneroni D, et al. (2010) Chemical composition of fine particles in Mexico City during 2003–2004. Atmospheric Pollution Research 2: 477-483.
- Xi J, Berlinski A, Zhou Y, Greenberg B, Ou X. (2012) Breathing resistance and ultrafine particle deposition in nasal-laryngeal airways of a newborn, an infant, a child, and an adult. J Biomed Engineering 40: 2579-2595.
- Stahlhofen W, Gebhart J, Heyder J (1980) Experimental determination of the regional deposition of aerosol particles in the human respiratory tract. See comment in PubMed Commons below Am IndHygAssoc J 41: 385-398a.
- Lippmann M, Yeates DB, Albert RE (1980) Deposition, retention, and clearance of inhaled particles. See comment in PubMed Commons below Br J Ind Med 37: 337-362.
- Brown JS, Gordon T, Price O, Asgharian B (2013) Thoracic and respirable particle definitions for human health risk assessment. See comment in PubMed Commons below Part Fibre Toxicol 10: 12.
- Ermund A, Schütte A, Johansson ME, Gustafsson JK, Hansson GC. (2013) Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am J PhysiolGastrointest Liver Physiol 305: G341-G347.
- Mabbott NA, Donaldson DS, Ohno H,Williams IR, Mahajan A. (2013)Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol 6: 666-677.
- Lu Z, Ding L, Lu Q, Chen YH3 (2013) Claudins in intestines: Distribution and functional significance in health and diseases. See comment in PubMed Commons below Tissue Barriers 1: e24978.
- De Lisle RC1 (2014) Disrupted tight junctions in the small intestine of cystic fibrosis mice. See comment in PubMed Commons below Cell Tissue Res 355: 131-142.
- Birchenough GM, Johansson ME, Stabler RA, Dalgakiran F, Hansson GC, et al. (2013) Altered innate defenses in the neonatal gastrointestinal tract in response to colonization by neuropathogenic Escherichia coli. Infect Immun 81: 3264-75.
- Collin P, Kaukinen K (2013) Celiac disease: clinch the diagnosis when it is just around the corner. See comment in PubMed Commons below Dig Dis Sci 58: 1165-1166.
- Mattila E, Kurppa K, Ukkola A, Collin P, Huhtala H, et al. (2013) Burden of illness and use of health care services before and after celiac disease diagnosis in children. See comment in PubMed Commons below J PediatrGastroenterolNutr 57: 53-56.
- Douglas-Escobar M, Elliott E, Neu J (2013) Effect of intestinal microbial ecology on the developing brain. See comment in PubMed Commons below JAMA Pediatr 167: 374-379.
- Hadjivassiliou M, Aeschlimann P, Sanders DS, Mäki M, Kaukinen K, et al. (2013) Transglutaminase 6 antibodies in the diagnosis of gluten ataxia. See comment in PubMed Commons below Neurology 80: 1740-1745.
- Thomas H, Beck K, Adamczyk M, Aeschlimann P, Langley M, et al. (2013) Transglutaminase 6: a protein associated with central nervous system development and motor function. See comment in PubMed Commons below Amino Acids 44: 161-177.
- Stenberg R, Hadjivassiliou M, Aeschlimann P, Hoggard N, Aeschlimann D3 (2014) Anti-transglutaminase 6 antibodies in children and young adults with cerebral palsy. See comment in PubMed Commons below Autoimmune Dis 2014: 237107.
- Moersch FP, Woltman HW (1956) Progressive fluctuating muscular rigidity and spasm (“stiff-man†syndrome); report of a case and some observations in 13 other cases. Proc Staff Meet Mayo Clin 31: 421-427.
- Blum P, Jankovic J (1991) Stiff-person syndrome: an autoimmune disease. See comment in PubMed Commons below MovDisord 6: 12-20.
- Gershanik OS (2009) Stiff-person syndrome. Parkinson and Rel Dis 1553: S130-S134.
- Boettler T, Pagni PP, Jaffe R, Cheng Y, Zerhouni P, et al. (2013) The clinical and immunological significance of GAD-specific autoantibody and T-cell responses in type 1 diabetes. See comment in PubMed Commons below J Autoimmun 44: 40-48.
- Dinkel K, Meinck HM, Jury KM, Karges W, Richter W (1998) Inhibition of gamma-aminobutyric acid synthesis by glutamic acid decarboxylase autoantibodies in stiff-man syndrome. See comment in PubMed Commons below Ann Neurol 44: 194-201.
- Levy LM, Levy-Reis I, Fujii M, Dalakas MC (2005) Brain gamma-aminobutyric acid changes in stiff-person syndrome. See comment in PubMed Commons below Arch Neurol 62: 970-974.
- Ali F, Rowley M, Jayakrishnan B, Teuber S, Gershwin ME, Mackay IR (2011) Stiff-person syndrome (SPS) and anti-GAD-related CNS degenerations: Protean additions to the autoimmune central neuropathies. J Autoimmunity 37: 79-87.
- Rosén A, Sandström O, Carlsson A, Högberg L, Olén O, et al. (2014) Usefulness of symptoms to screen for celiac disease. See comment in PubMed Commons below Pediatrics 133: 211-218.
- Kabbani TA, Vanga RR, Leffler DA, Villafuerte-Galvez J, Pallav K, et al. (2014) Celiac disease or non-celiac gluten sensitivity? An approach to clinical differential diagnosis. See comment in PubMed Commons below Am J Gastroenterol 109: 741-746.
- Diamond B, Honig G, Mader S, Brimberg L, Volpe BT (2013) Brain-reactive antibodies and disease. See comment in PubMed Commons below Annu Rev Immunol 31: 345-385.
- Levin EC, Acharya NK, Han M, Zavareh SB, Sedeyn JC, et al. (2010) Brain-reactive autoantibodies are nearly ubiquitous in human sera and may be linked to pathology in the context of blood-brain barrier breakdown. Brain Res 1345: 221-232.
- Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, et al. (2011) Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. See comment in PubMed Commons below Nature 479: 538-541.
- Teeling JL, Carare RO, Glennie MJ, Perry VH. (2012)Intracerebral immune complex formation induces inflammation in the brain that depends on Fc receptor interaction. Acta Neuropath 124: 479-490.
- Villarreal-Calderon R, Franco-Lira M, Gónzalez-Maciel A, Reynoso-Robles R, Harritt L, et al. (2013) Up-regulation of mRNA ventricular PRNP prion protein gene expression in air pollution highly exposed young urbanites: endoplasmic reticulum stress, glucose regulated protein 78 and nanosized particles. Int J MolSci 14: 23471-23491.
- Voigt J, Christensen J, Shastri VP (2014) Differential uptake of nanoparticles by endothelial cells through polyelectrolytes with affinity for caveolae. See comment in PubMed Commons below ProcNatlAcadSci U S A 111: 2942-2947.
- Howe SE, Lickteig DJ, Plunkett KN, Ryerse JS, Konjufca V.(2014) The uptake of soluble and particulate antigens by epithelial cells in the mouse small intestine. PLoS One 9: e86656.
- Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, et al. (2003) Staging of brain pathology related to sporadic Parkinson's disease. See comment in PubMed Commons below Neurobiol Aging 24: 197-211.
- Doty RL1 (2012) Olfactory dysfunction in Parkinson disease. See comment in PubMed Commons below Nat Rev Neurol 8: 329-339.
- Prediger RD, Aguiar AS Jr, Matheus FC, Walz R, Antoury L, et al. (2012) Intranasal administration of neurotoxicants in animals: support for the olfactory vector hypothesis of Parkinson's disease. See comment in PubMed Commons below Neurotox Res 21: 90-116.
- Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, et al. (2014) Gut microbiota are related to Parkinson's disease and clinical phenotype. See comment in PubMed Commons below MovDisord .
- Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W , Björklund T et al. (2014) Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. ActaNeuropathol 128:805-820.
- Kelly LP, Carvey PM, Keshavarzian A, Shannon KM, Shaikh M, et al. (2014) Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson's disease. See comment in PubMed Commons below MovDisord 29: 999-1009.
- Friedland RP1 (2015) Mechanisms of Molecular Mimicry Involving the Microbiota in Neurodegeneration. See comment in PubMed Commons below J AlzheimersDis .
- Brierley SM, Linden DR2 (2014) Neuroplasticity and dysfunction after gastrointestinal inflammation. See comment in PubMed Commons below Nat Rev GastroenterolHepatol 11: 611-627.
- Jellinger KA1 (2014) Thepathomechanisms underlying Parkinson's disease. See comment in PubMed Commons below Expert Rev Neurother 14: 199-215.
- Iadecola C1 (2004) Neurovascular regulation in the normal brain and in Alzheimer's disease. See comment in PubMed Commons below Nat Rev Neurosci 5: 347-360.
- Lampron A, Elali A, Rivest S (2013) Innate immunity in the CNS: redefining the relationship between the CNS and Its environment. See comment in PubMed Commons below Neuron 78: 214-232.
- Perry G, Castellani R2 (2014) Plaques and tangles: birthmarks of the aging soul. Preface. See comment in PubMed Commons below BiochemPharmacol 88: 423-425.
- Brandenberger C, Clift MJ, Vanhecke D, Mühlfeld C, Stone V, et al. (2010) Intracellular imaging of nanoparticles: is it an elemental mistake to believe what you see? See comment in PubMed Commons below Part Fibre Toxicol 7: 15.
- Levesque S, SuraceMJ, McDonald J, Block ML (2011) Air pollution and the brain: Subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J Neuroinflammationdoi: 10.1186/1742-2094-8-105.
- Levesque S, Taetzsch T, Lull ME, Johnson JA, McGraw C, et al. (2013) The role of MAC1 in diesel exhaust particle-induced microglial activation and loss of dopaminergic neuron function. See comment in PubMed Commons below J Neurochem 125: 756-765.
- Muza-Moons MM, Schneeberger EE, Hecht GA (2004)Enteropathogenic Escherichia coli infection leads to appearance of aberrant tight junctions strands in the lateral membrane of intestinal epithelial cells. Cell Microbiol 6: 783-793.
- Sedgmen BJ, Meeusen EN, Lofthouse SA (2004) Alternative routes of mucosal immunization in large animals. See comment in PubMed Commons below Immunol Cell Biol 82: 10-16.
- Dharmani P, Srivastava V, Kissoon-Singh V, Chadee K (2009) Role of intestinal mucins in innate host defense mechanisms against pathogens. J Innate Immun 1:123-135.
- Bilbo SD, Schwarz JM (2012) The immune system and developmental programming of brain and behavior. See comment in PubMed Commons below Front Neuroendocrinol 33: 267-286.
- Ren WY, Wu KF, Li X, Luo M, Liu HC, et al. (2014) Age-related changes in small intestinal mucosa epithelium architecture and epithelial tight junction in rat models. See comment in PubMed Commons below Aging ClinExp Res 26: 183-191.
- Kim JJ, Khan WI, (2013) Goblet cells and mucins: role in innate defense in enteric infections. See comment in PubMed Commons below Pathogens 2: 55-70.
- Hornig M, (2013) The role of microbes and autoimmunity in the pathogenesis of neuropsychiatric illness. See comment in PubMed Commons below CurrOpinRheumatol 25: 488-795.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 19319
- [From(publication date):
March-2015 - Apr 01, 2025] - Breakdown by view type
- HTML page views : 14575
- PDF downloads : 4744