The Interest of Adding Micronutrients to Docosahexaenoic Acid Supplementation to Prevent Age-Related Cognitive Decline
Received: 07-May-2018 / Accepted Date: 16-May-2018 / Published Date: 23-May-2018 DOI: 10.4172/2161-0460.1000438
Abstract
Aging is associated to cognitive decline that can lead to neurodegenerative diseases and constitutes one of the main social and economic issues of the 21st century. The loss of memory, orientation and processing abilities associated with aging are involved in the loss of autonomy and in the decline in the quality of life in the elderly. Brain structures involved in memory such as hippocampus, cortex and striatum, are particularly affected by molecular and cellular damage during this period. Lipid metabolism and neurofunctional alterations, including disturbances in synaptic plasticity and neurogenesis, chronic low-grade inflammation and increased oxidative stress, are partly to be involved in age-related cognitive decline. Actually, nutrition represents a strategy of choice to prevent or delay these impairments since many studies have provided valuable data concerning the effect of dietary patterns and specific nutrients on cognitive function. From all nutrients, some of them are particularly attractive. Indeed, n-3 polyunsaturated acids (PUFAs), especially docosahexaenoic acid (DHA), have been identified for their beneficial effects on cognition, notably by acting on brain plasticity (synaptic plasticity, neurogenesis), neuroinflammation and oxidative stress. Other nutrients such as vitamin A, vitamin E, vitamin D, polyphenols as well as pre- and probiotics have aroused a growing interest in decreasing cognitive disorders. As nutrition has to be taken as a whole, we first described the effects of the Mediterranean diet which constitutes the most complete association of nutrients and (DHA from fish, vitamins and polyphenols from fruits and vegetables) represents a global vision of nutrition, then we focused on the interest of combining DHA and micronutrients contained in this diet as well as pre- and probiotics, to prevent age-related cognitive decline and reported the synergistic effects of these associations. Finally, we completed with benefits from dairy products that increase DHA incorporation.
Keywords: Age-related cognitive decline; Docosahexaenoic acid; Vitamins; Polyphenols; Pre and probiotic; Dairy product; Mediterranean diet
Abbreviations
4-HHE: 4-hydroxy-2-hexenal; 4-HNE: 4-hydroxy- 2-nonenal; 5-MTHF: 5-methyltetrahydrofolic acid; AA: Arachidonic Acid; AD: Alzheimer’s Disease; AGEs: Advanced Glycation End Products; ALA: α-linolenic Acid; AMPA: α-amino-3-hydroxy-5- methyl-4-isoxazole propionic Acid; BDNF: Brain Derived Neurotrophic Factor; CaMKII: Calmodulin-dependent Kinase; CD11b: Complement Receptor 3; CNS: Central Nervous System; COX: Cyclooxygenase; CREB: cAMP-response element-binding protein; DAMPs: Damage- associated Molecular Patterns; DG: Dentate Gyrus; DHA: Docosahexaenoic Acid; EPA: Eicosapentaenoic Acid; ERK: Extracellular Signal-regulated Kinase; ICE: IL-1β Converting Enzyme; IL-10: Interleukin-10; IL- 18: Interleukin-18; IL-1β :Interleukin-1β; IL-4: Interleukin-4; IL-6: Interleukin-6; LA: Linoleic Acid; LC-PUFA: Long-chain Polyunsaturated Acid; LOX: Lipoxygenase; LTP: Long Term Potentiation; MAPK: Mitogen-activated Protein Kinases; MCI: Mild Cognitive Impairment; MDA: Malonaldehyde; MHC II: Major Histocompatibility Complex II; MMSE: Mini-mental State Exam; mtDNA: Mitochondrial DNA; n-3: Omega 3; n-6: Omega 6; NFκB: Nuclear Factor-kappa B; NGF: Nerve Growth Factor; NLRP3: NOD-like Receptor Protein 3; NMDA: N-methyl-D-aspartate; NPD-1: Neuroprotectin D1; PAMPs: Pathogen-associated Molecular Patterns; PC: Phosphatidylcholine; PD: Parkinson Disease; PE: Phosphatidylethanolamine; PPARs: Peroxisome Proliferator-activated Receptors; PPRs: Pattern Recognition Receptors; PS: Phosphatidylserine; PUFA: Polyunsaturated Fatty Acid; RA: Retinoic Acid; RAR: Retinoic Acid Receptor; RCT: Randomized Controlled Trial; ROS: Reactive Oxygen Species; RXR: Retinoid X Receptor; SNPs: Single Nucleotide Polymorphisms; SOD: Superoxide Dismutase; SPMs: Specialized Pro-resolving Mediators; TNF-α:Tumor Necrosis Factor α; TrkB: Tyrosine Kinase B
Introduction
The world population grows every year and gets older as the life expectancy increases. Indeed, in the last century, the elderly population (e.g. 65 years and older) tripled from 4% to 13% and it is expected to double over the next three decades to reach 20% of the population in 2025 and 33% in 2050 [1,2]. Thus, healthy aging constitutes one of the main social and economic challenges of the 21st century for the nations, especially since aging is associated to cognitive decline [3-5] that can lead to age-related disease such as neurodegenerative diseases. For example, Alzheimer’s disease (AD), the most common form of dementia, affects 10% of the elderly population in the US [1,6] and about 5% of the elderly population in Europe [7]. The loss of memory, orientation and processing abilities results in personal suffering, loss of autonomy and high costs of health care. Indeed, health care costs already account for between 8% and 10% of gross domestic product in developed countries [8]. Aging is a multifactorial process associated to molecular and cellular damage over time including retraction of synapses and loss of cellular components in the hippocampus, cortex and striatum, the critical brain regions for memory [4]. The mechanisms involved are not completely understood but include lipid metabolism as well as neurofunctional alterations, including disturbances in synaptic plasticity and neurogenesis, increased oxidative stress and activation of inflammatory signaling pathways which are linked to the diminution of cognitive performance and ultimately, to the development of neurodegenerative diseases [4,9].
Strategies to promote healthy brain aging including optimal cognitive function with a good quality of life are encouraged. Furthermore, lifestyle, especially diet and nutrition, may have a direct impact to prevent age-related cognitive decline by affecting multiple brain processes since many clinical and preclinical studies have provided valuable data concerning the effect of dietary patterns and/or specific nutrients on cognitive function [10-12]. Indeed, recent studies suggest that nutrition can slow the progression of age-related cognitive decline [4,12,13]. For example, n-3 polyunsaturated fatty acids (PUFAs) and especially docosahexaenoic acid (DHA), highly concentrated in the brain, have been largely studied. During aging, a large panel of studies shows a DHA deficit in brain membranes in humans and in rodents [14-18] due to impairments in lipid metabolism. Indeed, aging induces a reduction in the bioavailability and in the synthesis of fatty acids and phospholipids. Hence, n-3 long chain PUFAs (LCPUFAs) and particularly DHA have to be provided to the elderly, especially since aging is also characterized by a dysregulation in the cytokine homeostasis leading to a chronic pro-inflammatory state in the brain (“a low-grade inflammation”) [19] and since n-3 LC-PUFAs have anti-inflammatory properties [20-24]. However, results from n-3 PUFAs supplementation studies are conflicting either because of the insufficient anti-inflammatory properties of a single nutrient [25] or because of the high oxidative susceptibility of DHA, requiring the addition of antioxidants. Indeed, during aging, oxidative processes are higher [9], leading to a possible increase in DHA oxidation. Then adding antioxidants may be necessary for the elderly when supplying DHA, this population having particular nutritional requirements in term of key nutrients [25]. Hence, combining DHA and different micronutrients, antioxidants/polyphenols for example, represents a good strategy to enhance cognitive functions in the elderly [4,26]. Moreover, since the anti-inflammatory properties of a single nutrient at relevant concentrations are usually insufficient to achieve a therapeutic effect, combining nutrients avoids the use of high concentration of a single nutrient [26].In addition to the beneficial effect of DHA on cognition, there is a growing interest in vitamin A, vitamin D and vitamin E as well as phytonutrients found in fruits and vegetables such as polyphenols in decreasing cognitive decline [10,27-29]. An alternative strategy will be to increase the bioavailability of those nutrients, in particular of DHA. Recently, studies have demonstrated that probiotics and dairy products increase brain DHA content, possibly affecting cognitive decline [30- 34].
Hence, in this review, we focused on the interest of combining DHA and other micronutrients (vitamin A, D, E, polyphenols, pre- or probiotics) to prevent age-related cognitive decline. We first examined the Mediterranean diet that is rich in n-3 PUFA from fish and wholegrain cereals, in vitamins from fruits and vegetables and in polyphenols from olive oil because this diet has been found to be protective [1]. Then we focused on the benefits of DHA alone or with vitamins or polyphenols as antioxidants. Finally, we exposed the interest of adding pre- or probiotics to DHA or to consume dairy products to increase DHA bioavailability.
Brain Aging And Cognitive Decline
Memory alterations during aging
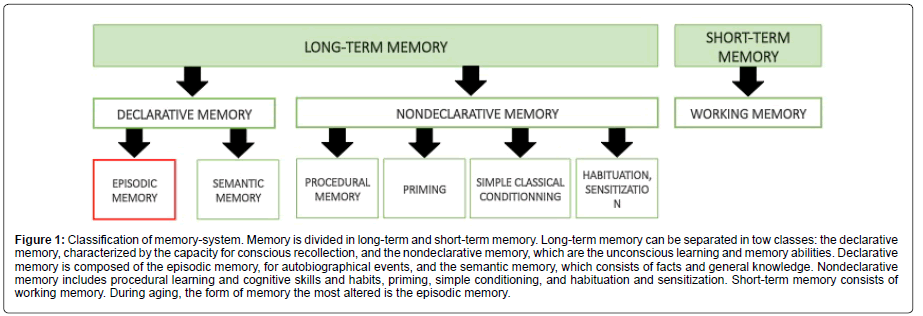
Brain aging is associated with different morphological and neurochemical changes leading to the development of memory deficits such as mild cognitive impairment (MCI) or AD. Studies from Salthouse show that notable age-related cognitive decline occurs in healthy individuals from the late 20 years old and continues throughout the adult lifespan (for review [35]). Normal aging is characterized by a decrease in cognitive performance which is defined by nonpathological memory alterations with a significant inter individual variability [36]. Hippocampal dependent memory has been shown to be strongly affected by age in humans [37,38] as well as in rodents [39,40]. However, aging does not impact equally all forms of memory (Figure 1).
Figure 1: Classification of memory-system. Memory is divided in long-term and short-term memory. Long-term memory can be separated in tow classes: the declarative memory, characterized by the capacity for conscious recollection, and the nondeclarative memory, which are the unconscious learning and memory abilities. Declarative memory is composed of the episodic memory, for autobiographical events, and the semantic memory, which consists of facts and general knowledge. Nondeclarative memory includes procedural learning and cognitive skills and habits, priming, simple conditioning, and habituation and sensitization. Short-term memory consists of working memory. During aging, the form of memory the most altered is the episodic memory.
It has been shown that working and episodic memories start declining from youth throughout the lifespan [41]. Results of longitudinal studies taking into account methodological biases related to “test-retest” suggest that both types of memory would undergo accelerated decline after 60 years [42]. Episodic memory is considered to be the form of long-term memory with the highest degree of agerelated decline, but conversely, knowledge (semantic memory) seems to be preserved or even improved [41].
Experimental data have shown that age-related memory deficits, including spatial learning deficits, are similar to those induced by hippocampal lesions [43]. In this context, many studies have identified age-related deficits in various tasks that evaluate spatial or working memory in rodents compared to their younger counterparts [44,45]. Furthermore, using a paradigm modeling human declarative memory, some authors have shown that older animals exhibit relational and working memory deficits which were associated with alterations in the activity of the hippocampus [46].
The link between the neurobiological alterations in the hippocampus and the genesis of memory deficits observed during aging is also accepted [5]. Indeed, changes in hippocampus occurring during aging involve disturbances in signaling pathways and alterations in brain plasticity which result in deficits in learning and memory. Cognitive impairments occurring during aging could be explained in part by neurofunctional alterations, chronic low grade inflammation and oxidative stress.
Lipid metabolism alterations during aging
The brain is highly enriched in LC-PUFAs that are essential fatty acids needed to be provided by the diet since they are poorly synthesized in mammals [47]. PUFAs are divided into two main families, the n-6 PUFAs (omega 6) and the n-3 PUFAs (omega 3). Linoleic acid (LA; 18:2 n-6) and α-linolenic acid (ALA; 18:3 n-3) are, respectively, the dietaryessential precursors of the LC-PUFAs arachidonic acid (AA; 20:4 n-6) and eicosapentaenoic acid (EPA; 20:5 n-3) and docosahexaenoic acid (DHA; 22:6 n-3) [48]. The synthesis of LC-PUFAs from their precursor requires the action of Δ6 and Δ5 desaturases and elongases as well as β-oxidation for the last step in DHA formation [49]. AA and DHA are largely esterified into phospholipids of cell membrane (DHA: 13-22% and AA: 5-11%) in the sn-2 position [47]. The main phospholipids of brain cell membrane are phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol, and plasmalogens which have specific PUFA profiles. Indeed, DHA for example, is mainly esterified in PE and PS in the brain [50].
During aging, changes in membrane phospholipid class composition as well as reduced brain levels of LC-PUFAs are observed, although all brain structures are not affected in the same way [48,51]. Indeed, several studies have reported the reduction with aging of DHA level in human brain [50,52] and rats [53,54]. In animals, a progressive decrease in cortical and hippocampal contents of PE and PS has been observed in aged rats [15,54,55]. Other studies performed in aged rodents have highlighted a reduction in DHA content in the whole brain [16,56] or only in certain brain areas such as hippocampus, cortex, hypothalamus as well as the cerebellum [17-19,55]. However, these data are controversial since further studies have shown in cortex of aged mice an increased level of AA and DHA in PC and PE which could be due to a decreased consumption of AA and DHA by the brain [57,58].
Aging is associated with the decrease of Δ6 desaturase activity in the liver and the brain which lead to the reduced efficiency of the conversion of the precursors, ALA to DHA [52]. Furthermore, enzymes involved in phospholipid synthesis are also impaired, thus hindering the incorporation of PUFAs into the membranes [59]. In addition, several studies in both humans and animals [60,61] have shown disturbances in the intestinal absorption of essential fatty acids suggesting changes in the bioavailability of n-3 PUFAs. Moreover, PUFAs are highly susceptible to free radical attack. Indeed, oxidative damage to lipids can occur through their peroxidation leading to the formation of reactive aldehydes such as malonaldehyde (MDA), 4-hydroxy-2-nonenal (4- HNE) or 4-hydroxy-2-hexenal (4-HHE). Increased levels of MDA and 4-HNE have been demonstrated in aged brain of humans and rodents [62,63]. Thus, the decrease in the activity of conversion enzymes inducing a reduction in the bioavailability of n-3 LC-PUFAs in the liver and in the synthesis in situ, associated with lipid peroxidation, can lead finally to disturbances in DHA metabolism and then to the development of cognitive decline. Several studies have also shown variations with age in genes involved in the biosynthesis and metabolism of PUFAs. Indeed, single nucleotide polymorphisms (SNPs) in desaturase genes FADS1 (Δ5 desaturase), FADS2 (Δ6 desaturase) as well as ELOVL2 (elongase 2) are linked to higher ALA and lower EPA plasma phospholipid levels with age, which suggest different rates of conversion [64].
All together, these data demonstrate the importance of the dietary intake of DHA for the elderly since changes in lipid metabolism have been linked to age-related cognitive decline.
Observational studies in elderly linked the age-related decrease in DHA blood concentration with cognitive disorders. Indeed, it has been shown in cohorts of elderly that the erythrocyte membrane content in n-3 PUFAs as well as their consumption are inversely correlated with age-related cognitive decline [65,66]. Interventional studies with a daily use of fish-oil supplements have shown increased levels of n-3 PUFAs, especially DHA, in erythrocytes which is associated with better cognitive performance in older age [67]. Another study performed by Barberger-Gateau [68] in elderly highlights a negative correlation between food consumption rich in n-3 PUFAs and cognitive decline. Similar relationships were found in the Framingham Offsprings Study with a positive correlation between serum DHA concentration and cognitive abilities. Another study performed in Japanese older individuals has shown a link between low DHA levels in serum and a higher risk of cognitive decline [69].
Modifications in lipid composition are suggested to have consequences for cellular functions of the central nervous system (CNS) [50]. This is supported by various animal studies demonstrating that a low-DHA diet for one or more generations is associated with deficits in cognitive function in rats [53,70]. In addition, data from our lab have shown that aged mice fed an unbalanced diet in n-3 PUFA alters synaptic plasticity, neuroinflammation as well as memory [57,71]. Furthermore, the decrease in brain DHA content sensitizes the brain to inflammatory stimuli [72], resulting in damage to neuroanatomical structures and inducing memory decline.
These age-related alterations of lipid metabolism contribute to reduce the PUFA content in phospholipids, especially DHA, in the brains of the elderly. This DHA reduction in cell membranes, especially of synaptic terminals, mitochondria and endoplasmic reticulum [73] has to be prevented since these structures are crucial for maintaining the function of the CNS, notably synaptic integrity, and limiting the age-related neuroinflammation and oxidative stress involved in cognitive decline [24,48].
Brain plasticity alterations during aging
Brain’s capacity to modify its neuronal circuit function is called plasticity. Synaptic plasticity refers to the activity-dependent changes in the strength and efficacy of synaptic transmission of synapses [74]. Long-term potentiation (LTP) represents one of the main form of longterm synaptic plasticity, defined as the strengthening of a synapse for a minute to a lifetime and is thought to play a key role in hippocampal function [75,76]. The establishment of LTP is highly dependent on the activation of the glutamatergic N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors which allow the recruitment of various signaling pathways and induce a remodeling and a specification of the synapse.
Cognitive alterations occurring during aging are partly due to morphological and physiological changes. Indeed, it has been reported a decrease in the density of NMDA and AMPA receptors associated with a decrease in synaptic transmission. Moreover, a demyelination of nerve fibers, and a reduction in the complexity of dendritic arborization as well as a decrease in the length and number of dendritic spines have been observed [77]. These disturbances in brain synaptic circuitry occurring during aging, especially in the hippocampus and prefrontal cortex, might promote relevant cognitive decline and the development of neurodegenerative diseases [78].
Several studies linked aging with impaired hippocampal LTP in rodents, which appears to be related with decreased ability to consolidate long-term memory [79,80]. Age-dependent decay of hippocampal LTP in rats has been correlated with poorer performance on spatial memory tasks [45,81]. LTP deficits occurring during aging can be explained by a neuronal reorganization, notably by structural changes of synapses and modifications in the morphology of neurons, in the prefrontal cortex and the hippocampus [82].
In addition, a large number of studies have observed a correlation between alterations of hippocampal LTP and structural changes of the synapse during aging. Indeed, synaptic loss, morphological alterations of dendrites, and a reduction in the expression of synaptic markers such as synaptophysin, are likely to be responsible of age-related reduction in hippocampal synaptic transmission and to contribute to cognitive deficits [83,84]. In humans, a reduction in spine number and length, as well as dysfunction of dendritic processes and changes in receptors and neurotransmitters release processes have also been described in the hippocampus associated with age-related cognitive impairments [85,86]. Studies in aged rats have shown a decrease in number of synaptic connections of about 30% in the dentate gyrus (DG), concomitantly with a decrease in their spatial memory performance [87,88]. Furthermore, a decrease in the expression of the synaptic markers synaptophysin, neurogranin as well as neuromodulin occurs during aging which is associated with altered LTP and spatial memory deficits in aged mice [89-92].
Other studies have shown a decreased expression of NMDA and AMPA receptors concomitantly with an increase in Ca2+ conductance in aged neurons, meaning that calcium signaling is also affected by age [93,94]. These changes in calcium homeostasis can consequently lead to synaptic plasticity alterations [93]. Among the factors located downstream of the calcium signal, other signaling pathways are affected by aging. The most implicated signaling pathway is the Mitogen-activated protein kinases (MAPK)/Extracellular signalregulated kinase (ERK)/cAMP-response element-binding protein (CREB) pathway which plays a crucial role in the formation of longterm memory and is highly conserved during evolution [95]. However, many studies have shown that aging is associated to an alteration of this pathway. Indeed, Simonyi et al. [96] showed a 20% reduction in the expression of ERK transcripts in the hippocampus of 13-month-old rats compared to 3-month-old rats. In addition, hippocampus of mice aged 23-24 months exhibits a reduction in CREB activation compared to 5-6 month-old adult mice, which is correlated with a poor spatial memory performance as measured in the Morris water maze [97].
Neurogenesis, also involved in brain plasticity, is defined as the processes leading to the formation of functional neurons. These processes include proliferation of neural stem cells/neural progenitor cells, migration of newly formed neurons, differentiation into a defined phenotype and its functional integration to the brain circuit [98]. In the hippocampus, new neurons generated are important for learning and memory [99]. Although hippocampal neurogenesis seems to persist throughout lifespan, an age-related decrease has been highlighted [100].
Indeed, an age-related reduction of the production of new neurons has been demonstrated in the hippocampus of old humans and rodents which has been associated with a decline in hippocampal-dependent spatial memory [101,102]. Despite the age-related decrease in the production of new hippocampal neurons, studies have shown that neurons integrated to the brain circuit appear functionally equivalent to those in young brain [103] suggesting that neurogenesis during aging is downregulated. In support, reduced cell proliferation and retarded neuronal maturation as well as an increased quiescence of neural stem cells have been demonstrated in hippocampus of aged rats [104]. These changes in the hippocampus could be explained by modifications in the neurogenic milieu occurring during aging, notably by the decreased expression of key neurotrophic factors.
Several neurotrophics factors involved in development, survival and functioning neurons are affected by aging [105]. Indeed, a decreased expression of the neurotrophin Brain Derived Neurotrophic Factor (BDNF) signaling, involved in memory formation, has been correlated with age-related reduction of neurogenesis, impaired spatial memory and cognitive deficits [106,107]. In both elderly and rodents, the agerelated decrease in BDNF levels concomitant with its receptor Tyrosine kinase B (TrkB) is associated with reduced cognitive functioning demonstrated by impaired performance on spatial memory tasks [106,108]. This decrease further contributes to age-related impairment of neurotrophic signaling in the hippocampus.
Recently, studies have shown using heterochronic parabiosis that young animals exposed to an old systemic environment containing blood-borne factors, or to plasma from old mice, displayed a decreased synaptic plasticity, reduced adult neurogenesis and impaired spatial learning and memory [109]. These observations suggests that agedependent systemic changes can modulate neurogenesis and synaptic plasticity which can potentially contribute to the decline in regenerative capacity observed in the normal aging brain and constitute a new strategy to target age-related dysfunctions.
All together, these findings suggest that alterations in hippocampal synaptic plasticity, especially of LTP, and neurogenesis may contribute to the age-related cognitive decline. Therefore, strategies capable of maintaining synapse integrity and restoring levels of factors involved in the processes of neurogenesis may be promising to prevent the development of memory deficits.
Chronic low-grade inflammation during aging
In physiological conditions, inflammation acts transiently, rising when needed then fading down, which is essential as it helps organisms to fight infections and plays crucial roles in repair as well as in maintenance of organs. Normal brain aging is associated with a chronic low-grade inflammation linked to the immunosenescence of the CNS also known as “inflammaging”. Franceschi et al. [110] defined that inflammaging is mainly due to dysfunctional organelles such as mitochondria, misplaced molecules (examples: lipofuscins, advanced glycation end products (AGEs), mitochondrial and nuclear DNA, etc), alterations of the ubiquitine/proteasome system as well as activation of the DNA damage response which lead to the low-grade activation of the immune system.
Microglial cells are the resident immune cells of the CNS involved in various physiological and pathophysiological functions [111]. These cells are equipped with various pattern recognition receptors (PRRs), including Toll-like receptors and NOD-like receptors which allow them to recognize both damage- (DAMPs) and pathogen- (PAMPs) associated molecular patterns and initiate an immune response [112]. During homeostasis, microglia are strictly regulated by factors within the CNS microenvironment but with aging, microglia are suggested to develop age-dependent cellular dystrophy and to become senescent [113]. Studies have reported dystrophic changes in microglia including appearance of microglial aggregates, altered morphology, and reduced arborization in addition with a decreased mobility in aged human and mice brain [114,115]. Furthermore, age-dependent microglia activation has been described in rodents [116,117]. Indeed, aged microglia take on a “primed” phenotype, characterized by an exaggerated and uncontrolled inflammatory response to an immune stimulus [118]. Aged microglia also display increased levels of lipofuscin granules, molecular sign of microglial senescence [119,120]. These lipofuscin granules have been recently shown to contain myelin fragments [121], suggesting impairments of its degradative pathways. Thus, microglial senescence has been linked to functional changes partly involved in the age-related increase in microglia-mediated neuroinflammatory response, driving the progression of neurodegenerative pathologies.
Inflammaging is characterized by the increased blood and brain levels expression of pro-inflammatory cytokines, including tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), and interleukin-18 (IL-18) under basal conditions and downregulation of the expression of anti-inflammatory factors such as interleukin-10 (IL-10), interleukin-4 (IL-4) or BDNF [122,123]. Studies have shown an increased expression of IL-6 in the hippocampus, cortex and cerebellum of aged mice compared to young adults [15,124] and a decreased expression of IL-10 [57]. Importantly, IL-6 serum levels predict incidence of disabilities and other deficits [125], including mobility and walking speed [126]. Other studies have shown that microglial cells from the brains of aged mice and rats produce more IL-1β and IL-6 than microglia from young animals [119,127-129]. The overproduction of IL-6 by aged mouse microglial cells is due to a sustained increase in NFκB transcription factor [130]. This chronic low-grade production of pro-inflammatory cytokines associated to microglial overactivationBioavailability has been linked to the development of cognitive impairments [131]. Inflammaging is also characterized by the upregulation of the expression of MHC II, CD68, caspase-1 as well as CD11b [132-134], representing the hallmarks of age-dependent microglia activation. Studies have shown that an age-related increase in cerebral IL-1β expression is observed in patients without neurological pathologies. This is associated with microglial cell activation, characterized by an increased MHC II expression and a change in morphology of microglial cells [135]. The number of microglial cells expressing MHC II also increases with age in rats [116].
Numerous studies have found a causal link between elevated cytokine levels in the blood and the brain and age-related cognitive dysfunctions. Epidemiological studies performed in elderly have correlated elevated plasma levels of IL-6 with cognitive decline including speed of information processing [131,136]. Furthermore, in hippocampus of aged rodents, significant increase in levels of IL-1β induced by infection or stress has been shown to impair learning and memory [137,138]. IL-6-deficient mice are protected from age-related decrease in cognitive performance [139] and display an attenuated induction of pro-inflammatory cytokines in their hippocampus in response to a bacterial endotoxin as compared to wild-type mice [140].
Overall, these findings support the idea that chronic low-grade inflammation occuring during aging may contribute to cognitive impairments. Thus, limiting the expression and the action of proinflammatory cytokines appears essential to prevent age-realated cognitive decline and the development of neurodegenerative pathologies.
Oxidative Stress during Aging
The “free radical theory of aging” has been described by Harman [141] as one of the most prominent model for age-related brain changes and cognitive decline. This theory proposes that aging is a consequence of free radical-induced damage on cell components and connective tissues, and the inability to counterbalance these changes by endogenous antioxidant defenses. Mitochondrial dysfunctions have also been involved in the physiological process of aging [142] since mitochondria generate a significant amount of cellular energy by consuming approximately 90% of intracellular oxygen through oxidative phosphorylation. According to the theory and although mitochondria are free-radical producers, they are also targets of ROS leading to increased oxidative damage. Consequently, damaged mitochondria become less efficient, loose their functional integrity and release more oxygen molecules thus increasing oxidative damage with age [143].
Under normal circumstances, oxidative stress is controlled by the balance between the production and the removal of ROS within the cell and in the local microenvironment. The level of oxidant molecules such as ROS plays a crucial role in signaling and synaptic plasticity by acting as cellular messenger [144] and growing evidence suggests a delicate balance between free radicals production and brain protection or damage [10,145]. Indeed, the maintenance of the intracellular redox state is essential for hippocampal neurogenic function [146] as well as LTP processes through the appropriate NMDA receptor function [147]. By contrast, dysregulation of the intracellular homeostasis, representing a common feature of the aging brain, results in accumulation of oxidative damage and can have deleterious consequences for neuronal function leading, in extreme conditions, to the cell death [99]. For example, studies have shown alterations in intracellular redox ratio [148] as well as LTP impairments associated with increased oxidative damage to cellular lipids in the hippocampus of aged rodents [149].
Several factors are involved in the age-related imbalance in the intracellular redox state. Indeed, studies performed in both aged humans and rodents have shown in the hippocampus disturbances of energy metabolism and an increased oxidative stress, protein oxidation and lipid peroxidation [62,150,151]. These observations have been corroborated using gene microarrays in hippocampus of aged rats [89]. In addition, an increased mitochondrial DNA (mtDNA) damage in the aged brain has been shown, probably due to a lack of mtDNA repair mechanisms, as well as the proximity of mtDNA to the inner mitochondrial membrane where ROS are generated [152]. Finally, a hippocampal decrease in the levels of endogenous antioxidants, including superoxide dismutase (SOD), catalase and other reductases, has been observed in both aged humans and rodents [150,153]. This reduction in antioxidant levels leads to an altered neuroprotection against oxidative damage. Interestingly, studies have shown in aged mice that an antioxidant treatment could moderate the age-related cognitive decline and the elevated hippocampal ROS levels [154]. That, together with age-related mitochondrial dysfunction, causes alterations of cellular architecture within the brain and raises the fact that uncontrolled free radical production is a major contributor to the loss of neuronal homeostasis, leading to accelerated aging and neurodegenerative disease development [155].
Nutrition as a potential protective factor of age-related cognitive decline
Actually, there is a growing interest to consider nutrients as a potential way to prevent or delay age-related cognitive decline. Nutrition is known to play a role in healthy aging since dietary and nutrient patterns have been linked to health outcomes related to aging [11]. It is particularly important in the elderly because of a decreased rate of digestion and absorption of nutrients [156]. The most complete association of nutrients is the Mediterranean diet combining n-3 LC-PUFAs from fish, with vitamins and polyphenols from fruits and vegetables and having a beneficial effect on age-related cognitive decline. Then we focused on n-3 PUFAs and different duos of nutrients identified in epidemiological and observational studies as acting synergistically and having beneficial effects towards brain aging: n-3 PUFAs and vitamin A, vitamin E, vitamin D, polyphenols, pre- or probiotics. We completed with benefits from dairy products that favored brain DHA incorporation.
Beneficial effects of the Mediterranean diet on memory performance during aging
The Mediterranean diet contains fish rich in n-3 LC PUFAs and plant foods (grains, vegetables, fruits, nuts and seeds, olive oil) rich in many nutrients such as vitamins and polyphenols. All together these nutrients can potentially contribute to better health and cognition.
Several longitudinal epidemiological studies have examined the relationship between consumption of the Mediterranean diet and cognitive performance. Indeed, a large panel of studies have shown that the Mediterranean diet is associated with a lower risk of cognitive decline, and development of MCI or AD [157-159]. In contrast, other studies do not find any beneficial effect of the Mediterranean diet on agerelated cognitive decline (reviewed in [160]). There are many potential reasons for such inconsistencies: the duration of the intervention, the different Mediterranean diets that are used do not always provide the same nutrients in the same amount, the lack of sensitivity of the methods used to evaluate a beneficial effect on age-related cognitive decline. Recently, a systematic review of randomized controlled trials (RCT) has confirmed evidence of a significant beneficial effect of the Mediterranean diet on cognitive functions related to global cognition, working memory, verbal and visual memory, visuospatial, language and executive function domains [161]. The benefits of the Mediterranean diet were always compared to the effect of a low-fat control diet that showed poorer cognitive performance. This means that the Mediterranean diet contains nutrients that can act synergistically to provide a powerful effect but the exact nutrients that induce such a synergistically effect are not known. However, n-3 PUFAs, vitamins and polyphenols seem to be good candidates as they have been shown to protect the brain from neuroinflammation and oxidative stress and to improve cerebral blood flow [160]. n-3 PUFAs are the only components of the Mediterranean diet which are immunomodulators so we focused first on the effects of n-3 PUFAs and on the interest to combine n-3 PUFAs, an anti-inflammatory nutrient, to antioxidants such as vitamins or polyphenols or to pre- and probiotics to increase their bioavailability.
Beneficial effects of n-3 PUFA on memory performance during aging
DHA represents the main n-3 PUFA in the brain and is involved in neurological function by modulating signal transduction pathways, neurogenesis, neuroinflammation, synaptic plasticity, membrane integrity as well as membrane organization [47].
The effects of n-3 PUFA supplementation have been assessed in humans. Many observational studies have associated dietary consumption of DHA with improved cognitive function and/or reduced cognitive decline in the elderly. Indeed, Gonzalez et al. [162] found in a cohort of 75 years old elderly residents a positive correlation between dietary consumption of DHA and mini-mental state exam (MMSE) scores, used to evaluate cognitive functions and memory abilities. A prospective observational study realized in a healthy elderly population reported that baseline dietary DHA intake levels at 70 years old is positively correlated with a better declarative memory test performance at age of 75 [163]. Furthermore, it has been shown in the elderly that high fish consumption is associated with a lower risk of developing neurological disorders [11,164]. Several epidemiological and observational studies have reported that patients with high blood levels of n-3 LC-PUFA had lower cytokine production [165,166]. A systematic meta-analysis peformed by Yurko-Mauro et al. [167] linked DHA intake and cognitive outcomes and showed a substantial improvement in episodic, working and semantic memory. More recently, an interventionnal study from McNamara et al. [168] showed in elderly with cognitive complaints that supplementation with fish oil is associated with reduced self-reported inefficiencies in everyday functioning as well as improved cognitive capability.
Evidence of beneficial effects have also been evaluated in animals. For example, aged mice fed a DHA/EPA diet display their brain accumulation that protects against neuroinflammation and cognitive impairment but also limits stress-related synaptic reduction [15,71]. Other studies have shown that DHA administration in old rodents improves spatial cognition ability, learning ability and memory [169,170].
DHA has been shown to promote neurogenesis, neural plasticity and LTP by increasing BDNF that affect the AkT and MAPK/ERK/ CREB signaling pathways [171,172]. n-3 PUFA supplementation in rodents has been shown to increase hippocampal synaptic plasticity and improve long-term memory in aged rodents [173]. Rodents exposed to n-3 PUFAs supplementation display facilitated hippocampal LTP and positive modulations of Calcium/calmodulin-dependent kinase II (CaMKII) and NMDA receptor function which are crucial for the maintenance of LTP [174,175]. Recently, Sidhu et al. [176] have shown in aged mice that DHA ameliorates the age-related loss of brain synaptic proteins, which are important for synaptic integrity and neurotransmission, crucial for proper cognitive function. Furthermore, DHA supplementation of 25-26 months old rats reverses age-related decrease in neurogenesis as well as transcription factors involved in learning and memory by enhancing the retinoid X receptor (RXR), retinoic acid receptor (RAR) and peroxisome proliferator-activated receptors (PPARs) which may improve cognition [177]. Tokuda et al. [178] have shown in rats that DHA supplementation may increase newborn neuron production and/or survival. DHA is also involved in the reduction or the resolution of inflammation occurring during aging. Indeed, aged rodents fed a fish oil-enriched diet displayed lower blood and brain levels of pro-inflammatory cytokines (IL-1β, TNF-α and IL-6) [179-181]. Chronic low-grade inflammation developped during aging can be modulated by DHA and its metabolites via several pathways, including activation of PPARs, inhibition of nuclear factor kappa-B (NFκB), and activation of the transmembrane receptor GPR120 [182,183]. Specialized pro-resolving mediators (SPMs) derived from DHA, including protectins, D-series resolvins, and maresins, are important in the resolution of inflammation since they have antiinflammatory and pro-resolving properties as well as neuroprotective functions [20-22]. They are derived from DHA via cyclooxygenase (COX) and lipoxygenase (LOX) pathways [23]. Although DHA has antiinflammatory properties, some studies performed in aged rodents have reported beneficial effects of fish oil on oxidative stress by promoting antioxidase activity, notably SOD and catalase [184,185].
Thus, promoting the accretion of n-3 PUFAs through diet, and especially DHA, which is able to influence many signaling pathways and brain systems would be a strategy of choice in the maintenance of the homeostasis of the CNS during aging and in the prevention of agerelated cognitive decline.
Beneficial effects of vitamin A and DHA on memory performance during aging
Vitamin A plays a key role in maintaining cognitive performance during all life and alterations of its metabolism are specifically associated to cognitive decline [27,186,187].
Vitamin A acts through its active metabolite, retinoic acid (RA). During aging, vitamin A metabolism is altered resulting in a decrease in RA bioavailability, and especially in brain in the hypoactivation of the retinoid pathway in peripheral blood mononuclear cells in humans and in brain of aged animals [90,188]. This hypoactivation of the retinoid pathway is involved in the etiology of age-related cognitive decline [91]. It is associated to a decrease in the expression of proteins involved in synaptic plasticity [90] that contributes to cognitive alterations in aged animals. Actually, we showed that a deficit in vitamin A induces alteration in synaptic plasticity and cognitive deficits associated to aging [46,189].
To the contrary, RA supplementation improves learning and memory performance in aged animals by restoring the expression of retinoid receptors (RAR and RXR) that regulate the expression of genes involved in synaptic plasticity and neurogenesis in hippocampus [91]. Moreover, a 4-month vitamin A supplementation in 13-monthold rats is able to increase the intracellular availability of RA, improves the arborization of newborn immature neurons and preserves spatial memory performance in 17-month-old rats [27]. Finally, recent data have revealed an anti-inflammatory effect of RA. Indeed, in microglial cell culture, RA, through its RAR and RXR receptors, induces a sharp decrease in LPS-induced production of proinflammatory cytokines [26]. Also RA possibly acts by protecting the integrity of the blood brain barrier against inflammatory insult [190].
n-3 PUFAs and vitamin A have synergistic effects in the promotion of neurobiological processes involved in cognitive performance [191]. Indeed, RA and DHA are able to bind equally RXR [192]. Thus, a n-3 PUFA rich diet has beneficial effects on cognitive performance and regulates the expression of genes involved in synaptic plasticity in rodents through nuclear receptors common to RA [193]. Moreover, Létondor et al. [191] highlighted for the first time a combined effect of a diet enriched in n-3 LC-PUFAs (0.7% EPA+0.7% DHA) and vitamin A (45IU/g of diet) on the age-relative cognitive decline, especially on reference memory performance. They showed that this beneficial synergic effect on memory could be in part mediated by RXR signaling pathway.
Beneficial effects of vitamin E and DHA on memory performance during aging
Vitamin E was first discovered by Evans and Bishop [194] and refers to a group of compounds that include both tocopherols and tocotrienols. Alpha-tocopherol is the main source in European diet (found in olive and sunflower oils) while gamma-tocopherol is the main source in American diet (found in soybean and corn oils) [195,196]. Tocopherols are radical scavengers and vitamin E has been well characterized as a powerful lipid-soluble antioxidant through its ability to scavenge ROS in cellular membranes [197].
In the aging brain, as well as in the case of several neurodegenerative diseases, there is a decline in the normal antioxidant defense mechanisms, which increases the vulnerability of the brain to the deleterious effects of oxidative damage [198]. Vitamin E administered to rodents can improve age-related impairments and improve cognitive behaviors [199]. The prospective epidemiological studies on dietary vitamin E effects consistently show statistically significant inverse associations with incident dementia and AD, and with cognitive decline [200].
Very few studies evaluate the synergistic effect between vitamin E and DHA in cognitive decline. A recent study demonstrates that in naturally aged rats, n-3 PUFAs and vitamin E supplementation improves the redox balance, lipid profile and liver function parameters. The antioxidant parameters are improved with a concomitant reduction in the lipid peroxidation products. Even if this study does not investigate the impact on cognitive function, the authors strongly suggest that dietary supplementation with n-3 PUFAs and α-tocopherol may reduce the pathological consequences of aging [201]. Nevertheless, a clinical study combining DHA and vitamin E (252 mg DHA, 60 mg EPA and 10 mg vitamin E) does not reveal an effect of this supplementation on cognitive function [202]. These results are consistent with data demonstrating a smaller increase in plasma concentrations in vitamin E in the presence of fish oil and an immuno-enhancing effect of vitamin E in the elderly dampened when concomitantly consumed with fish oil [203]. By the way, further studies are necessary to determine real beneficial effects of vitamin E and DHA on cognitive decline and if a better antioxidant can be supplemented with n-3 PUFAs.
Beneficial effects of vitamin D and DHA on memory performance during aging
Vitamin D is a fat-soluble vitamin that is naturally present in very few foods, added to others, and available as a dietary supplement. It is also produced endogenously when ultraviolet rays from sunlight strike the skin and trigger vitamin D synthesis. Vitamin D obtained from sun exposure, food, and supplements is biologically inert and must undergo two hydroxylations in the body for activation. The first occurs in the liver and converts vitamin D to 25-hydroxyvitamin D, also known as calcidiol and considered as a stable marker of vitamin D status. The second occurs primarily in the kidney and forms the physiologically active 1,25-dihydroxyvitamin D, also known as calcitriol [204].
Vitamin D plays a key role in the development and maintenance of the central nervous system [29,205]. Moreover, vitamin D is activated in neuronal and glial cells [206] and modulates the expression of neurotrophins for brain function [207]. Human studies reviewed in Tucker [29] conclude that vitamin D protects against age-related cognitive decline. Low concentrations of vitamin D are associated with significant cognitive decline. Vitamin D is important in elderly because the lack of vitamin D is prevalent in this population. Moreover, the activation of vitamin D is lower in older adults.
It was shown that vitamin D suppresses the inflammatory response in activated immune cells [26]. Vitamin D acts through the inhibition of nitrite oxide release from activated immune cells via interaction with vitamin D receptor [208], or through the inhibition of NFkB activity [209]. It has been shown that the relative risk of cognitive decline was 60% higher in elderly adults with severely deficiency in 25-hydroxy vitamin D when compared with those with sufficient levels [210]. The interaction between n-3 PUFA and vitamin D is still poorly investigated, especially in the elderly population. Itariu et al. [211] interestingly found that the inverse association between vitamin D deficiency and systemic inflammation is overcome by treatment with n-3 PUFA. These results allow to hypothesize that the mechanisms by which vitamin D and n-3 PUFA influence inflammation are strictly interconnected, and that the correction of a single nutritional deficiency may be sufficient to limit the negative effects of the other. A recent clinical trial on Chinese population suggests that lower 25-hydroxy vitamin D and DHA levels are risk factors for mild cognitive impairment, a syndrome that defined a cognitive decline greater than expected for an individual’s age and education level [212]. Recently, Kurtys et al. [26] study the combination of vitamin D, vitamin A and n-3 LC-PUFAs and they find an interesting combined effect of these nutrients at concentrations where they individually had little effect. This nutrient mix significantly reduced inflammation by increasing anti-inflammatory efficacy.
Beneficial effects of polyphenols and DHA on memory performance during aging
Polyphenols are phytochemicals now considered as essential micronutrients. Polyphenols can be divided into four main classes based on their structure: flavonoids, phenolic acids, stilbenes and lignans [213]. More and more evidences demonstrate the ability of dietary polyphenols to exert beneficial effects on brain aging. Polyphenol-rich fruits (e.g. blueberry, strawberry, concord grapes...) are now highly studied for their potential beneficial effects on memory [214,215].
Clinical studies have also observed an improvement of memory in older people with a supplementation with grape juice or with blueberry juice rich in flavonoids [216,217]. Moreover, flavonoid consumption has been associated with better cognitive performance in an epidemiological study over 10 years [218]. Although the mechanisms of action of flavonoids remain unclear, there is evidence that they modulate cellular and molecular processes by initiating neurogenesis and reducing neuronal damage, neurotoxicity and neuroinflammation [219].
In rodents, first evidence demonstrates their ability to reverse or allay age-related deficits in cognitive tasks, including those that require the use of spatial learning and memory in rodent [220-222]. Within the flavonoid class, anthocyanins, present in red berries as in blueberries, have been shown to prevent memory deficits in aged animals [220,223,224]. Recently, a polyphenol-rich diet from grape and blueberry with high contents of flavonoids, stilbenes and phenolic acid was successful to reverse age-induced effects in mice. This supplementation slightly improves memory of middle-aged mice by facilitating the use of spatial strategies. Moreover, this performance improvement can be linked to the restoration of CaMKII mRNA levels and to an increased hippocampal neurotrophic factor NGF expression [221].
Given such evidence of neurocognitive benefits in the context of aging, and the importance of developing interventions that are efficient and compatible with long-term adherence in advance of pathological cognitive decline, it appears necessary to explore the synergistic effect of DHA and polyphenols.
Preclinical studies demonstrate that intestinal uptake and blood concentrations of n-3 PUFAs are increased when co-administered with flavonoids [225,226]. Moreover, Hadad et al. [227] establish that concentrations of EPA with carotenoids and polyphenols are synergistically very efficient in inhibiting the transformation of microglia to M1 activated phenotype. Microglia activation toward the M1 phenotype has been reported to contribute to the neurodegenerative processes and cognition alterations due to the release of proinflammatory mediators and cytokines [228]. This result comforts the potential synergistic effect of DHA and polyphenols to prevent cognitive deficits. In addition to their potential role in neuroinflammation, the antioxidant property of flavonoids may prevent n-3 LC-PUFA from peroxidation to which they are particularly sensitive due to their multiple double bonds [229] and further supports a synergistic effect of both food compounds.
To our knowledge, only one study in humans evaluated the effect of blueberry and fish oil on cognitive functions but no synergistic effect has been observed, whereas fish oil group or blueberry group individually displayed fewer cognitive symptoms [168].
In animals, very few studies investigate the impact of combined flavonoids and fish oil on brain aging. Giunta et al. [230] demonstrate a significantly greater anti-amyloidogenic effect of fish oil and flavonoids, relative to each component fed separately in AD transgenic mice. Perez et al. [4] studied that combination of naturally occurring micronutrients α-tocopherol, citicholine, 5-methyltetrahydrofolic acid (5-MTHF), quercetin and the n-3 fatty acid containing PS-DHA reliably rejuvenates cognitive performance in aged mice, both males and females. The administration of these micronutrients alone or in simpler diets was not sufficient to induce a positive effect, revealing the importance of a synergistic effect. Results highlight the importance of including the flavonoid quercetin, a strong antioxidant and antiinflammatory agent [231,232] in the combination. But in this study, the addition of vitamins and minerals could increase the bioavailability of quercetin, which facilitates learning.
Beneficial effects of pre- or probiotics and DHA on memory performance during aging
The microbiota is a dynamic ecosystem and recent evidence demonstrates a clear association between changes in the microbiota and cognitive behaviors, notably learning and memory [233]. Probiotics are live microorganisms that are claimed to provide health benefits when consumed [234]. Probiotics have been the subject of various researches and appears to modulate notably inflammation [235]. However, data on the effects of probiotics on improving cognitive disorders in aging are scarce. Akbari et al. [236] determine that supplementation with probiotics (Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum (2×109 CFU/g each)) for 12 weeks positively affects cognitive function in AD patients. Interestingly the probiotic supplementation differently influences the lipid profiles with a substantial decrease of the triglyceride level in the probiotic-supplemented patients but the authors do not investigate DHA levels. Other recent studies also demonstrate a role of probiotic supplementation in cognitively impaired patients mediated by inflammation. Cattaneo et al. [237] demonstrate a link between stool abundance in Faecalibacterium prausnitzii and pro and antiinflammatory cytokines in patients with cognitive alteration. Leblhuber et al. [238] also evidence the impact of microbiota composition on lowgrade systemic and intestinal inflammation. All these findings together may indicate changes in the microbiota-gut-brain-axis correlated to neuroinflammation during cognitive decline.
Concerning interaction between DHA and probiotics, literature is rare. A recent RCT in an overweight Indian population has shown that supplementation with n-3 PUFAs plus a probiotic has a greater beneficial effect on insulin sensitivity, lipid profile, and atherogenic index than the probiotic alone, although n-3 PUFA supplementation shows only marginal effects on all these parameters [239].
Joffre et al. [31] demonstrate in rodents that a supplementation with Lactobacillus fermentum might have a key role in the protection of the brain via the brain accretion of n-3 PUFAs especially during n-3 PUFA deficiency (as occurred during aging).
Considering all these results, investigating the synergistic effect between DHA and probiotics appears to be a promising way to prevent age-related cognitive decline linked to age.
‘Prebiotic’ is a term used to describe a food component that may provide a health benefit when eaten because of changes it may bring about to the gut bacterial flora. Only one study investigated the role of prebiotics and DHA in cognitive impairment during aging but in a model of Parkinson Disease (PD). Interestingly, this study demonstrates that a diet containing specific prebiotic fibers such as fructo-oligosaccharides and galacto-oligosaccharides, DHA (0.75/100 g diet) and EPA (0.50/100 g diet) and other nutrients increases spatial recognition after a rotenone (a pesticide inducing PD in rodent model) injection compared to animals fed with a diet without prebiotics. This combination of nutrients could act synergistically to increase the synthesis of synaptic membranes [240] and could improve both neuronal connectivity and behavioral output [241].
Beneficial effects of dairy products on memory performance during aging
Dairy products play a key role in human diet because they contain many nutrients: proteins, minerals, vitamins…. However, during the last decades, they were criticized and accused to enhance cardiovascular diseases. Actually, dairy fat has been reconsidered [242]. Dairy fat contains about 400 fatty acids (2/3 of saturated fatty acids in milk). Among the saturated fatty acids, 16% are short or medium chain fatty acids (<C14) that have a biological role [243]. During the perinatal period, these fatty acids are specifically oriented toward energy suppliers and are beta-oxidized. They may thereby spare ALA, one of the best beta-oxidation substrates, from oxidation, and favour ALA partitioning towards the desaturation and elongation pathways and its conversion into very LC-PUFAs [244,245]. Therefore, this protective effect favors the endogen synthesis of DHA and potentiates its biological activity. Indeed, we showed that dairy fat promotes neurogenesis and cognitive processes at adulthood [246]. Moreover, perinatal dairy fat consumption protects from deleterious effect induced by LPS on spatial memory at adulthood [246].
Dairy products contain also important quantities of vitamin A. Milk provides ¼ of the preformed vitamin A [247] and we demonstrated above that vitamin A plays a key role in maintaining cognitive performance during aging.
Few studies have been conducted in the elderly to show the association between dairy product consumption and age-related cognitive decline. Park and Fulgoni [248] showed that dairy product consumption is associated to a better cognition in subjects >60 years. The review from Camfield et al. [1] concluded that low-fat dairy products, when consumed regularly as part of a balanced diet, may have a number of beneficial outcomes for neurocognitive health during aging. A meta-analysis on 7 publications involving 10941 participants indicates an inverse association between milk consumption and the risk of cognitive disorders: the highest level of milk consumption was significantly associated with a decreased risk of cognitive disorders [32]. To our knowledge, only one study conducted in 18-months-aged rats reports a beneficial effect of the consumption of buttermilk and krill oil rich in n-3 LC-PUFAs esterified in phospholipids [249]. The authors show that the combined nutrients are able to improve peripheral insulin sensitivity and to induce a significant increase of brainderived neurotrophic factor (BDNF) in the hippocampus favoring an improvement in energy state within neurons and facilitating both mitochondrial and protein synthesis, which are necessary for synaptic plasticity highly implicated in memory process.
Conclusion
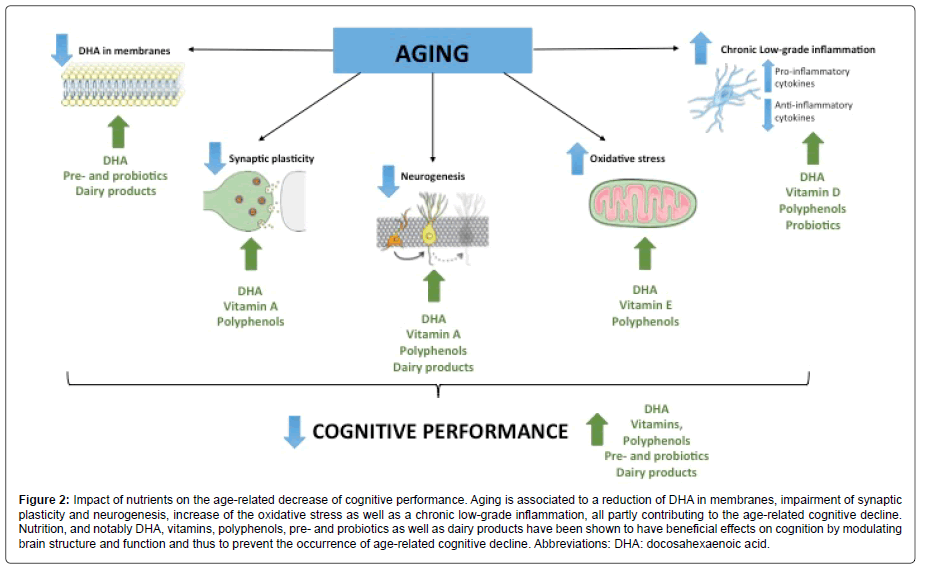
All together, these data provide new targets to prevent or delay brain aging through nutrition and demonstrate that combinations of dietary nutrients could represent an optimal way (Figure 2). The Mediterranean diet constitute the most complete association of nutrients combining LC-PUFAs, vitamins and polyphenols and having beneficial effect on age-related cognitive deficit. The consumption of DHA during aging is essential since it plays a key role for the reduction or resolution of chronic-low grade inflammation and the maintenance of brain’s plasticity. The association of DHA and vitamins is promising since it allows the promotion of neurobiological processes involved in cognitive performances by acting on synaptic plasticity and neurogenesis (vitamin A), oxidative stress (vitamin E) as well as neuroinflammation (vitamin D). Polyphenols have beneficial effect on memory during aging through their action on synaptic plasticity and neurogenesis, neuroinflammation and oxidative stress. The consumption of prebiotics and probiotics is thought to ameliorate neuroinflammation occurring during aging by enhancing the accretion of n-3 PUFAs in the brain. Dairy products seem to be promising in term of prevention of agerelated decline but still need to be studied. Thus, investigating the synergic effect between DHA and these micronutrients is a strategy of choice in the prevention of age-related cognitive decline.
Figure 2: Impact of nutrients on the age-related decrease of cognitive performance. Aging is associated to a reduction of DHA in membranes, impairment of synaptic plasticity and neurogenesis, increase of the oxidative stress as well as a chronic low-grade inflammation, all partly contributing to the age-related cognitive decline. Nutrition, and notably DHA, vitamins, polyphenols, pre- and probiotics as well as dairy products have been shown to have beneficial effects on cognition by modulating brain structure and function and thus to prevent the occurrence of age-related cognitive decline. Abbreviations: DHA: docosahexaenoic acid.
References
- Camfield DA, Owen L, Scholey AB, Pipingas A, Stough C (2011) Dairy constituents and neurocognitive health in ageing. Br J Nutr 106: 159-174.
- United Nations (2009) World population prospects: The 2008 revision, highlights, working paper, United Nations, Department of Economic and Social Affairs, New York.
- Bousquet J, Kuh D, Bewick M, Standberg T, Farrell J, et al. (2015) Operational definition of active and healthy ageing (AHA): A conceptual framework. J Nutr Health Aging 19: 955-960.
- Perez SD, Du K, Rendeiro C, Wang L, Wu Q, et al. (2017) A unique combination of micronutrients rejuvenates cognitive performance in aged mice. Behav Brain Res 320: 97-112.
- Erickson CA, Barnes CA (2003) The neurobiology of memory changes in normal aging. Exp Gerontol 38: 61-69.
- Mishra S, Palanivelu K (2008) The effect of curcumin (turmeric) on Alzheimer's disease: An overview. Ann Indian Acad Neurol 11: 13-19.
- Niu H, Ãlvarez-Ãlvarez I, Guillén-Grima F, Aguinaga-Ontoso I (2017) Prevalence and incidence of Alzheimer's disease in Europe: A meta-analysis. Neurologia 32: 523-532.
- Tabata K (2005) Population aging, the costs of health care for the elderly and growth. Journal of Macroeconomics 27: 472-493.
- Kelly L, Grehan B, Chiesa AD, O'Mara SM, Downer E, et al. (2011) The polyunsaturated fatty acids, EPA and DPA exert a protective effect in the hippocampus of the aged rat. Neurobiol Aging 32: 2318.
- Vauzour D, Camprubi-Robles M, Miquel-Kergoat S, Andres-Lacueva C, Bánáti D, et al. (2017) Nutrition for the ageing brain: Towards evidence for an optimal diet. Ageing Res Rev 35: 222-240.
- Allès B, Samieri C (2016) Nutrient patterns and their food sources in older persons from france and quebec: Dietary and lifestyle characteristics. Nutrients 8: 225.
- Ferry M, Roussel AM (2011) Micronutrient status and cognitive decline in ageing. European Geriatric Medicine 2: 15-21.
- Willis LM, Shukitt-Hale B, Joseph JA (2009) Modulation of cognition and behavior in aged animals: Role for antioxidant- and essential fatty acid-rich plant foods. Am J Clin Nutr 89: 1602S-1606S.
- Dyall SC, Michael GJ, Whelpton R, Scott AG, Michael-Titus AT (2007) Dietary enrichment with omega-3 polyunsaturated fatty acids reverses age-related decreases in the GluR2 and NR2B glutamate receptor subunits in rat forebrain. Neurobiol Aging 28: 424-439.
- Labrousse VF, Nadjar A, Joffre C, Costes L, Aubert A, et al. (2012) Short-term long chain omega3 diet protects from neuroinflammatory processes and memory impairment in aged mice. PLoS One 7: e36861.
- Barceló-Coblijn G, Högyes E, Kitajka K, Puskás LG, Zvara A, et al. (2003) Modification by docosahexaenoic acid of age-induced alterations in gene expression and molecular composition of rat brain phospholipids. Proc Natl Acad Sci U S A 100: 11321-11326.
- Latour A, Grintal B, Champeil-Potokar G, Hennebelle M, Lavialle M, et al. (2013) Omega-3 fatty acids deficiency aggravates glutamatergic synapse and astroglial aging in the rat hippocampal CA1. Aging Cell 12: 76-84.
- Little SJ, Lynch MA, Manku M, Nicolaou A (2007) Docosahexaenoic acid-induced changes in phospholipids in cortex of young and aged rats: A lipidomic analysis. Prostaglandins Leukot Essent Fatty Acids 77: 155-162.
- Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, et al. (2018) Age and age-related diseases: Role of inflammation triggers and cytokines. Front Immunol 9: 586.
- Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, et al. (2005) A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest 115: 2774-2783.
- Orr SK, Bazinet RP (2008) The emerging role of docosahexaenoic acid in neuroinflammation. Curr Opin Investig Drugs 9: 735-743.
- Rey C, Nadjar A, Buaud B, Vaysse C, Aubert A, et al. (2016) Resolvin D1 and E1 promote resolution of inflammation in microglial cells in vitro. Brain Behav Immun 55: 249-259.
- Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N (2015) Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta 1851: 397-413.
- Layé S, Nadjar A, Joffre C, Bazinet RP (2018) Anti-inflammatory effects of omega-3 fatty acids in the brain: Physiological mechanisms and relevance to pharmacology. Pharmacol Rev 70: 12-38.
- Bourre JM (2006) Effects of nutrients (in food) on the structure and function of the nervous system: Update on dietary requirements for brain. Part 1: Micronutrients. J Nutr Health Aging 10: 377-385.
- Kurtys E, Eisel ULM, Verkuyl JM, Broersen LM, Dierckx RJO, et al. (2016) The combination of vitamins and omega-3 fatty acids has an enhanced anti-inflammatory effect on microglia. Neurochem Int 99: 206-214.
- Touyarot K, Bonhomme D, Roux P, Alfos S, Lafenêtre P, et al. (2013) A mid-life vitamin A supplementation prevents age-related spatial memory deficits and hippocampal neurogenesis alterations through CRABP-I. PLoS One 8: e72101.
- Bonhomme D, Pallet V, Dominguez G, Servant L, Henkous N, et al. (2014) Retinoic acid modulates intrahippocampal levels of corticosterone in middle-aged mice: Consequences on hippocampal plasticity and contextual memory. Front Aging Neurosci 6: 6.
- Tucker KL (2016) Nutrient intake, nutritional status, and cognitive function with aging. Ann N Y Acad Sci 1367: 38-49.
- Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, et al. (2010) The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res 51: 1101-1112.
- Joffre C, Dinel AL, Aubert A, Fressange-Mazda C, Le Ruyet P, et al. (2016) Impact of lactobacillus fermentum and dairy lipids in the maternal diet on the fatty acid composition of pups' brain and peripheral tissues. Prostaglandins Leukot Essent Fatty Acids 115: 24-34.
- Wu L, Sun D (2016) Meta-analysis of milk consumption and the risk of cognitive disorders. Nutrients 8: 824.
- Ozawa M, Ohara T, Ninomiya T, Hata J, Yoshida D, et al. (2014) Milk and dairy consumption and risk of dementia in an elderly Japanese population: The hisayama study. J Am Geriatr Soc 62: 1224-1230.
- Vercambre MN, Boutron-Ruault MC, Ritchie K, Clavel-Chapelon F, Berr C (2009) Long-term association of food and nutrient intakes with cognitive and functional decline: A 13-year follow-up study of elderly french women. Br J Nutr 102: 419-427.
- Salthouse T (2010) Major Issues in Cognitive Aging, Oxford University Press, Oxford, New York.
- Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, et al. (2009) Age-associated cognitive decline. Br Med Bull 92: 135-152.
- Gallagher M, Rapp PR (1997) The use of animal models to study the effects of aging on cognition. Annu Rev Psychol 48: 339-370.
- Gazova I, Laczó J, Rubinova E, Mokrisova I, Hyncicova E, et al. (2013) Spatial navigation in young versus older adults. Front Aging Neurosci 5: 94.
- Barnes CA (1979) Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J Comp Physiol Psychol 93: 74-104.
- Markowska AL, Stone WS, Ingram DK, Reynolds J, Gold PE, et al. (1989) Individual differences in aging: Behavioral and neurobiological correlates. Neurobiol Aging 10: 31-43.
- Park DC, Reuter-Lorenz P (2009) The adaptive brain: Aging and neurocognitive scaffolding. Annu Rev Psychol 60: 173-196.
- Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L (2012) Memory aging and brain maintenance. Trends Cogn Sci 16: 292-305.
- Stoelzel CR, Stavnezer AJ, Denenberg VH, Ward M, Markus EJ (2002) The effects of aging and dorsal hippocampal lesions: Performance on spatial and nonspatial comparable versions of the water maze. Neurobiol Learn Mem 78: 217-233.
- Vandesquille M, Krazem A, Louis C, Lestage P, Béracochéa D (2011) S 18986 reverses spatial working memory impairments in aged mice: Comparison with memantine. Psychopharmacology (Berl.) 215: 709-720.
- Rosenzweig ES, Barnes CA (2003) Impact of aging on hippocampal function: Plasticity, network dynamics, and cognition. Prog Neurobiol. 69: 143-179.
- Etchamendy N, Desmedt A, Cortes-Torrea C, Marighetto A, Jaffard R (2003) Hippocampal lesions and discrimination performance of mice in the radial maze: Sparing or impairment depending on the representational demands of the task. Hippocampus 13: 197-211.
- Bazinet RP, Layé S (2014) Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci 15: 771-785.
- Joffre C, Grégoire S, De Smedt V, Acar N, Bretillon L, et al. (2016) Modulation of brain PUFA content in different experimental models of mice. Prostaglandins Leukot Essent Fatty Acids 114: 1-10.
- Sprecher H (2000) Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim Biophys Acta 1486: 219-231.
- Söderberg M, Edlund C, Kristensson K, Dallner G (1991) Fatty acid composition of brain phospholipids in aging and in Alzheimer's disease. Lipids 26: 421-425.
- McNamara RK, Able J, Jandacek R, Rider T, Tso P (2009) Inbred C57BL/6J and DBA/2J mouse strains exhibit constitutive differences in regional brain fatty acid composition. Lipids 44: 1-8.
- McNamara RK, Liu Y, Jandacek R, Rider T, Tso P (2008) The aging human orbitofrontal cortex: Decreasing polyunsaturated fatty acid composition and associated increases in lipogenic gene expression and stearoyl-CoA desaturase activity. Prostaglandins Leukot Essent Fatty Acids 78: 293-304.
- Suzuki H, Hayakawa S, Wada S (1989) Effect of age on the modification of brain polyunsaturated fatty acids and enzyme activities by fish oil diet in rats. Mech Ageing Dev 50: 17-25.
- Favrelère S, Stadelmann-Ingrand S, Huguet F, De Javel D, Piriou A, et al. (2000) Age-related changes in ethanolamine glycerophospholipid fatty acid levels in rat frontal cortex and hippocampus. Neurobiol Aging 21: 653-660.
- Favrelière S, Perault MC, Huguet F, De Javel D, Bertrand N, et al. (2003) DHA-enriched phospholipid diets modulate age-related alterations in rat hippocampus. Neurobiol Aging 24: 233-243.
- Arranz L, Naudà A, De la Fuente M, Pamplona R (2013) Exceptionally old mice are highly resistant to lipoxidation-derived molecular damage. Age (Dordr) 35: 621-635.
- Moranis A, Delpech JC, De Smedt-Peyrusse V, Aubert A, Guesnet P, et al. (2012) Long term adequate n-3 polyunsaturated fatty acid diet protects from depressive-like behavior but not from working memory disruption and brain cytokine expression in aged mice. Brain Behav Immun 26: 721-731.
- Delion S, Chalon S, Guilloteau D, Besnard JC, Durand G (1996) Alpha-Linolenic acid dietary deficiency alters age-related changes of dopaminergic and serotoninergic neurotransmission in the rat frontal cortex. J Neurochem 66: 1582-1591.
- Ilincheta de Boschero MG, Roque ME, Salvador GA, Giusto NM (2000) Alternative pathways for phospholipid synthesis in different brain areas during aging. Exp Gerontol 35: 653-668.
- Drozdowski L, Thomson AB (2006) Aging and the intestine. World J Gastroenterol 12: 7578-7584.
- Gao F, Taha AY, Ma K, Chang L, Kiesewetter D, et al. (2013) Aging decreases rate of docosahexaenoic acid synthesis-secretion from circulating unesterified a-linolenic acid by rat liver. Age (Dordr) 35: 597-608.
- Cini M, Moretti A (1995) Studies on lipid peroxidation and protein oxidation in the aging brain. Neurobiol Aging 16: 53-57.
- Dei R, Takeda A, Niwa H, Li M, Nakagomi Y, et al. (2002) Lipid peroxidation and advanced glycation end products in the brain in normal aging and in Alzheimer's disease. Acta Neuropathol 104: 113-122.
- Lemaitre RN, Tanaka T, Tang W, Manichaikul A, Foy M, et al. (2011) Genetic loci associated with plasma phospholipid n-3 fatty acids: A meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS Genet 7: e1002193.
- Heude B, Ducimetière P, Berr C; EVA Study (2003) Cognitive decline and fatty acid composition of erythrocyte membranes--The EVA Study. Am J Clin Nutr 77: 803-808.
- Solfrizzi V, Colacicco AM, D’Introno A, Capurso C, Torres F, et al. (2006) Dietary intake of unsaturated fatty acids and age-related cognitive decline: A 8.5-year follow-up of the italian longitudinal study on aging. Neurobiol Aging 27: 1694-1704.
- Whalley LJ, Fox HC, Wahle KW, Starr JM, Deary IJ (2004) Cognitive aging, childhood intelligence, and the use of food supplements: possible involvement of n-3 fatty acids. Am J Clin Nutr 80: 1650-1657.
- Barberger-Gateau P (2009) Association between mediterranean diet and late-life cognition. JAMA 302: 2433.
- Tan ZS, Harris WS, Beiser AS, Au R, Himali JJ, et al. (2012) Red blood cell ω-3 fatty acid levels and markers of accelerated brain aging. Neurology 78: 658-664.
- Catalan J, Moriguchi T, Slotnick B, Murthy M, Greiner RS, et al. (2002) Cognitive deficits in docosahexaenoic acid-deficient rats. Behav Neurosci 116: 1022-1031.
- Larrieu T, Hilal ML, Fourrier C, De Smedt-Peyrusse V, Sans N, et al. (2014) Nutritional omega-3 modulates neuronal morphology in the prefrontal cortex along with depression-related behaviour through corticosterone secretion. Transl Psychiatry 4: e437.
- Delpech JC, Thomazeau A, Madore C, Bosch-Bouju C, Larrieu T, et al. (2015) Dietary n-3 PUFAs deficiency increases vulnerability to inflammation-induced spatial memory impairment. Neuropsychopharmacology 40: 2774-2787.
- Suzuki H, Manabe S, Wada O, Crawford MA (1997) Rapid incorporation of docosahexaenoic acid from dietary sources into brain microsomal, synaptosomal and mitochondrial membranes in adult mice. Int J Vitam Nutr Res 67: 272-278.
- Citri A, Malenka RC (2008) Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology 33: 18-41.
- Bliss TV, Lomo T (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232: 331-356.
- Stuchlik A (2014) Dynamic learning and memory, synaptic plasticity and neurogenesis: An update. Front Behav Neurosci 8: 106.
- Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, et al. (2007) Changes in the structural complexity of the aged brain. Aging Cell 6: 275-284.
- Samson RD, Barnes CA (2013) Impact of aging brain circuits on cognition. Eur J Neurosci 37: 1903-1915.
- Barnes CA, McNaughton BL (1985) An age comparison of the rates of acquisition and forgetting of spatial information in relation to long-term enhancement of hippocampal synapses. Behav Neurosci 99: 1040-1048.
- Lister JP, Barnes CA (2009) Neurobiological changes in the hippocampus during normative aging. Arch Neurol 66: 829-833.
- Barnes CA, McNaughton BL (1980) Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol 309: 473-485.
- Burke SN, Barnes CA (2006) Neural plasticity in the ageing brain. Nat Rev Neurosci 7: 30-40.
- Wang R, Tang Y, Feng B, Ye C, Fang L, et al. (2007) Changes in hippocampal synapses and learning-memory abilities in age-increasing rats and effects of tetrahydroxystilbene glucoside in aged rats. Neuroscience 149: 739-746.
- Gureviciene I, Gurevicius K, Tanila H (2009) Aging and alpha-synuclein affect synaptic plasticity in the dentate gyrus. J Neural Transm (Vienna) 116: 13-22.
- Hatanpää K, Isaacs KR, Shirao T, Brady DR, Rapoport SI (1999) Loss of proteins regulating synaptic plasticity in normal aging of the human brain and in Alzheimer disease. J Neuropathol Exp Neurol 58: 637-643.
- von Bohlen Und Halbach O (2007) Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res 329: 409-420.
- Bondareff W, Geinisman Y (1976) Loss of synapses in the dentate gyrus of the senescent rat. Am J Anat 145: 129-136.
- Geinisman Y, Detoledo-Morrell L, Morrell F, Heller RE (1995) Hippocampal markers of age-related memory dysfunction: Behavioral, electrophysiological and morphological perspectives. Prog Neurobiol 45: 223-252.
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, et al. (2003) Gene microarrays in hippocampal aging: Statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci 23: 3807-3819.
- Féart C, Mingaud F, Enderlin V, Husson M, Alfos S, et al. (2005) Differential effect of retinoic acid and triiodothyronine on the age-related hypo-expression of neurogranin in rat. Neurobiol Aging 26: 729-738.
- Etchamendy N, Enderlin V, Marighetto A, Vouimba RM, Pallet V, et al. (2001) Alleviation of a selective age-related relational memory deficit in mice by pharmacologically induced normalization of brain retinoid signaling. J Neurosci 21: 6423-6429.
- Boucheron C, Alfos S, Enderlin V, Husson M, Pallet V, et al. (2006) Age-related effects of ethanol consumption on triiodothyronine and retinoic acid nuclear receptors, neurogranin and neuromodulin expression levels in mouse brain. Neurobiol Aging 27: 1326-1334.
- Foster TC, Norris CM (1997) Age-associated changes in Ca(2+)-dependent processes: Relation to hippocampal synaptic plasticity. Hippocampus 7: 602-612.
- Magnusson KR, Scruggs B, Zhao X, Hammersmark R (2007) Age-related declines in a two-day reference memory task are associated with changes in NMDA receptor subunits in mice. BMC Neurosci 8: 43.
- Weeber EJ, Sweatt JD (2002) Molecular neurobiology of human cognition. Neuron 33: 845-848.
- Simonyi A, Murch K, Sun GY (2003) Extracellular signal-regulated kinase 2 mRNA expression in the rat brain during aging. Neurochem Res 28: 1375-1378.
- Porte NB, Buhot MC, Mons N (2008) Alteration of CREB phosphorylation and spatial memory deficits in aged 129T2/Sv mice. Neurobiol Aging 29: 1533-1546.
- Yuan TF, Gu S, Shan C, Marchado S, Arias Carrión O (2015) Oxidative stress and adult neurogenesis. Stem Cell Rev 11: 706-709.
- Aimone JB, Deng W, Gage FH (2011) Resolving new memories: A critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron 70: 589-596.
- Christian KM, Song H, Ming GL (2014) Functions and dysfunctions of adult hippocampal neurogenesis. Annu Rev Neurosci 37: 243-262.
- Abrous DN, Koehl M, Le Moal M (2005) Adult neurogenesis: From precursors to network and physiology. Physiol Rev 85: 523-569.
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, et al. (2008) Spatial relational memory requires hippocampal adult neurogenesis. PLoS One 3: e1959.
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, et al. (2008) Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci 11: 901-907.
- Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK (2005) Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci 21: 464-476.
- Pencea V, Bingaman KD, Freedman LJ, Luskin MB (2001) Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp Neurol 172: 1-16.
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, et al. (2010) Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci 30: 5368-5375.
-  Rex CS, Lauterborn JC, Lin CY, Kramár EA, Rogers GA, et al. (2006) Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol 96: 677-685.
- Calabrese F, Guidotti G, Racagni G, Riva MA (2013) Reduced neuroplasticity in aged rats: A role for the neurotrophin brain-derived neurotrophic factor. Neurobiol Aging 34: 2768-2776.
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, et al. (2011) The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477: 90-94.
- Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S (2017) Inflammaging and 'Garb-aging'. Trends Endocrinol Metab 28: 199-212.
- Spittau B (2017) Aging microglia-phenotypes, functions and implications for age-related neurodegenerative diseases. Front Aging Neurosci 9: 194.
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, et al. (2015) Neuroinflammation in Alzheimer's disease. Lancet Neurol 14: 388-405.
- Streit WJ (2006) Microglial senescence: Does the brain's immune system have an expiration date? Trends Neurosci 29: 506-510.
- Davies DS, Ma J, Jegathees T (2017) Microglia show altered morphology and reduced arborization in human brain during aging and Alzheimer's disease. Brain Pathol 27: 795-808.
- Hefendehl JK, Neher JJ, Sühs RB, Kohsaka S, Skodras A, et al. (2014) Homeostatic and injury-induced microglia behavior in the aging brain. Aging Cell 13: 60-69.
- Â Ogura K, Ogawa M, Yoshida M (1994) Effects of ageing on microglia in the normal rat brain: Immunohistochemical observations. Neuroreport 5: 1224-1226.
- Perry VH, Matyszak MK, Fearn S (1993) Altered antigen expression of microglia in the aged rodent CNS. Glia 7: 60-67.
- Perry VH, Holmes C (2014) Microglial priming in neurodegenerative disease. Nat Rev Neurol 10: 217-224.
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K (2007) Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 55: 412-424.
- Eichhoff G, Busche MA, Garaschuk O (2008) In vivo calcium imaging of the aging and diseased brain. Eur J Nucl Med Mol Imaging 35 Suppl 1: S99-106.
- Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, et al. (2016) Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci 19: 995-998.
- von Bernhardi R, Tichauer JE, EugenÃn J (2010) Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J Neurochem 112: 1099-1114.
- Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, et al. (2012) Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: A microarray study. J Neuroinflammation 9: 179.
- Ye SM, Johnson RW (2001) An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation 9: 183-192.
- Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, et al. (2016) Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res Rev 31: 1-8.
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, et al. (1999) Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 47: 639-646.
- Rozovsky I, Finch CE, Morgan TE (1998) Age-related activation of microglia and astrocytes: In vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiol Aging 19: 97-103.
- Ye SM, Johnson RW (1999) Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol 93: 139-148.
- Yu WH, Go L, Guinn BA, Fraser PE, Westaway D, et al. (2002) Phenotypic and functional changes in glial cells as a function of age. Neurobiol Aging 23: 105-115.
- Ye SM, Johnson RW (2001) Regulation of interleukin-6 gene expression in brain of aged mice by nuclear factor kappaB. J Neuroimmunol 117: 87-96.
- Rafnsson SB, Deary IJ, Smith FB, Whiteman MC, Rumley A, et al. (2007) Cognitive decline and markers of inflammation and hemostasis: The Edinburgh Artery Study. J Am Geriatr Soc 55: 700-707.
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, et al. (2006) The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem 99: 1263-1272.
- VanGuilder HD, Bixler GV, Brucklacher RM, Farley JA, Yan H, et al. (2011) Concurrent hippocampal induction of MHC II pathway components and glial activation with advanced aging is not correlated with cognitive impairment. J Neuroinflammation 8: 138.
- Harry GJ (2013) Microglia during development and aging. Pharmacol Ther 139: 313-326.
- Â Sheng JG, Mrak RE, Griffin WS (1998) Enlarged and phagocytic, but not primed, interleukin-1 alpha-immunoreactive microglia increase with age in normal human brain. Acta Neuropathol 95: 229-234.
- Schram MT, Euser SM, de Craen AJ, Witteman JC, Frölich M, et al. (2007) Systemic markers of inflammation and cognitive decline in old age. J Am Geriatr Soc 55: 708-716.
- Buchanan JB, Sparkman NL, Chen J, Johnson RW (2008) Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology 33: 755-765.
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, et al. (2009) Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun 23: 46-54.
- Braida D, Sacerdote P, Panerai AE, Bianchi M, Aloisi AM, et al. (2004) Cognitive function in young and adult IL (interleukin)-6 deficient mice. Behav Brain Res 153: 423-429.
- Sparkman NL, Buchanan JB, Heyen JRR, Chen J, Beverly JL, et al. (2006) Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci 26: 10709-10716.
- Â Harman D (1956) Aging: A theory based on free radical and radiation chemistry. J Gerontol 11: 298-300.
- Harman D (1972) The biologic clock: The mitochondria? J Am Geriatr Soc 20: 145-147.
- Poddar J, Pradhan M, Ganguly G, Chakrabarti S (2018) Biochemical deficits and cognitive decline in brain aging: Intervention by dietary supplements. J Chem Neuroanat .
- Finkel T (1998) Oxygen radicals and signaling. Curr Opin Cell Biol 10: 248-253.
- Chakrabarti S, Munshi S, Banerjee K, Thakurta IG, Sinha M, et al. (2011) Mitochondrial dysfunction during brain aging: Role of oxidative stress and modulation by antioxidant supplementation. Aging Dis 2: 242-256.
- Huang TT, Zou Y, Corniola R (2012) Oxidative stress and adult neurogenesis--effects of radiation and superoxide dismutase deficiency. Semin Cell Dev Biol 23: 738-744.
- Bodhinathan K, Kumar A, Foster TC (2010) Intracellular redox state alters NMDA receptor response during aging through Ca2+/calmodulin-dependent protein kinase II. J Neurosci 30: 1914-1924.
- Â Stebbings KA, Choi HW, Ravindra A, Llano DA (2016) The impact of aging, hearing loss, and body weight on mouse hippocampal redox state, measured in brain slices using fluorescence imaging. Neurobiol Aging 42: 101-109.
- Â Lynch MA (1998) Analysis of the mechanisms underlying the age-related impairment in long-term potentiation in the rat. Rev Neurosci 9: 169-201.
- Venkateshappa C, Harish G, Mahadevan A, Srinivas Bharath MM, Shankar SK (2012) Elevated oxidative stress and decreased antioxidant function in the human hippocampus and frontal cortex with increasing age: Implications for neurodegeneration in Alzheimer’s disease. Neurochem Res 37: 1601-1614.
- Nicolle MM, Gonzalez J, Sugaya K, Baskerville KA, Bryan D, et al. (2001) Signatures of hippocampal oxidative stress in aged spatial learning-impaired rodents. Neuroscience 107: 415-431.
- Mecocci P, MacGarvey U, Kaufman AE, Koontz D, Shoffner JM, et al. (1993) Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann Neurol 34: 609-616.
- Â Alabrese V, Butterfield DA, Stella AMG (2008) Aging and oxidative stress response in the CNS.
- Souza LC, Antunes MS, Filho CB, Del Fabbro L, de Gomes MG, et al. (2015) Flavonoid chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol Biochem Behav 134: 22-30.
- Wang X, Wang W, Li L, Perry G, Lee H, et al. (2014) Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochimica et Biophysica Acta (BBA) 1842: 1240-1247.
- Milan AM, Cameron-Smith D (2015) Digestion and postprandial metabolism in the elderly. Adv Food Nutr Res 76: 79-124.
- Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, et al. (2013) Mediterranean diet, cognitive function, and dementia: A systematic review. Epidemiology 24: 479-489.
- Feart C, Samieri C, Barberger-Gateau P (2015) Mediterranean diet and cognitive health: An update of available knowledge. Curr Opin Clin Nutr Metab Care 18: 51-62.
- Sofi F, Abbate R, Gensini GF, Casini A (2010) Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr 92: 1189-1196.
- Knight A, Bryan J, Wilson C, Hodgson JM, Davis CR, et al. (2016) The mediterranean diet and cognitive function among healthy older adults in a 6-month randomised controlled Trial: The medLey study. Nutrients 8.
- Radd-Vagenas S, Duffy SL, Naismith SL (2018) Effect of the Mediterranean diet on cognition and brain morphology and function: a systematic review of randomized controlled trials. Am J Clin Nutr 107: 389-404.
- González S, Huerta JM, Fernández S, Patterson AM, Lasheras C (2010) The relationship between dietary lipids and cognitive performance in an elderly population. Int J Food Sci Nutr 61: 217-225.
- Titova OE, Sjögren P, Brooks SJ, Kullberg J, Ax E, et al. (2013) Dietary intake of eicosapentaenoic and docosahexaenoic acids is linked to gray matter volume and cognitive function in elderly. Age (Dordr) 35: 1495-1505.
- Devore EE, Grodstein F, van Rooij FJ, Hofman A, Rosner B, et al. (2009) Dietary intake of fish and omega-3 fatty acids in relation to long-term dementia risk. Am J Clin Nutr 90: 170-176.
- Alfano CM, Imayama I, Neuhouser ML, Kiecolt-Glaser JK, Smith AW, et al. (2012) Fatigue, inflammation, and ω-3 and ω-6 fatty acid intake among breast cancer survivors. J Clin Oncol 30: 1280-1287.
- Farzaneh-Far R, Harris WS, Garg S, Na B, Whooley MA (2009) Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: The heart and soul study. Atherosclerosis 205: 538-543.
- Yurko-Mauro K, Alexander DD, Van Elswyk ME (2015) Docosahexaenoic acid and adult memory: A systematic review and meta-analysis. PLoS One 10: e0120391.
- McNamara RK, Kalt W, Shidler MD, McDonald J, Summer SS, et al. (2018) Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiol Aging 64: 147-156.
- Gamoh S, Hashimoto M, Hossain S, Masumura S (2001) Chronic administration of docosahexaenoic acid improves the performance of radial arm maze task in aged rats. Clin Exp Pharmacol Physiol 28: 266-270.
- Petursdottir AL, Farr SA, Morley JE, Banks WA, Skuladottir GV (2008) Effect of dietary n-3 polyunsaturated fatty acids on brain lipid fatty acid composition, learning ability, and memory of senescence-accelerated mouse. J Gerontol A Biol Sci Med Sci 63: 1153-1160.
- Boneva NB, Yamashima T (2012) New insights into “GPR40-CREB interaction in adult neurogenesis†specific for primates. Hippocampus 22: 896-905.
- Gururajan A, van den Buuse M (2014) Is the mTOR-signalling cascade disrupted in Schizophrenia? J Neurochem 129: 377-387.
- Joffre C, Nadjar A, Lebbadi M, Calon F, Laye S (2014) n-3 LCPUFA improves cognition: The young, the old and the sick. Prostaglandins Leukot Essent Fatty Acids 91: 1-20.
- Calon F, Lim GP, Morihara T, Yang F, Ubeda O, et al. (2005) Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer’s disease. Eur J Neurosci 22: 617-626.
- Connor S, Tenorio G, Clandinin MT, Sauvé Y (2012) DHA supplementation enhances high-frequency, stimulation-induced synaptic transmission in mouse hippocampus. Appl Physiol Nutr Metab 37: 880-887.
- Sidhu VK, Huang BX, Desai A, Kevala K, Kim HY (2016) Role of DHA in aging-related changes in mouse brain synaptic plasma membrane proteome. Neurobiol Aging 41: 73-85.
- Dyall SC, Michael GJ, Michael-Titus AT (2010) Omega-3 fatty acids reverse age-related decreases in nuclear receptors and increase neurogenesis in old rats. J Neurosci Res 88: 2091-2102.
- Tokuda H, Kontani M, Kawashima H, Kiso Y, Shibata H, et al. (2014) Differential effect of arachidonic acid and docosahexaenoic acid on age-related decreases in hippocampal neurogenesis. Neurosci Res 88: 58-66.
- Jia Q, Zhou HR, Shi Y, Pestka JJ (2006) Docosahexaenoic acid consumption inhibits deoxynivalenol-induced CREB/ATF1 activation and IL-6 gene transcription in mouse macrophages. J Nutr 136: 366-372.
- Orr SK, Palumbo S, Bosetti F, Mount HT, Kang JX, et al. (2013) Unesterified docosahexaenoic acid is protective in neuroinflammation. J Neurochem 127: 378-393.
- Minogue AM, Lynch AM, Loane DJ, Herron CE, Lynch MA (2007) Modulation of amyloid-beta-induced and age-associated changes in rat hippocampus by eicosapentaenoic acid. J Neurochem 103: 914-926.
- Sun GY, Simonyi A, Fritsche KL, Chuang DY, Hannink M, et al. (2017) Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot Essent Fatty Acids S0952-3278(16)30213-7.
- Wellhauser L, Belsham DD (2014) Activation of the omega-3 fatty acid receptor GPR120 mediates anti-inflammatory actions in immortalized hypothalamic neurons. J Neuroinflammation 11: 60.
- Chen J, Wei Y, Chen X, Jiao J, Zhang Y (2017) Polyunsaturated fatty acids ameliorate aging via redox-telomere-antioncogene axis. Oncotarget 8: 7301-7314.
- Dasilva G, Pazos M, GarcÃa-Egido E, Gallardo JM, RodrÃguez I, et al. (2015) Healthy effect of different proportions of marine ω-3 PUFAs EPA and DHA supplementation in wistar rats: Lipidomic biomarkers of oxidative stress and inflammation. J Nutr Biochem 26: 1385-1392.
- Bonhomme D, Minni AM, Alfos S, Roux P, Richard E, et al. (2014) Vitamin A status regulates glucocorticoid availability in Wistar rats: Consequences on cognitive functions and hippocampal neurogenesis? Front Behav Neurosci 8: 20.
- Mingaud F, Mormede C, Etchamendy N, Mons N, Niedergang B, et al. (2008) Retinoid hyposignaling contributes to aging-related decline in hippocampal function in short-term/working memory organization and long-term declarative memory encoding in mice. J Neurosci 28: 279-291.
-  Pallet V, Azaïs-Braesco V, Enderlin V, Grolier P, Noël-Suberville C, et al. (1997) Aging decreases retinoic acid and triiodothyronine nuclear expression in rat liver: exogenous retinol and retinoic acid differentially modulate this decreased expression. Mech Ageing Dev 99: 123-136.
- Bonnet E, Touyarot K, Alfos S, Pallet V, Higueret P, et al. (2008) Retinoic acid restores adult hippocampal neurogenesis and reverses spatial memory deficit in vitamin A deprived rats. PLoS One 3: e3487.
- Mizee MR, Nijland PG, van der Pol SMA, Drexhage JAR, van Het Hof B, et al. (2014) Astrocyte-derived retinoic acid: A novel regulator of blood-brain barrier function in multiple sclerosis. Acta Neuropathol 128: 691-703.
-  Létondor A, Buaud B, Vaysse C, Richard E, Layé S, et al. (2016) EPA/DHA and vitamin A supplementation improves spatial memory and alleviates the age-related decrease in hippocampal RXR? and kinase expression in rats. Front Aging Neurosci 8: 103.
- Wietrzych-Schindler M, Szyszka-Niagolov M, Ohta K, Endo Y, Pérez E, et al. (2011) Retinoid x receptor gamma is implicated in docosahexaenoic acid modulation of despair behaviors and working memory in mice. Biol Psychiatry 69: 788-794.
- McCann JC, Ames BN (2005) Is docosahexaenoic acid, an n-3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am J Clin Nutr 82: 281-295.
- Evans HM, Bishop KS (1922) On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 56: 650-651.
- Wagner KH, Kamal-Eldin A, Elmadfa I (2004) Gamma-tocopherol--an underestimated vitamin? Ann Nutr Metab 48: 169-188.
- Jiang Q, Christen S, Shigenaga MK, Ames BN (2001) gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr 74: 714-722.
- Esterbauer H, Dieber-Rotheneder M, Striegl G, Waeg G (1991) Role of vitamin E in preventing the oxidation of low-density lipoprotein. Am J Clin Nutr 53: 314S-321S.
- Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408: 239-247.
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, et al. (1999) Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci 19: 8114-8121.
- Morris MC (2012) Nutritional determinants of cognitive aging and dementia. Proc Nutr Soc 71: 1-13.
- Narayanankutty A, Kottekkat A, Mathew SE, Illam SP, Suseela IM, et al. (2017) Vitamin E supplementation modulates the biological effects of omega-3 fatty acids in naturally aged rats. Toxicol Mech Methods 27: 207-214.
- Stough C, Downey L, Silber B, Lloyd J, Kure C, et al. (2012) The effects of 90-day supplementation with the omega-3 essential fatty acid docosahexaenoic acid (DHA) on cognitive function and visual acuity in a healthy aging population. Neurobiol Aging 33: 824.e1-3.
- Wu D, Han SN, Meydani M, Meydani SN (2006) Effect of concomitant consumption of fish oil and vitamin E on T cell mediated function in the elderly: A randomized double-blind trial. J Am Coll Nutr 25: 300-306.
- Â Institute of Medicine (US) Committee to review dietary reference intakes for vitamin D and calcium (2011) dietary reference intakes for calcium and Vitamin D, National Academies Press (US), Washington (DC).
- Harms LR, Burne TH, Eyles DW, McGrath JJ (2011) Vitamin D and the brain. Best Pract Res Clin Endocrinol Metab 25: 657-669.
- Â Neveu I, Naveilhan P, Jehan F, Baudet C, Wion D, et al. (1994) 1,25-dihydroxyvitamin D3 regulates the synthesis of nerve growth factor in primary cultures of glial cells. Brain Res Mol Brain Res 24: 70-76.
- Clemens TL, Garrett KP, Zhou XY, Pike JW, Haussler MR, et al. (1988) Immunocytochemical localization of the 1,25-dihydroxyvitamin D3 receptor in target cells. Endocrinology 122: 1224-1230.
- Hur J, Lee P, Kim MJ, Cho YW (2014) Regulatory effect of 25-hydroxyvitamin D3 on nitric oxide production in activated microglia. Korean J Physiol Pharmacol 18: 397-402.
- Chen Y, Zhang J, Ge X, Du J, Deb DK, et al. (2013) Vitamin D receptor inhibits nuclear factor κB activation by interacting with IκB kinase β protein. J Biol Chem 288: 19450-19458.
- Dickens AP, Lang IA, Langa KM, Kos K, Llewellyn DJ (2011) Vitamin D, cognitive dysfunction and dementia in older adults. CNS Drugs 25: 629-639.
- Itariu BK, Zeyda M, Leitner L, Marculescu R, Stulnig TM (2013) Treatment with n-3 polyunsaturated fatty acids overcomes the inverse association of vitamin D deficiency with inflammation in severely obese patients: A randomized controlled trial. PLoS One 8: e54634.
- Yin Y, Fan Y, Lin F, Xu Y, Zhang J (2015) Nutrient biomarkers and vascular risk factors in subtypes of mild cognitive impairment: A cross-sectional study. J Nutr Health Aging 19: 39-47.
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: Food sources and bioavailability. Am J Clin Nutr 79: 727-747.
- Kean RJ, Lamport DJ, Dodd GF, Freeman JE, Williams CM, et al. (2015) Chronic consumption of flavanone-rich orange juice is associated with cognitive benefits: an 8-wk, randomized, double-blind, placebo-controlled trial in healthy older adults. Am J Clin Nutr 101: 506-514.
- Bensalem J, Dal-Pan A, Gillard E, Calon F, Pallet V (2015) Protective effects of berry polyphenols against age-related cognitive impairment. Nutrition and Aging 3: 89-106.
- Krikorian R, Shidler MD, Nash TA, Kalt W, Vinqvist-Tymchuk MR, et al. (2010) Blueberry supplementation improves memory in older adults. J Agric Food Chem 58: 3996-4000.
- Krikorian R, Boespflug EL, Fleck DE, Stein AL, Wightman JD, et al. (2012) Concord grape juice supplementation and neurocognitive function in human aging. J Agric Food Chem 60: 5736-5742.
- Letenneur L, Proust-Lima C, Le Gouge A, Dartigues JF, Barberger-Gateau P (2007) Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol 165: 1364-1371.
- Spencer JPE (2009) Flavonoids and brain health: Multiple effects underpinned by common mechanisms. Genes Nutr 4: 243-250.
- Shukitt-Hale B, Carey A, Simon L, Mark DA, Joseph JA (2006) Effects of concord grape juice on cognitive and motor deficits in aging. Nutrition 22: 295-302.
- Bensalem J, Servant L, Alfos S, Gaudout D, Layé S, et al. (2016) Dietary polyphenol supplementation prevents alterations of spatial navigation in middle-aged mice. Front Behav Neurosci 10: 9.
- Malin DH, Lee DR, Goyarzu P, Chang YH, Ennis LJ, et al. (2011) Short-term blueberry-enriched diet prevents and reverses object recognition memory loss in aging rats. Nutrition 27: 338-342.
- Cho J, Kang JS, Long PH, Jing J, Back Y, et al. (2003) Antioxidant and memory enhancing effects of purple sweet potato anthocyanin and cordyceps mushroom extract. Arch Pharm Res 26: 821-825.
- Rendeiro C, Vauzour D, Rattray M, Waffo-Téguo P, Mérillon JM, et al. (2013) Dietary levels of pure flavonoids improve spatial memory performance and increase hippocampal brain-derived neurotrophic factor. PLoS One 8: e63535.
- Maestre R, Douglass JD, Kodukula S, Medina I, Storch J (2013) Alterations in the intestinal assimilation of oxidized PUFAs are ameliorated by a polyphenol-rich grape seed extract in an in vitro model and Caco-2 cells. J Nutr 143: 295-301.
- Toufektsian MC, Salen P, Laporte F, Tonelli C, de Lorgeril M (2011) Dietary flavonoids increase plasma very long-chain (n-3) fatty acids in rats. J Nutr 141: 37-41.
- Hadad N, Levy R (2017) Combination of EPA with carotenoids and polyphenol synergistically attenuated the transformation of microglia to M1 phenotype via inhibition of NF-κB. Neuromolecular Med 19: 436-451.
- Lull ME, Block ML (2010) Microglial activation and chronic neurodegeneration. Neurotherapeutics 7: 354-365.
- Cazzola R, Camerotto C, Cestaro B (2011) Anti-oxidant, anti-glycant, and inhibitory activity against a-amylase and a-glucosidase of selected spices and culinary herbs. Int J Food Sci Nutr 62: 175-184.
- Giunta B, Hou H, Zhu Y, Salemi J, Ruscin A, et al. (2010) Fish oil enhances anti-amyloidogenic properties of green tea EGCG in Tg2576 mice. Neurosci Lett 471: 134-138.
- Kanter M, Unsal C, Aktas C, Erboga M (2016) Neuroprotective effect of quercetin against oxidative damage and neuronal apoptosis caused by cadmium in hippocampus. Toxicol Ind Health 32: 541-550.
- Rinwa P, Kumar A (2013) Quercetin suppress microglial neuroinflammatory response and induce antidepressent-like effect in olfactory bulbectomized rats. Neuroscience 255: 86-98.
- Gareau MG (2014) Microbiota-gut-brain axis and cognitive function. Adv Exp Med Biol 817: 357-371.
- Rijkers GT, de Vos WM, Brummer RJ, Morelli L, Corthier G, et al. (2011) Health benefits and health claims of probiotics: Bridging science and marketing. Br J Nutr 106: 1291-1296.
- Reid G, Jass J, Sebulsky MT, McCormick JK (2003) Potential uses of probiotics in clinical practice. Clin Microbiol Rev 16: 658-672.
- Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, et al. (2016) Effect of probiotic supplementation on cognitive function and metabolic status in alzheimer's disease: A randomized, double-blind and controlled trial. Front Aging Neurosci 8: 256.
- Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, et al. (2017) Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging 49: 60-68.
- Leblhuber F, Egger M, Schuetz B, Fuchs D (2018) Commentary: Effect of probiotic supplementation on cognitive function and metabolic status in alzheimer's disease: A randomized, double-blind and controlled trial. Front Aging Neurosci 10: 54.
- Rajkumar H, Mahmood N, Kumar M, Varikuti SR, Challa HR, et al. (2014) Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: A randomized, controlled trial. Mediators Inflamm 2014: 348959.
- van Wijk N, Broersen LM, de Wilde MC, Hageman RJJ, Groenendijk M, et al. (2014) Targeting synaptic dysfunction in Alzheimer’s disease by administering a specific nutrient combination. J Alzheimers Dis 38: 459-479.
- Perez-Pardo P, de Jong EM, Broersen LM, van Wijk N, Attali A, et al. (2017) Promising effects of neurorestorative diets on motor, cognitive, and gastrointestinal dysfunction after symptom development in a mouse model of parkinson’s disease. Front Aging Neurosci 9: 57.
- Thorning TK, Raben A, Tholstrup T, Soedamah-Muthu SS, Givens I, et al. (2016) Milk and dairy products: Good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr Res 60: 32527.
- Astrup A, Rice Bradley BH, Brenna JT, Delplanque B, Ferry M, et al. (2016) Regular-fat dairy and human health: A synopsis of symposia presented in europe and north america (2014-2015). Nutrients 8.
- Ide T (2000) Effect of dietary alpha-linolenic acid on the activity and gene expression of hepatic fatty acid oxidation enzymes. Biofactors 13: 9-14.
- Dinel AL, Rey C, Bonhomme C, Le Ruyet P, Joffre C, et al. (2016) Dairy fat blend improves brain DHA and neuroplasticity and regulates corticosterone in mice. Prostaglandins Leukot Essent Fatty Acids 109: 29-38.
- Dinel AL, Rey C, Baudry C, Fressange-Mazda C, Le Ruyet P, et al. (2016) Enriched dairy fat matrix diet prevents early life lipopolysaccharide-induced spatial memory impairment at adulthood. Prostaglandins Leukot Essent Fatty Acids 113: 9-18.
- Van Loo-Bouwman CA, Naber TH, Schaafsma G (2014) A review of vitamin A equivalency of β-carotene in various food matrices for human consumption. Br J Nutr 111: 2153-2166.
- Park KM, Fulgoni VL (2013) The association between dairy product consumption and cognitive function in the National Health and Nutrition Examination Survey. Br J Nutr 109: 1135-1142.
- Tomé-Carneiro J, Carmen Crespo M, Burgos-Ramos E, Tomas-Zapico C (2018) Buttermilk and krill oil phospholipids improve hippocampal insulin resistance and synaptic signaling in aged rats. Mol Neurobiol.
Citation: Chataigner M, Dinel AL, Pallet V, Layé S, Joffre C (2018) The Interest of Adding Micronutrients to Docosahexaenoic Acid Supplementation to Prevent Age-Related Cognitive Decline. J Alzheimers Dis Parkinsonism 8:438. DOI: 10.4172/2161-0460.1000438
Copyright: © 2018 Chataigner M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5867
- [From(publication date): 0-2018 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 5028
- PDF downloads: 839