Research Article Open Access
The Induction of Pregnancy Block in Mice by Bodily Fluids via the Vomeronasal Organ
| Roger N Thompson1, Murtada Taha2, Audrey Napier1 and Kennedy S Wekesa1* | |
| 1Department of Biological Sciences, Alabama State University, Montgomery, AL, 36104, USA | |
| 2Department of Biology, Albany State University, Albany, GA, 31705, USA | |
| *Corresponding Author : | Kennedy S Wekesa Department of Biological Sciences Alabama State University, Montgomery Alabama 36101-0271, USA Tel: 334-229-4196 Fax: 334-229-1007 E-mail: wekesa@alasu.edu |
| Received April 06, 2013; Accepted May 02, 2013; Published May 06, 2013 | |
| Citation: Thompson RN, Taha M, Napier A, Wekesa KS (2013) The Induction of Pregnancy Block in Mice by Bodily Fluids via the Vomeronasal Organ. Biochem Physiol 2:109. doi:10.4172/2168-9652.1000109 | |
| Copyright: © 2013 Thompson RN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Animals have evolved specific communication systems to identify and attract mates, and to discern the social status of conspecifics. In mice, these exchanges of information involve the emission and detection of pheromones. These pheromones are detected by the vomeronasal system. While urine has long been identified as the primary source of pheromones, including those responsible for pregnancy block, recent evidence indicates that there are other sources. These sources contain MHC class I peptides from the immune system and ESP1 from an exocrine gland. The MHC class I peptides have been identified as compounds that elicit the pregnancy block effect via the vomeronasal system, similar to the effect elicited by urine from male mice, including castrated or juvenile males. Here we provide evidence which shows bodily fluids such as saliva, blood serum or fecal extract, along with tissue extracts are capable of inducing the pregnancy block (Bruce Effect) paradigm, in a manner equivalent to female mice exposed to whole urine. While there appears to be a number of sources that can induce pregnancy block, one exception is the nervous system. Therefore, we conclude that pregnancy block can be mediated by stimuli from several different sources in the same manner as whole male urine.
| Keywords |
| Bruce effect; Pregnancy block; MHC; Vomeronasal organ |
| Introduction |
| Pheromones are chemical signals which provide conspecifics information about gender, dominance and reproductive status [1]. They elicit innate and stereotypical reproductive and social behaviors, along with neuroendocrine and physiological changes. The vomeronasal organ (VNO), as part of the accessory olfactory system, is the site for pheromone binding to specific receptors, thus initiating a signal transduction pathway leading from the vomeronasal neurons to the hypothalamus-pituitary axis via the medial amygdala [2]. Molecular evidence has led to the isolation of two independent families of vomeronasal receptor genes (VR), known as V1Rs [3] and V2Rs [4-6], that encode putative pheromone receptors. Vomeronasal neurons are classified based on the vomeronasal receptor type and the G-protein to which they are coupled. The apical layer expresses the G-protein Gαi2, along with members of the V1R family of vomeronasal receptors. The basal layer expresses the G-protein Gαo and members of the V2R receptor family [7]. More recent studies have introduced the formyl peptide receptor (FPR) as a possible chemosensory receptor [8]. Possible roles for the FPRs include the assessment of conspecifics or other species based on variability in normal bacterial or mitochrondrial proteins [9]. FPRs are selectively expressed in the neuroepithelium, express either Gαi2 or Gαo, and are highly dispersed throughout the neuroepithelium [10]. |
| Despite the importance of the VNO in eliciting stereotypic social behaviors and despite the identification of a large family of putative pheromone receptors expressed selectively in the vomeronasal neurons [3-6], few biological compounds have been identified that act directly on the VNO. Most of the studies on identifying the ligands for pheromone receptors have focused on chemicals present in urine, seminal fluid and vaginal secretions [11]. |
| Signaling pheromones account for many behavioral responses in the mouse. For example, male, as well as lactating female, aggressiveness towards a male intruder is believed to be triggered by molecules present in male urine [12]. Territorial markings and alarm scents are other examples of signaling pheromones which have an effect on the recipient. Early studies have documented a number of effects for primer pheromones in mice. It has been shown that grouped female mice modify or suppress their estrous cycle [13], while male urine can restore and synchronize the estrus cycle of noncycling females [14], male urine can also accelerate the onset of puberty in females [15]. In addition, the exposure of a recently mated female mouse to a male of a different strain from that of the stud, prevents implantation of fertilized eggs [16], implying that the stud or his individual odor must be memorized at the moment of mating to avoid the pregnancy block effect later. These pheromonal effects are commonly believed to be mediated by stimuli present in urine, which act on the vomeronasal system. |
| Male mouse urine contains an unusually high quantity of proteins, called major urinary proteins (MUPs). They form a large family of highly homologous androgen-dependent proteins that are synthesized in the liver and excreted with urine [17,18]. MUPs are also expressed in exocrine glands such as mammary, parotid, sublingual, submaxillary, lachrymal, nasal, and in modified sebaceous glands like preputial and perianal glands [19]. MUPs have been shown to elicit aggressive behavior [20]. Other compounds found in urine which act as primer pheromones include 2-sec-butyl-4,5-dihydrothiazole and 2,3-dihydroexo- brevicomin found in male urine and are known to accelerate puberty, induce estrus synchronization and increase aggression [21-23]. 6-hydroxy-6-methyl-3-heptanone is also found in male urine and has been shown to accelerate puberty, while isobutylamine from male urine accelerates estrus and aids vaginal opening [22,23]. Only a single compound has been characterized exclusively in female urine. That compound is 2,5-dimethyl pyrazine, and has been shown to suppress female estrus [24,25]. Compounds found in both male and female urine include 2-heptanone and n-pentyl acetate. The effect contributed to these compounds is estrus extension and estrus suppression, respectively [24,26]. The farnesene (α and β) are secreted from the preputial gland into the urine and have been shown to accelerate puberty, serve as territorial markings, increase male attractiveness to females and suppress estrus in females [21-23]. All of these compounds are considered volatile and have been shown to activate V1R receptors. The lists of compounds which activate the V2R receptors, though smaller, have similarly characterized behaviors as the aforementioned. Specifically, MHC class I peptides which are found in both male and female urine have been shown to increase attractiveness between individuals of different strains, and induce pregnancy block [27,28]. In 2005, the exocrine gland secreting peptides (ESP1 and ESP36), which are secreted by the extraorbital lacrimal gland were introduced as chemosensory compounds, for their ability to elicit electrical responses in vomeronasal sensory neurons [29]. |
| We have been able to show that chemosensory cues from male urine and MHC class I peptides are capable of stimulating the production of IP3 in the VNO, and inducing pregnancy failure [30]. In 2004, Leinders- Zufall et al. [27] showed that stimulation with MHC peptides resulted in an influx of calcium and an excitatory electrical response These peptides (MHC-complexes) are released into the extracellular space and appear in urine and other bodily secretions [31]. The MHC peptides are believed to carry information about gender, sexual and social status, dominance hierarchy and individuality. It is this individuality which has been shown to influence pregnancy block or Bruce Effect [16]. According to the Bruce Effect paradigm, the urine scent from a strange male blocks embryo implantation by inhibiting prolactin (PRL) secretion. While it has been previously shown that the Bruce Effect is mediated via the VNO, the inhibition of PRL by the odorous components of urine is mediated via the main olfactory system. Thus, suggesting that both structures are essential for pheromone mediated responses through specific and non-redundant mechanisms [32]. |
| In the present study, we induce pregnancy failure in C57BL/6 female mice using BALB/c male urine and saliva. In addition, we investigated other possible sources for pregnancy failure induction. Here, we show that we are able to induce pregnancy failure (Bruce Effect), using blood serum, fecal waste and liver tissue extract from a BALB/c male mouse, when administered to the mated female of a C57BL/6 pairing. Stimulation with an extract of BALB/c male brain tissue failed to induce pregnancy failure for the C57BL/6 pairing. |
| Materials and Methods |
| Animals |
| C57BL/6 and BALB/c mice were obtained from Charles River Laboratories (Kingston, NY, USA), and maintained in a breeding colony at the Department of Biological Sciences, Alabama State University. Animals were housed in Institutional Animal Care and Use Committee (IACUC), inspected and approved facilities, and cared for according to NIH Guidelines for care and use of laboratory animals. Food and water were provided ad libitum. |
| Chemical stimuli |
| The MHC class I ligands were chosen to correspond to prototypical representatives for the two disparate H-2 haplotypes, namely AAPDNRETF (for the H-2b haplotype of C57BL/6 mice), and SYFPEITHI (for the unrelated H-2d haplotype of BALB/c mice) [27]. |
| The synthetic peptides were purchased from Sigma-Genosys (The Woodlands, TX, USA). Peptide concentration was 250 μm in PBS. For pregnancy block assays, peptide mixtures in PBS (H- 2b haplotype AAPDNRETF; H-2d haplotype SYFPEITHI) were mixed 1:1 with saliva, prior to use. Peptides were also used without mixing with saliva. Saliva was collected from male mice, following the administration of carbachol (Sigma-Aldrich, USA). Whole urine and saliva were collected from adult males, pooled by strain, and stored frozen at -80°C until needed. In addition, tissue extracts were prepared following the method outlined in Ma et al. [25] for mouse brain, liver, and blood serum. Feces were collected from the cage of a BALB/c male, crushed and suspended in dH2O. |
| Pregnancy failure test |
| Animals included adult, virgin female mice (mus musculus) of the C57BL/6 strain, and sexually experienced males of either C57BL/6 or BALB/c strains. Mice were kept in Nalgene cages 26 cm×21 cm×14 cm, at 20°C room temperature, and a 12/12 hour light/dark cycle. Each C57BL/6 female was paired with a single C57BL/6 or BALB/c male and checked four times during the ensuing 12 hour period for vaginal plugs, indicating that mating had occurred. Mated females remained with the males, until 0800 hours the following day, at which time the females were moved to clean cages and assigned to groups based on stimulus to be administered. Exposure to urine, saliva, prepared extracts, supplemented urine and saliva, or peptide alone was achieved by depositing 30 μl of liquid on the oronasal groove, while holding the female by the nape of the neck. Stimulants were delivered 4 times per day for two days at regular intervals. Nine days after mating, the females were sacrificed, the uteri surgically removed and examined for implantation sites or the presence of embryos. After completing the dissections, results for each group were recorded and the percentage of pregnancy failures calculated. |
| Results |
| Pregnancy block experiments |
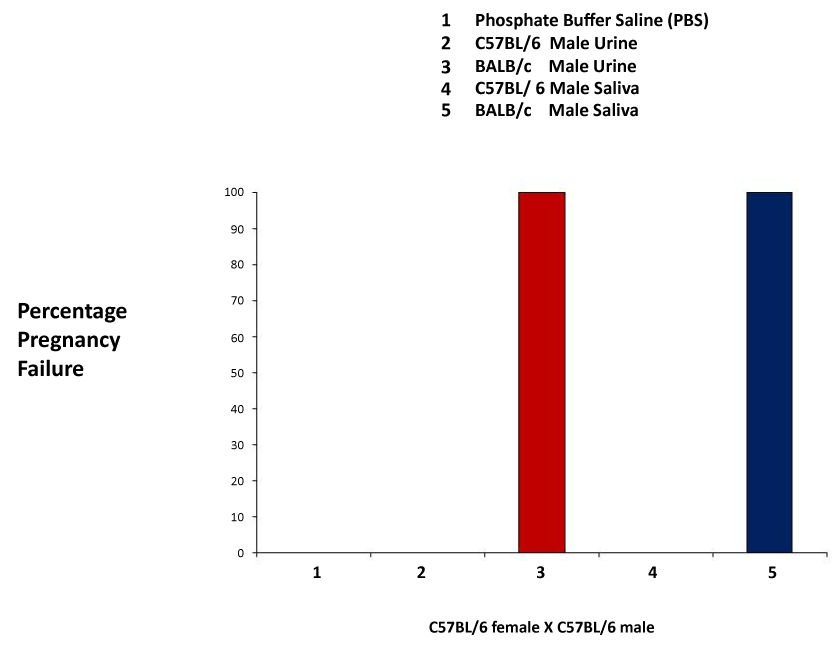
| These experiments closely followed those previously published by Leinders-Zufall et al. [27] and Thompson et al. [30]. We first performed a series of experiments to examine pregnancy failure, which occur when recently mated females are exposed to phosphate buffer saline (PBS), male urine or male saliva of the same strain, or that of a different strain. Application of the unfamiliar urine or saliva resulted in a 100% pregnancy failure rate, whereas application of PBS, familiar urine or saliva, did not result in pregnancy failure (Figure 1). |
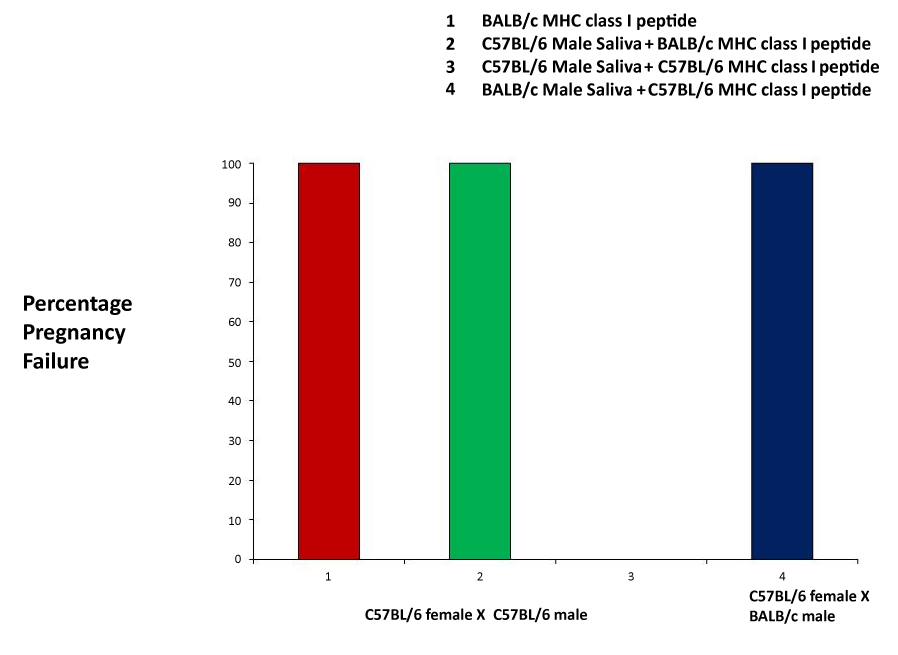
| The MHC class I (H2d haplotype) peptide was used as a positive control, and was successful in producing pregnancy failure at a rate of 100% (Figure 2). Next, we mixed the MHC peptides with male saliva, prior to application. Using a C57Bl/6 female crossed with a C57Bl/6 male, we administered a mixture of C57Bl/6 male saliva and BALB/c MHC peptide, and observed a 100% pregnancy failure rate. As another control, the pairing of C57Bl/6 mice and administration of a mixture of C57Bl/6 saliva and the C57 MHC peptide resulted in no pregnancy block. Finally, we mixed C57Bl/6 MHC peptide with BALB/c male saliva, and applied the mixture to a C57Bl/6 female mated with a BALB/c male. This combination resulted in a 100% pregnancy failure rate (Figure 2). |
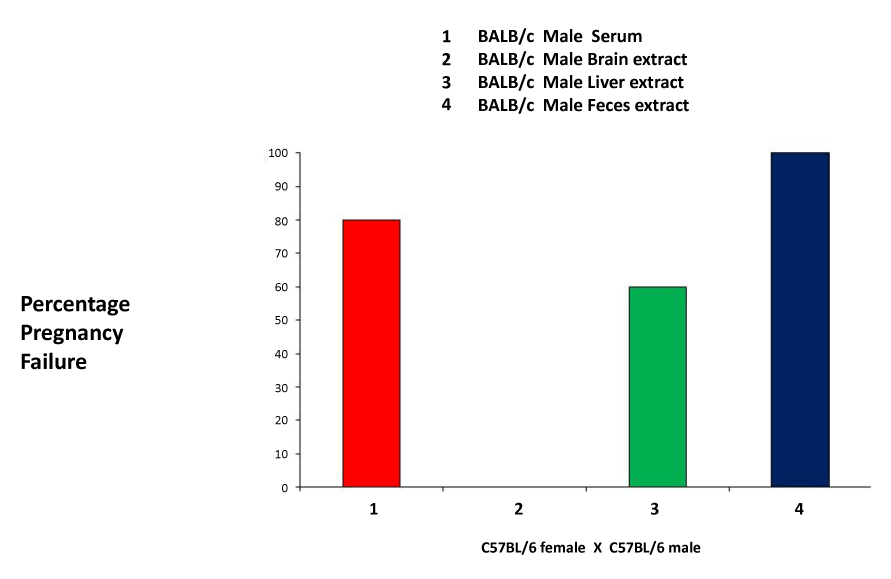
| In order to further investigate the ability of bodily fluids or secretions to induce pregnancy failure, we used tissue extracts from the brain and liver, blood serum and feces. The results show that the liver extract, the blood serum and the fecal extract were all able to induce pregnancy failure at rates of 60, 80 and 100%, respectively, whereas the brain extract was not capable of inducing pregnancy failure (Figure 3). Our results indicate the presence of the pregnancy failure stimulus in most tissue and fluids, in agreement with previous findings. The exclusion of the stimulus from the nervous system was surprising, but not totally unexpected, given the role of the blood brain barrier. |
| Discussion |
| Classical MHC class I antigens are glycoproteins integrally embedded in the membrane of nearly all cells. They are best known for their role of tissue transplantation incompatibility. MHC complexes cause extreme rejection of grafts. The polymorphic MHC represents a barrier in crossmatching of tissues from unrelated donors, and contributes to the variability at the cell surface. In 1996, Medawar [33] referred to this as “the uniqueness of the individual”. Due to this characteristic trait, only close relatives share similarity at the MHC locus, and individual’s requiring transplantation focus on family members to obtain closely matched tissue [34]. MHC class I molecules are used by T lymphocytes of the immune system as associative recognition molecules. Their use has directed attention to an immunological explanation for their polymorphisms. The current explanation holds that MHC polymorphisms within a population ensure that a lethal pathogen such as a virus, cannot exterminate a species by epidemic infection [35]. |
| The discovery that MHC associated odor signals are present in mice, came from the use of genetically defined inbred strains [36]. An inbred strain consists of animals which are genetically uniform and homozygous for all their genes. Each chromosome pair is identical; therefore, two strains that are identical except for allelic differences at a particular locus are called congenic strains. Any measurable characteristic that differs between the two congenic strains can be contributed to the genes at that locus. Therefore, the spectrum of peptides present in any one individual is unique. |
| Pregnancy failure in mice is a prime example of a physiological response to male pheromones. This physiological response is linked to an increase in LH and a reduction in prolactin [37]. Pregnancy failure occur when a recently impregnated female is exposed to a male (or his scent), other than the male to whom she was mated. The effect is a laboratory phenomenon, and therefore, is an excellent tool for investigating signal transduction via the vomeronasal organ. The vomeronasal system provides a neural pathway linking the periphery to the hypothalamus, and modulation of LH and prolactin release. Pheromonal stimulants have proven elusive, with only a few compounds clearly identified and linked to specific behaviors. Many of the known compounds are found in the urine; however, other studies have shown that exocrine glands are sources of pheromones. Pregnancy failure (the Bruce Effect) was first described in 1959, and was believed to be testosterone dependent. Since then a number of discoveries have lead to a better and yet still incomplete understanding of the process. Currently, the signaling pathway includes the vomeronasal organ and V2R receptors, the accessory olfactory bulb, the hypothalamus and the pituitary gland. Evidence for the roles of prolactin and progesterone during pregnancy are well documented and understood. |
| In 2004, Leinders-Zufall et al. [27] introduced the MHC class I peptides as chemosensory cues, which activate the V2R receptors of basal layer vomeronasal sensory neurons. While a great deal of information is known about the MHC peptide’s immunological role, less is known about their ability to activate the vomeronasal organ. Although pregnancy failure (the Bruce Effect) was initially believed to be testosterone dependent, in 2007, we provided evidence showing that pregnancy failure is not testosterone dependent, indicating an androgenic effect on the immune system as unlikely [30]. In the current study, new evidence suggests that while the pregnancy failure stimulus appears ubiquitous in most tissues and fluids, one area excluded is the nervous system. |
| The ion channel TRPC2 is expressed in the microvilli of vomeronasal neurons and plays an important role in the transduction process of sensory sensory neurons [38]. Studies by Stowers et al. [39] show that genetic ablation of TRPC2 either eliminates or strongly reduces the sensory response of the VNO to urine or small, volatile pheromones [40]. Another study by Kelliher et al. [28] shows that pregnancy block can still occur in the absence of the TRPC2 channel. Thus, indicating the presence of an unknown or as yet unidentified mechanism responsible for pregnancy failure. Removal of the VNO results in the lack of or absence of pregnancy failure, indicating an intact VNO is required. In a previous study, we were able to induce pregnancy failure using prototypical MHC class I peptides, male urine and mixtures of peptides with urine [30]. The present study adds numerous other sources to that list. While the compound responsible for pregnancy failure remains elusive, the generation of an action potential is based on the influx of calcium into the sensory neuron. There are still unanswered questions concerning the role of calcium, once it has entered the cell. In 2003, Liman [41] described a Ca2+ activated non-selective cation channel which does not discriminate between Na+ or K+ ions. Activation appears to be via the phosphatidyalinositol signaling pathway. That study describes VNO microvilli as having calcium activated cation channels, which may be opened by calcium ions that either enters the cell through the TRPC2 channel (not in the case for the Bruce Effect), or from the endoplasmic reticulum. More recently, the discovery of the formyl peptide receptor offers yet another opportunity for calcium entry, following pheromonal stimulation. Opening of these channels would enhance the receptor potential, and therefore, amplify the signal. While the current dogma identifies the TRPC2 as the primary channel for signal transduction, there may still be a role for the release of IP3. It is possible that the IP3 produced might function in the classical manner, by which it binds to the IP3 receptor on the endoplasmic reticulum (ER), initiating the release of calcium from within the ER, or possibly activate an as yet unidentified channel responsible for pregnancy failure. |
| Previous studies indicate that for pregnancy failure to occur, an intact VNO is a prerequisite, while the event does not require involvement of the main olfactory epithelium. In addition, both the apical and basal layers express the TRPC2 channel, which evidence indicates is necessary for signal transduction in the VNO. Yet, based on findings by Kelliher et al. [28], such is not the case for pregnancy failure. This suggests that not all pheromonal responses in the VNO are mediated by the TRPC2 channel. To date, evidence indicates a definite role for V2R receptors of the VNO, yet the exclusion of TRPC2 would suggest a mechanism of transduction, involving an as yet unidentified channel or method of calcium entry. |
References
|
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 13797
- [From(publication date):
May-2013 - Dec 03, 2024] - Breakdown by view type
- HTML page views : 9398
- PDF downloads : 4399
