The In vitro and In vivo Efficacy of Gold Nanoparticle in Comparison to the Glucantime as a Therapeutic Agent against L. major
Received: 20-May-2018 / Accepted Date: 13-Jul-2018 / Published Date: 24-Jul-2018 DOI: 10.4172/2332-0877.1000373
Keywords: Gold nanoparticle; Leishmania major; In vitro; In vivo
Introduction
The infection of the cutaneous leishmaniasis, caused by a protozoan of the genus Leishmania, is transmitted by some species of female phlebotomine sandfly. This disease is observed in three forms including cutaneous, mucocutaneous and visceral Leishmaniasis [1]. The cutaneous leishmaniasis is the most common form of the Leishmaniasis that it can observe in both dry (urban) and wet (rural) forms. Annually, 1.5 million people are infected by the cutaneous leishmaniasis in the world and it is estimated that about 12 million people are suffering from the cutaneous leishmaniasis in different regions of the world [2]. The number of people, who is annually infected with cutaneous leishmaniasis in Iran, has been reported about 15 thousand that the actual number of the infected people is 4-5 times higher than reported numbers [3].
The wet ulcer is generally formed by L. major which is also called the cutaneous leishmaniasis because this type of leishmaniasis is mostly found in rural areas where the infectious agent tends to the rodents rather than the humans [4]. The treatment of people who live in endemic areas is not considered as a serious issue because the spontaneous recuperation is associated with creating a protective immune in these people; however, the treatment should be performed when the ulcers are created on people's faces or inflammation of the lymphatic vessels be observed [5,6]. The local treatment should be prescribed for the primary and non-inflamed ulcers and the systemic treatment should be performed for the multiple or undetectable ulcers [7]. The antimony meglumine (Glucantime) and Sodium stibogluconate (Pentustam) are considered as the selective drugs to treat the disease; but, their performance has reduced from 20%-50% in recent years [8]. The emergence of the resistant forms against these drugs is the major problem of the treatment which is caused to introduce new anti-leishmanial such as Ketoconazole, Paromomycin, Amphotericin B, Miltefosine and other chemical compounds [9]. However, all of these drugs exhibit various side effects. The toxicity of these drugs, the sustainability of the adverse effects even after the modification of dose and long-term treatment are of their defects [10]. Besides, these treatments, especially in rural areas, are not suitable due to the high costs and lack of access. Hence, the use of new compounds without the mentioned issues seems to be essential.
Nanotechnology is the understanding and controlling of the material at dimensions of 1 to 100 nm, which is brought unusual physical, chemical and biological properties and is led to the possibility of the unique and innovative applications [11]. The gold nanoparticles are utilized as anti-HIV [12], anti-arthritic [13], anti-malarial [14] and angiogenesis [13]. In addition, since the nanoparticles are used in various fields, especially in the biological and medical sciences, the electrical properties of gold nanoparticles can be changed in various aspects such as size, nature of coating agent and the distance between the nanoparticles that it is dependent on the synthesis method used [12]. There are several methods for the synthesis of gold nanoparticles which is classified in chemical, physical and biological groups [15]. The chemical reduction has been identified as a most practical and effective method for the preparation of metal nanoparticles [16]. Similar to the silver, the gold is a chemical element which has the anti-bacterial properties in addition to its application for jewellery. It has achieved more popularity to use as drug in industry and medical and etc due to the anti-bacterial properties [17,18]. This metal is used as medicines to treat some diseases such as rheumatoid arthritis. Recently, the antitumor effect of gold has detected and it has also used in the treatment of cutaneous leishmaniasis [19,20]. In addition, various researches have been conducted to study the effect of metals such as gold, as ligand, along with some drugs including chloroquine, Pentaamine, clotrimazole and Ketoconazole to treat the malaria, leishmaniasis and trypanosomiasis [21]. This study was to investigate the effect of nanogold against L. major . The results of this study can be used to discover a suitable alternative drug in the treatment of L. major which it is without the disadvantages of drugs used to treat, especially the side effects of chemical drugs.
Materials And Methods
Nano gold preparation
Gold nanoparticle was prepared by heating 20 ml of HAuCl4 (1.0 Mm) on a moving hot plate and bringing the solution just to 140ºC by thawing process and to it 1% sodium citrate solution was added to the heated solution. Then the solution became dark red and the nanoparticles were detached [22].
In vitro anti-leishmanial activity study
Promastigotes at log phase were centrifuged at 2000 rpm for 5 min, diluted in fresh culture medium to a final density of 2 × 106 cells/mL. 200 μL of parasites were added in different wells and 20 μL of each extract were added in medium. Two rows of 96-well plate were left for 1% (w/v) acetic acid and 1 mol NaOH as negative controls. Live and dead promastigotes were counted after adding 0.1% eosin stain with light microscope.
In vivo anti-leishmanial activity study
Fifteen 6-7-weeks-old female BALB/c mice were used in current investigation. All mice were inoculated subcutaneously in the base of tail with approximately 1.6 × 106 in stationary stages promastigotes of L. major . Mice were divided into three groups with each group containing 5 mice. The groups were categorized as follows: Group 1 was treated with the 100 μg/ml of nano gold; Group 2 was treated with the 400 μg/ml of nano gold; Group 3 was untreated (control group). Treatment was started when local lesions were obvious. The mice were treated topically once in a day for 28 continuous days. The lesion size was measured weekly before and after treatment by dial micrometer in two diameters (a,b) and comparing it with that of untreated lesions. The wound size was considered by the formula:
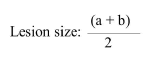
Correspondingly; before treatment and 7, 14, 21 and 28 days after treatment, slides of infected lesions were prepared and fixed with methanol, stained with Giemsa and examined by oil immersion and light microscope (1000X). Nano gold effectiveness was evaluated by comparing the diameters of wounds and the number of slides with amastigotes, between treated and untreated groups.
Cytotoxicity assay
The 2, 5-diphenyl tetrazolium bromide (MTT) colorimetric technique was utilized to display the cytotoxic activity of nano gold. Summery, the macrophage cells (1 × 104 cells/ml) were cultured for 24 hours on cell culture plates and were then exposed to 3 different concentrations (100, 500 and 1000 ppm) of nano gold. Additionally; metronidazole and DMSO were used as positive and negative controls, respectively. For analysis of the viability, 10 μL MTT reagents in 5.0 mg/mL PBS was added to each well and then kept at 37°C for 3 h and after incubation, centrifugation at 1300 rpm for 5 min was done. DMSO was added to the sediment and after 20 min, the optical density (OD) of each well was measured at 550 nm. All experiences were done in triplicates [23].
Statistical analysis
All In vitro experiments were performed in three times. The mean and standard error of at least three experiments were determined. Statistical analysis of the differences between mean values gotten from experimental groups was done by means of χ2, ANOVA and Paired T test. In this study, p<0.05 was considered as the significant level.
Results
In vitro study
The effect of low molecular weight nano gold at different concentrations (50, 100, 200 and 400 ppm) on promastigotes of L. major after 30, 60, 120 and 180 min is presented in Table 1. In all concentrations, the effect of nano gold is time dependent that in all times, living promastigote decreased in different tubes in comparison to past time. Also, the effect of nano gold on L. major was significantly differenced from negative control controls (P<0.001). In all time intervals, the use of any concentration of nano gold-controlled L. major more successfully by increasing in concentration.
| Times | Nano gold concentration | Controls | |||
|---|---|---|---|---|---|
| 100 ppm | 500 ppm | 1000 ppm | 1% acetic acid | 1 mol NaOH | |
| 30 min | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 |
| 60 min | 52.16 ± 2.88 | 80.48 ± 2.16 | 95.47 ± 1.49 | 93.6 ± 1.04 | 96.3 ± 1.09 |
| 120 min | 39.25 ± 1.44 | 26.77 ± 2.12 | 21.14 ± 3.25 | 91.64 ± 1.32 | 91.54 ± 0.95 |
| 180 min | 6.35 ± 0.75 | 4.66 ± 2.56 | 0.00 ± 3.25 | 91.9 ± 1.62 | 89.01 ± 1.45 |
| P-value | 0.002 | 0.001 | 0.001 | ||
Table 1: The effects of nano gold in concentrations of 100, 500 and 1000 ppm on the viability of the promastigotes of L. major after 30, 60, 120 and 180 min, in vitro , in comparison to 1% acetic acid and 1 mol NaOH as negative controls.
In vivo study
The mean size of lesions was decreased in the groups treated with nano gold in comparison with the control group (Table 2). The mean lesions size in the control groups was 8.74 mm, whereas it was 2.07 mm and 1.05 mm in groups treated with the 100 ppm and 1000 ppm concentration of nano gold, respectively. The mean difference between treated and control groups was significant (p<0.05).
| Groups | Concentration | Lesion size (mm) | Lesion size after intervention | |||
|---|---|---|---|---|---|---|
| µg/ml | Before intervention | After 7 days | After 14 days | After 21 days | After 28 days | |
| Nano gold | 100 ppm | 7.12 ± 2.41 | 5.9 ± 1.35 | 4.12 ± 0.8 | 2.25 ± 1.25 | 2.07 ± 0.86 |
| Nano gold | 1000 ppm | 11.2 ± 1.28 | 10.8 ± 2.50 | 7.66 ± 2.77 | 5.24 ± 2.00 | 1.05 ± 1.05 |
| Control group | untreated | 8.25 ± 2.18 | 8.1 ± 2.2 | 8.9 ± 2.12 | 8.8 ± 2.85 | 8.47 ± 1.42 |
Table 2: The effects of nano gold in concentrations of 100 and 1000 ppm in BALB/c mice infected with Iranian strain of L. major after 7, 14, 21 and 28 days of treatment, in vivo , in comparison to controls groups.
Cytotoxicity
In this study cytotoxicity was evaluated on macrophage cells, nano gold showed no hemolytic activity, with less than 5% lysis within 180 min of incubation when it was used at 1000 ppm. Treatment of the positive control with glucantime showed a hemolytic effect, with 17% lysis after 180 min, whereas 0.5% DMSO did not cause lysis.
Discussion
The findings of this study showed that the gold nanoparticles have the appropriate antileishmanial effects. The gold nanoparticles are a series of metallic components with different sizes. Today, the gold particles are successfully applied to treat a broad spectrum of microorganisms [12]. In this study, the effect of different dosages of gold nanoparticles on the elimination of L. major has evaluated based on its properties at In vivo and In vitro condition. Based on the results of present work, it was detected that the different dosage of gold nanoparticles has a significant effect on the promastigotes of L. major ; so that the increasing of the gold nanoparticles dosages in the certain times, especially in 60 and 180 min, increase the lethality percentage. Furthermore, it was observed that the increasing of the contact time increases the lethality percentage and the elimination level of the promastigotes of the L. major , especially in dosages of 500 ppm; so that the highest lethality percentage is observed in the gold nanoparticle dosage of 1000 ppm in the contact time of 180 min which it was higher than the control group. Moreover, the results showed that the lethality effect of the glucantime is 99% in the 180 min. As a result, the comparison of the observed performance of the gold nanoparticles with the performance of glucantime, which the medicinal dosage of glucantime, illustrates that the lethality effect of gold nanoparticles in lower dosage, was close to the results of glucantime as the selective drug for treatment of Leishmania.
In the study, the gold nanoparticles has used as a therapeutic agent for cutaneous leishmaniasis caused by Iranian strains of L. major . In this study, the effect of two different dosages of gold nanoparticles has studied against L. major strains in the 25 mice. The gold nanoparticles in dosages of 500 ppm and 1000 ppm were consumed to treat the mice twice in a day for 28 days. The results showed that the number of parasite amastigote in the ulcers is significantly decreased. Also, the gold nano-particle solution has a significant role in reducing the death rate in mice. In addition, despite the significant reduction of the amastigote, the lethality percentage of the parasite has not been reported.
In recent decades, the researchers have conducted several In vitro studies to evaluate the anti-parasitic properties of metal nanoparticles e.g. silver and also other substances e.g. chitosan on L. major [24,25]. For example, Saeed et al. has surveyed the potential of silver nanoparticle and curcumin chitosan to eliminate the L. major in 200 rats [26]. Their study illustrated that the simultaneous use of silver nanoparicle and chitosan nanoparticles had greater efficiency to destroy the promastigotes of L. major . Mohebali et al. has investigated the effect of different dosages of nanosilver on the In vitro and In vivo elimination of L. major [27]. The results of their study showed that the various dosages of silver nanoparticles can reduce the amastigotes compared to control group, but it was not significant. Also, the different dosage of nanosilver has not significant effect to decrease the mean size of the ulcers.
In the study of Karima et al., the use of the silver nanoparticle has significantly reduced the mean growth of ulcers diameters; so that the ulcers sizes in the treated group with silver nanoparticles decreased to about one second of the negative control group (without treatment) after 5 weeks. Since, a single strain has studied in both of these studies, thus the strain has not effective role on the performance of the nanosilver against L. major [28]. Jebelli et al. have compared the potential of nano-materials including silver, gold, TiO2, MnO2 and ZnO2 under ultraviolet and infrared lights on Leishmania species. They have observed that the highest antileishmania activity is related to silver nanoparticles [29]. It is also found out that the antileishmania activity of the both ultraviolet and infrared is lesser than the silver nanoparticles activity. They also reported that the silver nanoparticles were better than gold in the context of the elimination of leishmaniasis. However, based on the results of Jamee and Jebelli and also the results of recent studies, it seems that the gold has the better antileishmania effect than the silver. The use of gold nanoparticles can be considered as one of the innovative therapeutic method with low side-effects issues to eliminate the CL.
Conclusion
According to the results of this study, the 1000 ppm of the gold nanoparticles can effectively use to eliminate the L. major in both In vivo and In vitro . Thus, it can be concluded that the metal nanoparticles such as gold, in suitable dosages, can be used for In vivo elimination of the promastigotes of L. major in order to the therapeutic uses or to perform the complimentary studies on other blood-tissue parasites in the animals. In addition, this study can be considered as a suitable approach to find the proper alternative to the drugs used as a therapeutic agent for the CL since it can be a method without the issues related to the chemical drugs such as glucantime.
References
- Oliveira M, Barreira L, Gangadhar KN, Rodrigues MJ, Santos T, et al. (2016) Natural products from marine invertebrates against Leishmania parasites: a comprehensive review. Phytochemistry Reviews 15: 1-35.
- Ferreira F, Marcili A, Horta CM (2016) Epidemiological aspects and risk factors for infection by Leishmania infantum chagasi in dogs from municipality of Petrolina, Northeastern Brazil.
- Norouzinezhad F, Ghaffari F, Norouzinejad A, Kaveh F, Gouya MM (2016) Cutaneous leishmaniasis in Iran: Results from an epidemiological study in urban and rural provinces. Asian Pacific Journal of Tropical Biomedicine 6: 614-619.
- Holakouie-Naieni K, Mostafavi E, Boloorani AD, Mohebali M, Pakzad (2017) Spatial modeling of cutaneous leishmaniasis in Iran from 1983 to 2013. Acta Tropica 166: 67-73.
- Butsch F, Lorenz B, Goldinger A, Stebut EV (2016) Topical treatment with a two component gel releasing nitric oxide cures C57BL/6 mice from cutaneous leishmaniasis caused by Leishmania major. Experimental dermatology 25: 914-916.
- van Griensven J, Gadisa E, Aseffa A, Hailu A, Beshah AM, et al. (2016) Treatment of Cutaneous Leishmaniasis Caused by Leishmania aethiopica: A Systematic Review. PLoS Negl Trop Dis 10: e0004495.
- Sarkari B, Sattari H, Moein MR, Tamadon AM, Rad RS, et al. (2016) Effect of topical gel prepared with hydroalcoholic extract of Echinacea purpurea on treatment of Leishmania major-induced cutaneous leishmaniasis in BALB/C mice. J Pharm Negat Results 7: 12.
- Alborzi A, Pouladfar G, Attar A, Falahi F, Jafarpour Z, et al. (2016) Effectiveness of Short-Course Meglumine Antimoniate (Glucantime®) for Treatment of Visceral Leishmaniasis: A 13-Year, Multistage, Non-Inferiority Study in Iran. Am J Trop Med Hyg 96: 16-0345.
- Ghoyonlo VM, Jafari MR, Yazdanpanah MJ, Esmaili H, Noori S, et al. (2016) Lack of efficacy of liposomal glucantime in the treatment of cutaneous leishmaniasis. Indian J Dermatology, Venereology, and Leprology 82: 347.
- Gutiérrez V, Seabra AB, Reguera RM, Khandare J, Calderón M, et al. (2016) New approaches from nanomedicine for treating leishmaniasis. Chem Soc Rev 45: 152-168.
- Ghosh P, Mondal S, Bera T (2016) preparation and characterization of andrographolide nanoparticles for visceral leishmaniasis chemotherapy: in vitro and in vivo evaluations. Int J of Pharm Pharm Sci 8: 102-107.
- das Neves J, Nunes R, Rodrigues F, Sarmento B (2016) Nanomedicine in the development of anti-HIV microbicides. Adv drug deliv rev 103: 57-75.
- Balakrishnan S, Bhat FA, Raja Singh P, Mukherjee S, Elumalai P, et al. (2016) Gold nanoparticle–conjugated quercetin inhibits epithelial–mesenchymal transition, angiogenesis and invasiveness via EGFR/VEGFRâ€2â€mediated pathway in breast cancer. Cell Prolif 49: 678-697.
- Shanker K, Khare P, Tiwari N, Mohanty S, Bawankule DU, et al. (2016) Synthesis of Gold Mediated Biocompatible Nanocomposite of Lactone Enriched Fraction from Sahadevi (Vernonia cinerea Lees): An Assessment of Antimalarial Potential. Curr top med chem 16: 2043-2050.
- Bavand Z, Gholami S, Honari S, Esboei BS, Torabi N, et al. (2014) Effect of Gold Nanoparticles on Giardia Lamblia Cyst Stage in In vitro. Arak Medical University J 16: 27-37.
- Azubel M, Kornberg RD (2016) Synthesis of Water-Soluble, Thiolate-Protected Gold Nanoparticles Uniform in Size. Nano letters 16: 3348-3351.
- Rajeshkumar S, Malarkodi C, Vanaja M, Gnanajobitha G, Paulkumar K, et al. (2013) Antibacterial activity of algae mediated synthesis of gold nanoparticles from Turbinaria conoides. Der. Pharma. Chemica 5: 224-229.
- Hajipour MJ, Fromm KM, Ashkarran AA, Jimenez de Aberasturi D, de Larramendi IR, et al. (2012) Antibacterial properties of nanoparticles. Trends in biotechnology 30: 499-511.
- Koonce NA, Quick CM, Hardee ME, Jamshidi-Parsian A, Dent JA, et al. (2015) Combination of Gold Nanoparticle-Conjugated Tumor Necrosis Factor-α and Radiation Therapy Results in a Synergistic Antitumor Response in Murine Carcinoma Models. Int J Radiat Oncol Biol Physics 93: 588-596.
- Das S, Roy P, Mondal S, Bera T, Mukherjee A (2013) One pot synthesis of gold nanoparticles and application in chemotherapy of wild and resistant type visceral leishmaniasis. Colloids Surfaces B Biointerfaces 107: 27-34.
- Lalitha A, Subbaiya R, Ponmurugan P (2013) Green synthesis of silver nanoparticles from leaf extract Azhadirachta indica and to study its anti-bacterial and antioxidant property. Int J Curr Microbiol App Sci 2: 228-235.
- Zhao P, Li N, Astruc D (2013) State of the art in gold nanoparticle synthesis. Coordination Chemistry Reviews 257: 638-665.
- Pratsinis A, Hervella P, Leroux JC, Pratsinis SE, Sotiriou GA, et al. (2013) Toxicity of silver nanoparticles in macrophages. Small 9: 2576-2584.
- Salah R (2015) Antileishmanial activities of chitin and chitosan prepared from shrimp shell waste.
- Rahimi MT, Ahmadpour E, Rahimi Esboei B, Spotin A, Kohansal Koshki MH, et al. (2015) Scolicidal activity of biosynthesized silver nanoparticles against Echinococcus granulosus protoscolices. Int J Surgery: 128-133.
- Saeed M, Omar A, Hussein MZ, Elkhidir IM, Al-Qubais, et al. (2014) In-Vitro Evaluation of Chitosan's Capacity in Delivering Calcium Phosphate Nano-Adjuvant: A Novel Mucosal Vaccine Carrier. J Chitin Chitosan Sci 2: 259-266.
- Mohebali M, Sarkar S, Gilani K, Akhoundi, Esmaeili, et al. (2015) Nanosilver in the treatment of localized cutaneous leishmaniasis caused by Leishmania major (MRHO/IR/75/ER): an in vitro and in vivo study. DARU J Pharma Sci 17: 285-289.
- Karimi M, Dalimi A, Jameie F, Dalimi A, Ghafarifar F (2016) Healing effect of induction of direct electric currents plus selenium and silver nanoparticle on skin lesions caused by leishmanial major in balb/c mice. urmia med j 26: 828-835.
- Jebeli MS, NMB Mohamed (2013) HTML: Synthesis and Charactrization of Ni-Zn Ferrite Based Nanoparticles by Sol-Gel Technique.
Citation: Vazini H (2018) The In vitro and In vivo Efficacy of Gold Nanoparticle in Comparison to the Glucantime as a Therapeutic Agent against L. major. J Infect Dis Ther 6: 373. DOI: 10.4172/2332-0877.1000373
Copyright: © 2018 Vazini H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4151
- [From(publication date): 0-2018 - Feb 22, 2025]
- Breakdown by view type
- HTML page views: 3438
- PDF downloads: 713
