Case Report Open Access
The Importance of EGFR Mutation Detection Method for the Correct Clinical Management of Patients with Non-Small Cell Lung Cancer: A Case Report
Zizzi A1*, Barbisan F1, Laici G2, Postacchini E1, Biagiotti L1, Tozzo S1, Mei F3 and Scarpelli M11Department of Biomedical Sciences and Public Health, Pathologic Anatomy and Histopathology Division, Azienda Ospedaliero-Universitaria Ospedali Riuniti, Ancona, Italy
2Medical Oncology Unit, S. Croce Hospital, Fano, Italy
3Department of Immunoallergic and Respiratory Diseases, Pulmonary Diseases Unit, Azienda Ospedaliero-Universitaria Ospedali Riuniti, Ancona, Italy
- *Corresponding Author:
- Antonio Zizzi
Department of Biomedical Sciences and Public Health
Pathologic Anatomy and Histopathology Division
AOU Ospedali Riuniti, Via Conca, 71–Ancona, Italy
Tel: +395964825
Fax: +390715964809
E-mail: antonio.zizzi@ospedaliriuniti.marche.it
Received date: March 09, 2017; Accepted date: March 14, 2017; Published date: March 17, 2017
Citation: Zizzi A, Barbisan F, Laici G, Postacchini E, Biagiotti L, et al. (2017) The Importance of EGFR Mutation Detection Method for the Correct Clinical Management of Patients with Non-Small Cell Lung Cancer: A Case Report. J Clin Exp Pathol 7:305. doi: 10.4172/2161-0681.1000305
Copyright: © 2017 Zizzi A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
In patients with non-small cell lung cancer (NSCLC), the naïve double EGFR mutation of exon 18 represents an unusual event and the few papers in the literature demonstrated the attenuated response to different tyrosine kinase inhibitors (TKIs). We presented a case of a 73-year-old female smoker patient with adenocarcinoma in the right upper lung lobe diagnosed in another Italian Hospital Center and the first molecular analysis, performed by the pyrosequencing platform in a cytological smear, identified a G719A mutation of EGFR gene. The patient was immediately treated with afatinib at a daily dose of 40 mg as TKI. During the normal follow up it was noted a partial response (PR) to treatment and, a new Computer Tomography, revealed the presence of an abnormal shadow in the right lower lung. A new Transbronchial Needle Aspiration (TBNA) and the consequent cyto-histological diagnosis revealed a relapse of the disease. A new molecular analysis made in our Center with Sequenom platform, showed a double EGFR mutation (G719A+E709A), both of exon 18. We also tested the EGFR with Sequenom platform in the cytological smear used for the first diagnosis, kindly sent us by Pathological Anatomy Unit of the Hospital Center, revealing both the double EGFR mutation G719A+E709A. Therefore, both mutations were the naïve double EGFR mutation of exon 18.
This result explained the PR to afatinib treatment and highlighted the importance of multiplex and sensitive methods used to identify the correct EGFR activating mutations in NSCLC, on the right clinical management of these patients.
Keywords
Lung; Adenocarcinoma; EGFR mutation; Afatinib; Sequenom platform; Pyrosequencing
Abbreviations
NSCLC: Non-small-cell lung cancer; EGFR: Epidermal growth factor receptor; TKIs: Tyrosine kinase inhibitors; TBNA: Transbronchial Needle Aspiration; PR: Partial response; FFPE: Formalin-fixed paraffin-embedded.
Introduction
Non-small-cell lung cancer (NSCLC) is the most common form of lung cancer [1], often diagnosed at advanced stages when surgery is no longer a viable choice. Several studies on NSCLC have shown that 10-15% of the patients are carriers of activating somatic mutations in the epidermal growth factor receptor gene (EGFR), which point mutations in exon 18 (including mutations in codon 709 and 719) short in-frame deletions in exon 19, clustered around the amino-acid residues 746-750 and a specific exon 21 point mutation (above all L858R) [2-4]. There is a correlation between these mutations, which together they account for 90% of the total of activating mutations, and increased efficacy of treatment with tyrosine kinase inhibitors (TKIs, e.g.: gefitinib, afatinib and erlotinib) for these patients [1-3,5].
Therefore, the identification of activating somatic mutations of EGFR gene in patients with NSCLC is critical step to choice the best therapy with TKIs.
Historically, direct sequencing of DNA extracted from formalinfixed paraffin-embedded (FFPE) tumour tissue blocks during biopsy or resection were the gold standard methods to identify the somatic mutations of EGFR gene in NSCLC patients [1].
Actually, alternative and less invasive sources of tumour material can be collected for diagnosis of NSCLC, such as cytology simple or liquid biopsy, and a number of multimarker methods with improved sensitivity (eg. sequenom MassARRAY platform, real time quantitative PCR, next generation sequencing, etc.) are currently used in alternative to direct sequencing due to its less sensitivity [6-8].
Herein, we present an uncommon case of adenocarcinoma of the lung with a partial response (PR) to fist-line treatment with afatinib as TKI and harboring double EGFRactivating mutation of exon 18, found in a second Transbronchial Needle Aspiration (TBNA).
Case Report
We presented a case of a 73-year-old female smoker patient (10-15 cigarettes daily from 1963 to 2015) with a history of poorly differentiated adenocarcinoma of the lung.
Briefly, the patient had worked as coating of furniture. Due to unspecified cardiac arrhythmia, patient was through treatment with Sotalex (Bristol-Myers Squibb Srl) 160 mg daily and for an arterial hypertension with two drugs: Micardis Plus (Boehringer Ingelheim Italia SpA) and Norvasc (Pfizer Italia Srl). In January, 2015 appearance of esophageal motility disorder, at epigastric site and radiating to back, allows decreased appetite and weight loss. At March, 2105 an esophagogastroduodenoscopy documented a vegetating lesion at 28 cm from the teeth and a widespread chronic gastritis with mucosal erosions. The histological evaluation of gastric biopsies revealed an extensive intestinal metaplasia of gastric mucosa and the squamous epithelium showed no significant morphological alterations.
A Computer Tomography (CT) scan conducted after 15 days, evidenced a massive expansion process lobed in the right upper lung lobe, together with lymphadenopathies and adjacent esophagus compression and an adenopathy along the celiac artery. The patient revealed serum level of CEA >1500 ng/ml and of CA19.9=322 U/ml. In April, 2015, the patient performed a TBNA of subcarinal lymph nodes sent at a Pathological Anatomy Section of another Italian Hospital Center for the cytological and molecular diagnoses. The cytological examination showed rare groups of atypical epithelial cells likely adenocarcinoma of the lung (TTF-1 positive and p40 negative).
Molecular analysis using a cytological smear, performed with the pyrosequencing procedure, identified a G719A mutation of EGFR gene. Based on these cytological and molecular results, the patient received first-line treatment with afatinib at a daily dose of 40 mg. It was noted a slight clinical benefit already after 2 weeks of therapy. In July, 2015 patient performed a new CT scan that documented a slight reduction of all clinical disease parameters, likely partial response (PR) to treatment.
The patient well tolerated the treatment for six month, with moderate symptoms of diarrhea and oral stomatitis. Due to evident nasal crusts, the treatment was discontinued for some days in December, 2015. Another CT scan made in January, 2016 revealed the presence of an abnormal shadow in the right lower lung. The patient was admitted to the Pulmonary Diseases Unit, Department of Internal Medicine, United Hospitals, Ancona, Italy in February, 2016 and received a TBNA with a transbronchial biopsy of right lower lobe for a new cyto-histological and molecular diagnosis. Cytological simple, sent to the Cytopathology Unit, United Hospitals, Ancona, Italy, revealed the presence of the voluminous atypical epithelial cells related to lung adenocarcinoma.
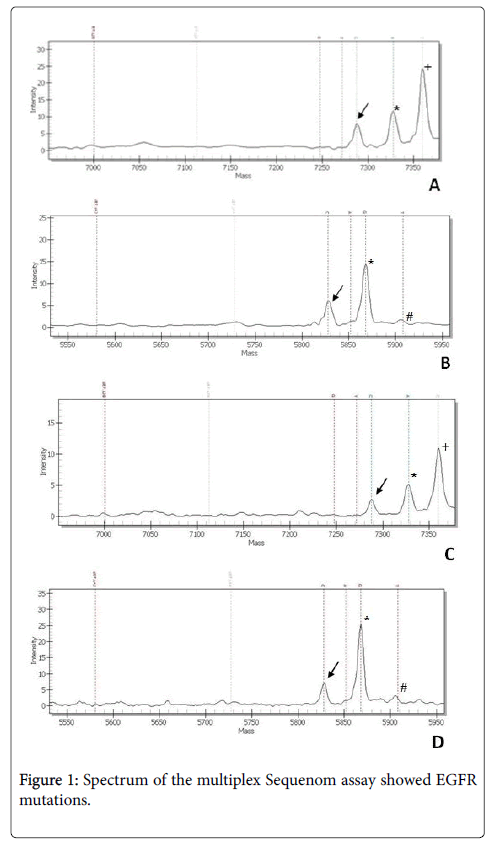
The biopsy, sent to the Pathological Anatomy and Histopathology Unit, Department of Internal Medicine, United Hospitals, Ancona, Italy for detection of the EGFR T790M mutation, revealed a bronchial mucosa infiltrated by poorly differentiated adenocarcinoma, with TTF1 positive and p63 negative cells. Allele-specific single base extension products were quantitatively analyzed in a cytological smear by matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) according to the user’s manual of the MassARRAY system (Sequenom, Inc., San Diego, CA). We identified a double EGFR mutation: at codon 719 (G719A) and at codon 709 (E709A), both in the exon 18 (Figure 1), without evidence of the EGFR T790M mutation. We also tested the EGFR with Sequenom platform in the cytological smear used for the first diagnosis, kindly sent us by Pathological Anatomy Unit of the Hospital Center, revealing both the double EGFR mutation G719A+E709A (Figure 1). The patient receive a dose of 30 mg daily of afatinib as treatment and reveal serum level of CEA=53 ng/ml and of CA19.9=22.8 U/ml (Figure 1).
A) arrow shows the mutant peak at the position 2126 consistent with an E709A mutation (2126A>C) on cytological smear used for the second molecular diagnosis, asterisk marks the wild-type peak and +indicate other SNPs/mutations. B) arrow shows the mutant peak at the position 2156 consistent with an G709A mutation (2156G>C) on cytological smear used for the second molecular diagnosis, asterisk marks the wild-type peak and # indicate dotted lines of unextended probes. C) Arrow shows the mutant peak at the position 2126 consistent with an E709A mutation (2126A>C) on cytological used for the first molecular diagnosis, asterisk marks the wild-type peak and +indicate other SNPs/mutations. D) arrow shows the mutant peak at the position 2156 consistent with an G709A mutation (2156G>C) on cytological smear used for the first molecular diagnosis, asterisk marks the wild-type peak and # indicate dotted lines of unextended probes.
A CT scan made in May, 2016 revealed disease progression to mediastinal lymph-nodes, lung and adenal gland. Therefore, the patient started a first line chemotherapy with cisplatin plus gemcitabine, performing three cycles with apparent clinical benefit. Follow up will be continued. The patient provided written informed consent for the publication of her case details.
Discussion
De novo resistance (or primary resistance) to TKI therapy can occur in the 5% of lung cancers, even in the presence of an activating mutation in EGFR. Primary TKI resistance may also be mediated by other rarer mutations in EGFR that occur together with drug-sensitive mutations [9]. In addition, several studies reported that, in the majority of patients who initially respond positively to EGFR TKI treatment, occur the mechanisms of acquired resistance (secondary resistance) to the TKI drugs themselves, with consequent progression of the disease, mostly due to the presence of the missense EGFR mutation T790M of exon 20 [10].
Herein, we report an unusual case of a female smoker patient with lung adenocarcinoma, presenting a PR to afatinib treatment as TKI therapy and sent to our Center for detection of EGFR T790M mutation. The Sequenom platform revealed the drug sensitive G719A and a rare E709A mutation of EGFR gene. This E709A mutation, undetected in the first molecular diagnosis made by pyrosequencing procedure in another Italian Hospital Center, was then a naïve EGFR mutation of exon 18 of EGFR gene, together with G719A. The attenuated response or less sensitivity to TKIs and downstream effector phosphorylation profiles than the EGFR mutation of codon 719 alone were demonstrated only by two studies [11,12], in tumor simple and in vitro NSCLC patients, respectively, while the more attenuated response to TKIs due to naïve co-presence of the mutations at 719 and 709 codons of the same exon was found and/or described by few reports [8,9,13-15]. In particular, Tam et al. (2009), found that double mutant (E709A+G719C) of EGFR, showed a response profile intermediate between those of E709A and G719C, suggesting that the presence of the uncommon E709A, in addition to the activating G719C, would confer relative resistance to gefitinib treatment [12]. Thus, the attenuated response to afatinib found in our patient was due to presence of the E709A mutation in addition to the G719A of EGFR gene, and it was not due to the mechanisms of secondary resistance occurred after TKI treatment.
Recent evidences in multimarker sequencing methods, such as Sequenom or next generation technologies, could provide a multiplexed and high range detection of EGFR and other activating genes in NSCLC patients [6-8]. In our experience, the usefulness and feasibility of the Sequenom platform is due to the simplest, multimarker, most rapid, sensitive and cost-effective method to evaluate many genes and several hundred mutations per case only in 2-5% of the DNA isolated from archival and routinely processed FFPE tumour tissue blocks in more patients.
Pyrosequencing could represented a potential alternative to the Sequenom platform to detect small gene portions of some genes only, but the lack of gene and codons multiplexing made the procedure more time consuming, required more DNA [7] and above all it could not identify the important mutations for the best clinical management of these patients. The sensitivity of method used for gene mutations is an important requirement to optimize the initial target therapy and to define the right clinical management of these patients, as well as to avoid repeated and invasive surgery procedures for the patients.
Moreover, we agree with other author [8,16] suggesting the potential benefit of using of the new sensitive technologies to non-invasively screen of activating mutations in NSCLC patients, such as in plasma or other human fluid cell-free DNA. Thus, these findings may provide another aspect on future targeted molecular therapy.
References
- Ellison G, Zhu G, Moulis A, Dearden S, Speake G, et al. (2013) EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol 66: 79-89.
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, et al. (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350: 2129-2139.
- Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, et al. (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304: 1497-1500.
- Sharma SV, Bell DW, Settleman J, Haber D (2007) Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 7: 169-181
- Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, et al. (2005) Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 97: 339-346.
- Wu K, Huang RS, House L, Cho WC (2013) Next-generation sequencing for lung cancer. Future Oncol 9: 1323-1336.
- Fumagalli D, Gavin PG, Taniyama Y, Kim SI, Choi HJ, et al. (2010) A rapid, sensitive, reproducible and cost-effective method for mutation profiling of colon cancer and metastatic lymph nodes. BMC Cancer 10: 101.
- Sozzi G, Roz L, Conte D, Mariani L, Andriani F, et al. (2009) Plasma DNA Quantification in Lung Cancer Computed Tomography Screening. Five-Year Results of a Prospective Study. Am J Respir Crit Care Med 179: 69-74.
- Pao W, Chmielecki J (2010) Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer 10: 760-774.
- Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, et al. (2005) Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2: 73.
- Kobayashi S, Canepa HM, Bailey AS, Nakayama S, Yamaguchi N, et al. (2013) Compound EGFR mutations and response to EGFR tyrosine kinase inhibitors. J Thorac Oncol 8: 45-51.
- Tam IY, Leung EL, Tin VP, Chua DT, Sihoe AD, et al. (2009) Double EGFR mutants containing rare EGFR mutant types show reduced in vitro response to gefitinib compared with common activating missense mutations. Mol Cancer Ther 8: 2142-2151.
- Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, et al. (2004) Mutations of the Epidermal Growth Factor Receptor Gene in Lung Cancer: Biological and Clinical Implications. Cancer Res 64: 8919-8923.
- Tam IY, Chung LP, Suen WS, Wang E, Wong MC, et al. (2006) Distinct epidermal growth factor receptor and KRAS mutation patterns in non-small cell lung cancer patients with different tobacco exposure and clinicopathologic features. Clin Cancer Res 12: 1647-1653.
- Sequist LV, Besse B, Lynch TJ, Miller VA, Wong KK, et al. (2010) Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: results of a phase II trial in patients with advanced non-small-cell lung cancer. J Clin Oncol 28: 3076-3083.
- Couraud S, Vaca-Paniagua F, Villar S, Oliver J, Schuster T, et al. (2014) BioCAST/IFCT-1002 investigators. Noninvasive diagnosis of actionable mutations by deep sequencing of circulating free DNA in non-small cell lung cancer: Findings from BioCAST/IFCT-1002. Clin Cancer Res 20: 4613-4624.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 3100
- [From(publication date):
April-2017 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 2268
- PDF downloads : 832