Review Article Open Access
The Importance of Dietary Behavior to the Health of Monozygotic Twins
Cardoso C*, Afonso C and Bandarra NMDivision of Aquaculture and Upgrading, Department of Sea and Marine Resources, Portuguese Institute for the Sea and Atmosphere, Portugal
- *Corresponding Author:
- Carlos Cardoso
Division of Aquaculture and Upgrading, Department of Sea and Marine Resources
Portuguese Institute for the Sea and Atmosphere–IPMA, Avenida Brasília
1449 006, Lisbon, Portugal
Tel: 00351 21 302 7034
Fax: 00351 21 301 5948
E-mail: carlos.cardoso@ipma.pt
Received date: May 08, 2017; Accepted date: June 01, 2017; Published date: June 12, 2017
Citation: Cardoso C, Afonso C, Bandarra NM (2017) The Importance of Dietary Behavior to the Health of Monozygotic Twins. J Community Med Health Educ 7:526. doi:10.4172/2161-0711.1000526
Copyright: © 2017 Cardoso C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Community Medicine & Health Education
Abstract
The development, health, and phenotype of monozygotic twins can be decisively affected by dietary behaviour. Several channels of biochemical, microbiological, and physiological differentiation between twins are affected by the particular traits of any given diet and are to be evaluated under a new perspective and point of view. The nutritional factors have a more direct impact in the gut flora, obesity and its associated health problems. Diabetes and cardiovascular diseases show a strong dependency on the dietary options often trumping the genetic aspects may be affected leading to the metabolic syndrome and other diseases. Other systems such as the endocrine and the immune systems also give further examples of the differentiation of health outcomes as a result of dietary patterns. Moreover, cancer frequency, onset, and development are partially related to food constituents, especially in the case of cancer diseases arising in the gastrointestinal tract. The epigenetic changes that occur during lifetime be partially due to nutrition and may contribute to the pathogenesis of cancer. Indeed, some evidence ascribes phenotypic discordance between monozygotic twins partially to epigenetic factors. However, the role of diet in the development, ageing, and health status of monozygotic twins is still not fully understood and warrants further study. It is possible that over the next decade a full characterization of human genomic, epigenomic, and transcriptomic data will be within the reach of most researchers and shed much light onto the interplay of genetic determined processes and nutrition effects.
Keywords
Monozygotic twins; Behaviour; Nutrition; Gut flora; Health endpoints
Introduction
Unquestionably, diet has a large impact on the development, wellbeing, and health of monozygotic twins, thereby decisively being one of the most important factors modeling phenotypic variations between them. This happens through diverse channels and according to very distinct class of phenomena. Indeed, these encompass such different aspects as the gut flora, the direct and indirect effect on cardiovascular, metabolic, and endocrine physiology of the human body, the immune system, challenging diseases as cancer, or very subtle changes at the epigenetic level. All these sensitive areas together with the science underpinning such realities are the subject of a thorough analysis in this chapter. A final section deals with those subjects not totally understood or still open to further research progress as well as with the evolution perspectives for this particular field of scientific endeavor.
The role of the diet in the development of humans and their health and the large impact of recent dietary changes are well known [1]. The link between food and health has been evaluated and related with scientific studies [1]. According to popular wisdom, “we are what we eat”. Of course, this is exaggerated if it is to be interpreted as meaning that diet determines all human phenotypical features and health. In fact, genetics, biological factors such as the intrauterine environment, upbringing, education, child and adolescent environment, pollution, occupational hazards, social aspects and situations, and many other factors exert a strong influence on the specific set of characteristics of an human adult and its propensity to disease [2,3].
History tells us that a great importance was always attached to food and human evolution was largely influenced by food resources and strategies to use the nutrient-richest foods [4]. The agricultural Neolithic revolution and the emergence of the so-called Western diet have profoundly affected the nutritional balance of human diet and are deemed responsible for several diseases [1]. Indeed, ‘seven deadly sins’ have been ascribed to the diet of sedentary human populations, particularly, in the more developed countries: Glycemic load (blood glucose raising potential), fatty acid composition (for instance, low ω3/ ω6 fatty acid ratios and an excess of saturated fat), macronutrient composition (excessive fat and easily absorbed sugars), micronutrient density (for instance, low zinc), acid-base balance (net acid generation after metabolism), sodium-potassium ratio (too much sodium), and fibre content (too low) [1]. Besides being perceived as an essential condition for survival and health, early observers reported food importance to mental or physical fitness [5].
In the particular case of twins, its study started in earnest in the XIX century with the objective of estimating the relative powers of nature and nurture [6] that is, the relative role of genetics and environment (diet, education, etc.). Francis Galton’s ‘The history of twins’ was a seminal work in this field [6]. This Victorian era researcher concluded that heredity mattered more than environment. However, the introduced and developed methodologies were more important than this conclusion. As concepts evolved during historical time, these methodologies were later further developed with the establishment of the classical twin method of comparing the similarities of monozygotic and dizygotic twins [7]. In this context, diet is a fundamental factor that operates independently of genetics to a large extent, since it has been shown that food preferences are primarily attributed to environment and not genetic predispositions [8]. The first, systematic, clinically-ascertained twin studies of the diet-genetics binomial are mainly found in the second half of the XX century, for instance, regarding eating disorders [9] with first studies pointing to higher concordance rates in monozygotic twins than in dizygotic twins [10].
The studies between monozygotic twins enable to exclude the genetic factor as a variable and, as such, are very useful whenever environmental factors for instance, diet-need to be examined. This may be very important when critical issues and the connections between diet and obesity [11], diabetes [12], cardiovascular [13-15], metabolic [16], endocrine [17] immune system [18] and cancer [19] diseases are to be highlighted. Accordingly, the gold standard for distinguishing genetic from environmental traits has been the comparison between twins [20]. The following sections will cover these several connections and systems, thereby highlighting the complex interplay between genome, epigenetic factors, and nutrition and underlining how much a given diet may differentiate the nature and fate of monozygotic twins.
Gut Flora: A Factor of Variability between Twins
It is well known that human gut flora as well as other human microbiomes is quite rich and diverse [21]. The area most colonized by microbes is the distal gut [22]. The typical gut flora alone consists of hundreds of bacterial species, collectively encoding an enormous gene set that is 150 times larger than the human set of genes [23]. The gut microbiome is fundamental for many essential processes, including vitamin and amino acid biosynthesis, dietary energy harvest, and immune development [24]. This importance and the observed large natural variability leads to an obvious question: does gut flora differ between monozygotic twins?
It must be remarked that human microbiome projects are being initiated throughout the world, with the goal of correlating human physiological phenotypes with the structures and the functions of their indigenous microbial communities [25]. These authors have endeavoured to sequence in a more thorough way the DNA of the organisms composing the gut microbiome. This was also done with the purpose of understanding how much of the observed organismal diversity is due to methodological insufficiencies. Indeed, it was confirmed a high level of species diversity after reduction of methodological noise [25]. It was shown that the 54 studied twin pairs had very different species assemblages (Table 1). There were important differences at the genetic microbiome level and at other biochemical levels. Namely, differences within twins were found in the set of carbohydrate active enzymes (for instance, cellulases) and in transcriptional activities [25]. It was observed that the microbial community genes encoding for the carbohydrate-active enzymes were highly enriched in the Faecalibacterium bins found in the microbiome of one of the twins, but not in the other. Even genes widely distributed could lead to variability as a result of abundance variation. Therefore, results highlighted another level of genetic variation between humans, imparting variability to otherwise genetically identical twins [25]. Though these twins were at least 5 km apart [26], they were both obese and had a very similar life history: they had been vaginally delivered and had no history of intestinal disease.
| Health Aspect | Main Findings | Reference |
|---|---|---|
| Gut flora | Twin pairs had very different species assemblages, different carbohydrate active enzymes, and different transcriptional activities | [25] |
| Fermented milk products affected human fecal metatranscriptome during consumption period, but did not change bacterial species composition in the gut | [27] | |
| GI diseases | The concordance rates between twin pairs were 6-17% in ulcerative colitis and 37-58% in Crohn’s disease | [28,30-33] |
| Obesity | Twin studies enabled to estimate heritability impact on BMI to be between 40 and 70% | [43,44] |
| Environmental aspects may generate important differences in obesity according to a study on monozygotic twins (diet richer in fat leads to obesity regardless of the genetic background) | [50] | |
| Type 2 diabetes | Prospective study on monozygotic twins reported that the observed rate of concordance is, at least, 76%-very strong genetic component | [53] |
| CVD | Important role of genetics based on data from monozygotic and dizygotic twins, but studies on monozygotic twins have also shown that diet is a decisive factor | [60,61] |
| Adverse effect of dietary sodium on coronary flow and, thus, on the cardiovascular system | [61] | |
| A monozygotic twin study showed that dietary n-3 PUFA increased high-density lipoprotein 2b, which is protective against CVD | [67] | |
| Twin study established a connection between sugar and low n-3 PUFA and high triglyceride levels | [68] | |
| Metabolic syndrome | Twin studies have circumscribed the impact of genetic factors to 50% | [72] |
| Goiter | Strong genetic dependence was indicated by twin studies, being concordance rate in monozygotic twins over 50% higher than in dizygotic twins | [74,75] |
| Autoimmune diseases | Studies on twins have estimated concordance rates of 30% for multiple sclerosis | [18] |
| Study on monozygotic twins showed that besides genetic factors environmental aspects are important in the genesis of autoimmune thyroid diseases | [78] | |
| The importance of 25-hydroxyvitamin D in multiple sclerosis was corroborated by a twin study | [83] | |
| In rheumatoid arthritis, concordance rate in monozygotic twins did not exceed 30% | [87,88] | |
| A twin study claimed that dietary supplementation with calcium and vitamin D benefited bone mineral density | [92] | |
| Type 1 diabetes had a disease concordance rate of less than 40% in identical twins | [94] | |
| For Hashimoto’s thyroiditis, concordance rates varied between 35 and 70% in identical twins | [100] | |
| Cancer | A study on cohorts of twins concluded that environment has the main role in causing sporadic cancer | [107] |
| A study on testicular cancer in twins showed that factors other than diet affect disease incidence | [119] | |
| Epigenetics | Studies have shown that phenotypic discordance between monozygotic twins is partially due to epigenetic factors | [124] |
| A study on obesity-discordant monozygotic twins reported several differences in the transcription profiles in adipose tissue between twins | [132] |
Table 1: Main findings in the scientific literature concerning dietary effects on the health and well-being of monozygotic twins.
Of course, physical separation meaning living in different households and environments and experiencing slight differences in diets and other aspects seems to be important in generating human microbiome diversity in monozygotic twins. For instance, an interesting study [27] showed that commercially available fermented milk products (FMP) containing a consortium of bacterial strains, such as Bifidobacterium animalis , Lactobacillus delbrueckii , Lactococcus lactis and Streptococcus thermophilus , were able to have an impact in the human fecal metatranscriptome though confined to the period of FMP consumption. However, no significant effect was detected on the bacterial species composition or in the proportional representation of genes encoding known enzymes [27]. Hence, effects were circumscribed to changes at the expression level of microbiomeencoded enzymes involved in several metabolic routes, namely concerning carbohydrate metabolism. A different caloric content in the diet over many years may not only lead to different outcomes concerning obesity, but also affect the composition and characteristics of the human gut microbiome [22] Such effects were identified in a dataset composed of twin-mother trios [22].
Gastrointestinal (GI) diseases and, in particular, inflammatory bowel diseases (IBD), seem to be associated to specific bacterial assemblages in the gut
28]. This was shown for ileal Crohn’s disease. These diseases are only to a limited extent affected by human genetics [29] genetic susceptibility, being common twin pairs discordant for disease [28]. In fact, concordance rates for monozygotic twins range from 6 to 17% for ulcerative colitis [30-33] and from 37% to 58% for Crohn’s disease [31,33]. Concerning this IBD subject, significant differences in the gut microbiomes of identical twins according to Crohn’s disease status have been found [34,35]. Hence, differences in diet that may cause specific bacterial assemblages in the GI tract are conducive (together with other factors) to higher probability of developing IBD and thus become a source of variation in the quality of living of monozygotic twins. Nevertheless, studies are not fully conclusive and the dietary aspects leading to IBD are not clearly identified [36]. There is some evidence associating a higher intake of ω6 fatty acids [37] as well as frequent fast-food intake [38] with enhanced IBD risk.
Future research in this novel scientific field is needed. Particularly, the characterization of the enzymatic activity of these systems, the breadth of their distribution of organisms, the host and environmental parameters (namely diet) determining their abundance in the human gut microbiome, and their effects on host nutrient/energy budget are all aspects whose study is warranted.
Obesity, Diabetes and Cardiovascular Disease: Nutrition and Twins
Overweight and obesity are an important worldwide concern in terms of clinical and public health since they are associated to an increased risk of other diseases, such as type-2 diabetes and cardiovascular diseases (CVD), which are major causes of mortality [39]. In 2005, the estimated world’s overweight adult population were 937 million and the obese reached the 396 million. Yet, it is expected that in 2030 the overweight and obese adult’s figures surpass a total of 2 and 1 billion of individuals, respectively [39]. In what concerns children, the prevalence has augmented significantly in developed and developing countries [40]. It has been estimated that overweight and obesity caused more than 3 million deaths worldwide in 2010, with huge public health losses as measured in disability-adjusted life-years (DALYs) [40].
It is well known that the onset of obesity is triggered by an imbalance between energy intake and expenditure [41] and that the quantity of calories ingested has a direct impact on human weight. Indeed, there are several nutritional recommendations to prevent weight gain and other diseases, like CVD and type 2 diabetes. In fact, it has been recommended the consumption of whole grains, vegetables, fish, fruits, and nuts, whereas the consumption of refined grains and sugary drinks is deemed pernicious.
Twin studies have shown that there are genetic influences on obesity [42]. Twin studies estimate heritability of body mass index (BMI) to be 40-70% in children and adults [43,44] (Table 1) and other anthropometric measures of obesity and fat distribution, such as skinfold thickness, waist circumference, and waist to hip ratio display a similar impact of heritability [43-46]. Twins studies have also demonstrated a considerable influence of the genes on eating patterns of adults [47].
On the other hand, nutrition seems to be decisive for weight variation and obesity outcomes [48]. Of course, shared environmental influences during the infancy of twins contribute to reinforce the genetic driver to similarity between twins [49]. However, environmental differences after leaving their parents’ home may generate important differences, namely concerning obesity [50]. This study on monozygotic twins highlighted the great importance of diet for obesity development. In fact, the obese twins reported preference for fatty foods three times more frequently than the lean co-twin [50]. Furthermore, when recalling taste preference for fat at the time the twins left their parental homes, both the obese and lean co-twins consistently recalled that the obese twin had greater preference for fatty foods than the lean twin. Otherwise, psychological traits of the lean and obese co-twins did not diverge [50]. Hence, the conclusion is that preference for fatty foods and consequentially a diet richer in fat leads to obesity regardless of the genetic background. This preference may be due to several different factors, being possible that education exerts some effect on BMI [51].
Food preferences, dietary patterns, and obesity are influential in the development of type 2 diabetes. As with obesity, genetically based processes and environmental influences are both important. It has been claimed that heritability is high for slowness in eating (over 80% share) and satiety responsiveness (over 65%) and not so high for food responsiveness [less than 65%) [52]. A prospective study on monozygotic twins reported that the observed rate of concordance for type 2 diabetes can reach, at least, 76%, thereby pointing to a very strong genetic component [53]. However, the fact that not all monozygotic twins are concordant for this disease suggests that environmental factors may be relevant. A review [12] has established that an increased risk for developing type 2 diabetes is associated with overweight and obesity, abdominal obesity, physical inactivity, and maternal diabetes. These authors also found probable that a high intake of saturated fat contributes to an increased risk. Moreover, they claimed that from existing evidence it is also possible that n-3 polyunsaturated fatty acids (n-3 PUFA) and low glycemic index foods may reduce disease risk, while total fat intake and trans fatty acids may enhance risk [12]. It should also be remarked that the monochorionic intrauterine nutrition of most monozygotic twins has been shown to favor growth retardation [54] and that low birth weight is associated with increased risk of type 2 diabetes later in life [55,56].
Obesity is also a risk factor for CVD [57]. Data showed that weight gain after the young adult years conveyed an increased risk of CVD in both sexes that could not be ascribed either to the initial weight or the levels of the risk factors that may be due to weight gain. Besides obesity, there are other risk factors for CVD that can be directly-for instance, excessive salt [58] or indirectly diabetes [59] linked to diet. Nevertheless, genetic factors also play a role [15]. For instance, a study on the genetic and environmental effects on susceptibility to heart diseases for males and females with data from monozygotic and dizygotic twins confirmed the existence of an important role for genetics (Table 1) [60]. Indeed, it was shown that individual susceptibility to mortality due to heart diseases and coronary heart diseases had a strong genetic influence in both males and females.
However, studies on monozygotic twins have also shown that genetic factors do not explain all, being diet a decisive factor [61]. The influence of nutrition starts in the womb [62]. It has been proposed that fetal malnutrition in middle to late gestation may lead to permanent changes in metabolism and physiology that raise the risk of CVD in adulthood [63]. But, intrauterine nutrition is as shared as genes by the monozygotic twins and does not act as a differentiating variable.
Among nutritional parameters, mounting evidence suggests that too much sodium in a diet is conducive to a higher risk of stroke and CVD [64,65]. This was corroborated by a recent study [61] involving 286 male middle-aged twins. It was shown that habitual dietary sodium is inversely associated with coronary flow reserve independent of other factors, thus meaning an adverse effect of sodium on the cardiovascular system [61]. Regarding this issue, a recent study involving twins pointed out that both genetic predisposition and shared environment contribute to sodium intake [66]. However, salt consumption habits must be an informed choice of each individual.
It has also been reported that n-3 PUFA typically found in fish and other seafood have a positive effect on the lipoprotein profile [67]. This study on monozygotic twin pairs showed that n-3 PUFA increased the high-density lipoprotein 2b, which is deemed to be protective against CVD. Conversely, another twin study established a connection between a sweet-laden and fatty diet poor in n-3 PUFA and a pernicious variation of the triglyceride levels as well as of the particle size of very low-density lipoprotein [68]. Such effects were brought about by a ‘junk food’ diet encompassing French fries, hamburger, pizza, salty snacks, and sweets.
Therefore, though genetic, intrauterine, and infancy environment factors have an effect on the cardiovascular system, CVD is not predetermined by genes, being possible by choosing a healthy fat and low sodium diet to reduce disease risk. Furthermore, the inclusion of nutraceuticals in the diet may counteract dyslipidemia and reduce cardiovascular risk factor for coronary heart disease [69]. In this way, two identical twins may experience very different health outcomes if they choose different diets.
Nutrition and its Impact on Metabolic and Endocrine Diseases in Twins
The relationship between nutritional aspects and the development of metabolic and endocrine diseases is another important area of research also involving monozygotic twin studies.
Some of these diseases are related to obesity, diabetes, and CVD, which were addressed in previous section. Namely, the metabolic syndrome, a problem affecting energy utilization and storage, is considered to increase the risk of developing type 2 diabetes mellitus and CVD [70]. There is convincing evidence that diet plays an important role in the development and progression of the metabolic syndrome. Obesity is a key aetiological factor in the development of this health problem [70]. Monozygotic twin studies help understanding the biological impact of gene–nutrient interactions and provide a key insight into the pathogenesis and progression of dietrelated polygenic disorders, including the metabolic syndrome [16,70] The difference in concordance rates between monozygotic and dizygotic twins shows that this disease is at the crossroads of diet and genetics. In contrast, the heritability estimates for hyperinsulinaemia, hypertension, and hypertriacylglycerolaemia are low, thus pointing to a larger role of environmental influence on these components of the metabolic syndrome [16]. Several twin studies have emphasized the importance of environmental factors in different aspects related to metabolic syndrome [71,72] thereby circumscribing the impact of genetic factors to approximately 50% (Table 1) [72].
With regard to endocrine system diseases, there are quite diverse, ranging from diabetes mellitus (previously addressed) to goiter (largely related to iodine deficiency) and other thyroid diseases [some also autoimmune such as Graves’ disease and Hashimoto’s thyroiditis), Cushing’s disease, or premature ovarian failure. The relative importance of diet, other environmental factors, and genetic causes in the pathogenesis of this diverse array of diseases varies widely. Moreover, the interaction between environmental and genetic factors may be quite complex as in the case of Graves’ disease, which is a specific form of hyperthyroidism [73].
In the case of goiter, a strong genetic dependence is indicated by twin studies [74]. There is a higher concordance rate (over 50%) for goiter in monozygotic than in dizygotic twins [75]. Nevertheless, important influence is exerted by the diet, either by the iodine content in food or the presence of goitrogenic constituents in diet, such as flavonoids in millet and soybean, cyanogenic glucosides in cassava, or glucosinolates in vegetables belonging to the genus Brassica [76].
Sensitivity of the Twins Immune System to Dietary Variation and Autoimmune Disease
The immune system of humans and in particular, of monozygotic twins also responds to dietary patterns and their changes. As it is well known, this system has evolved to protect us from disease caused by microorganisms. This requires differentiation between materials that belong to the human organism (self) and those that do not belong (non-self). In some cases, there is a defective reaction of the immune system and so-called autoimmune disease develops as a result of reactivity to self. Complex interactions between genome and the environment determine which individuals will be affected by any given autoimmune disease. Important diseases in this class include celiac disease, lupus erythematosus, multiple sclerosis, psoriasis, rheumatoid arthritis, Sjögren’s syndrome, vasculitis, Hashimoto’s thyroiditis, and many others.
The influence of environmental factors on the genesis of these diseases has been shown by studies on identical twins [18]. For instance, these twins have disease concordance rates of only 50% (Crohn’s disease) or less (30%, for multiple sclerosis) (Table 1). In multiple sclerosis (MS), environmental factors such as vitamin D intake [18] and sunlight exposure [77] seem to be important as shown by comparison between monozygotic twins. A Danish study on monozygotic twins has also shown that besides genetic factors environment is of etiological importance for the genesis of autoimmune thyroid diseases [78]. Among environmental variables, diet can strongly influence the human body and its susceptibility to disease [79]. The mechanisms for this influence may be direct or indirect, even involving epigenetic changes (see below).
Concerning MS, there is a gradient of growing risk with higher latitude, which is coincident with sunlight exposure reduction and more frequent vitamin D deficiency [80] Vitamin D supplementation [81] and high concentrations of serum 25-hydroxyvitamin D in adulthood [82] were linked to lower MS risk. The importance of the latter substance was corroborated by a twin study approach [83]. However, in this case, gene factors may influence the trait. Nevertheless, the importance of dietary factors in the genesis of MS seems to be significant-consumption of fish three or more times a week was protective against MS for individuals with low sunlight exposure [84] and further studies on twins, especially using a casecontrol approach have been advised [85].
With regard to rheumatoid arthritis, there is a clear genetic component [86], but the concordance rate in monozygotic twins does not exceed 30%, leaving 70% to be explained by other factors [87,88]. The severity of the disease may also differ among concordant twins [89]. Dietary factors have been suggested to play a role [90]. Namely, higher consumption of caffeine and red meat has been associated with an increased risk of rheumatoid arthritis. On additionally, a higher level of intake of cooked, but not raw, vegetables has been claimed to protect against the onset of disease [91]. Moreover, a randomized placebo-controlled trial conducted on twenty pairs of identical twins over a period of six months showed that dietary supplementation with calcium and vitamin D greatly improved bone mineral density, a parameter that is associated to the severity of rheumatoid arthritis and that worsens life quality of patients [92].
Type 1 diabetes is also an immune-mediated disease, since β cell destruction is due to autoimmune reactivity [93]. Scientific evidence such as low disease concordance (less than 40%) in identical twins points to a critical role of environment in the disease etiology [94]. Nutritional factors are thought to be of high importance [95] Gut incretin hormones are secreted in different amounts as a response to glucose in twins with the disease, but not in their healthy co-twins [96] Increased weight gain in infancy has been associated to greater risk of type 1 diabetes. Moreover, cow milk is considered diabetogenic [97]. On the other hand, some vitamins and minerals have been proposed to protect against type 1 diabetes [95]. Recent results [98] have shown that Nε-carboxymethyl-L-lysine (CML), a glycotoxin possibly related to heat-treated dietary factors [99], is a diabetes risk factor. In twins, familial environment explained 75% of CML variance, thus pointing to the importance of diet in the development of disease.
For the Hashimoto’s thyroiditis, concordances rates varying widely between 35% and 70% in identical twins have been reported [100], thus suggesting a role for nutritional factors. However, the identification of possible dietary parameters that enhance the risk of this and other endocrine system diseases is still in its infancy.
In general, immune system studies using twin pairs have highlighted the relative importance of genetic and environmental factors. For instance, regarding food allergies not mediated by immunoglobulin E (IgE), high concordance rates of approximately 75% among identical twins have been reported in different studies [101] Despite this high value, it is considered that environmental factors are also decisive in these and other (IgE-mediated) food allergies. Indeed, various twin studies stress the importance of genome, but show that there is significant variation due to environmental exposure [102].
Diet as a Fundamental Cause Affecting Cancer in Twins
The effect of diet on the generation of cancer is considered significant along with other factors [103,104]. Indeed, important organizations such as the American Cancer Society have encouraged changes in diet, namely lower saturated fat and higher fruit and vegetable consumption [103]. There are several important dietary substances, which may be influential in the incidence of cancer, such as, resveratrol (in red grapes and berries), genistein (soybean), allicin (garlic), lycopene (tomato), β-carotene (carrots), and dietary fibre [105]. However, epidemiological available data are not consistent for many foods, remaining associations with cancer risk unclear [106]. Owing to the impact on some critical aspects (obesity, ω3/ω6 fatty acids ratio, meat consumption, polyphenol and sulphur compounds, vitamins, minerals, isoflavones, fibre and others), dietary options may lead to an enhanced or reduced cancer risk [106].
Therefore, dietary factors are expected to lead to different cancer risk in identical twins if these chose distinct diets over a long time period. On the other hand, as was previously mentioned, studies on monozygotic twins eliminate the genetic factor as a variable and allow the assessment of the importance of environmental factors. However, there are some shared environmental background aspects in identical twins such as smoking or diet in childhood family [107]. Even though part of the diet effect may act as a non-differentiating factor, whenever genetically identical twins move to distinct surroundings and have different diets, cancer risk factors may change [107]. Indeed, a study on cohorts of twins from Sweden, Denmark, and Finland [107] has concluded that environment has the main role in causing sporadic cancer (Table 1) [especially for colorectal and breast cancer), being the contribution of inherited genetic factors considered minor to most types of neoplasms. Nevertheless, there are some cancer diseases (prostate and colorectal), which show statistically significant effects of heritability [107].
The cancer diseases related to the GI tract are the most obvious connection between diet and carcinogenesis. Important factors in the genesis of cancer like Helicobacter pylori infection for gastric carcinoma [108] are independent of the genetic makeup [109]. These authors found that among monozygotic twins reared apart and discordant for H. pylori status, infected twins consumed more ascorbic acid than their unaffected co-twins. GI carcinogenesis can also be induced by obesity [110]. The mechanisms whereby obesity leads to several types of GI cancer diseases (particularly, esophageal, gastric, pancreatic, and colorectal) involve changes in insulin levels, adipokines secretion, inflammatory cytokines contents and other important physiological parameters [110]. Diverticular disease is another potential GI carcinogenic factor that is partially caused by low dietary fibre [111]. Though results of a twin comparison by these authors show that 53% of susceptibility to diverticular disease is ascribable to genetic factors, a significant impact of food choices is acknowledged. Furthermore, the risk for esophageal adenocarcinoma is increased by gastroesophageal reflux disease, which in turn was shown to be a condition sensitive to BMI and, as such, dietary aspects, in an important study involving monozygotic twin pairs [112].
Gut microbiota may also influence the incidence of cancer, namely colorectal cancer [113]. As mentioned above (section 2), diet may model the gut microbiota [114] and as such, produce an effect on cancer risk. Hence, dietary options may be a cause for a differential cancer risk and mortality in monozygotic twins. There are other indirect connections between diet and cancer. Namely, DNA is methylated over time and monozygotic twins’ studies show that the methylation status diverges with age, thereby demonstrating that this phenomenon is susceptible to environmental factors [115]. These DNA changes may have an important role in cancer [116] and are affected by diet and nutrient intake [117,118]. It should be emphasized that such DNA alterations may be relevant for cancer diseases other than GI cancer. This issue is further developed below in the diet and epigenetics section (section 7).
There are also cancer types where the case for a dietary effect is weak, such as testicular cancer [119]. These authors found factors other than diet causing a difference in cancer incidence in twins. For other cancer diseases, it is difficult to separate the genetic and environmental factors through monozygotic twins’ comparison because several prenatal aspects are intertwined. For infant leukemia, the connection between maternal diet and disease remains a hypothesis [120]. It is assumed as possible that dietary exposure to substances that inhibit topoisomerases could lead to disease. However, it has been only shown a prenatal origin for some childhood leukemias [121,122].
All these studies and the associated progress made on the understanding of the cancer process point to the importance of primary prevention including a healthy diet as the most effective way to reduce risk and mortality of some types of cancer [123].
Food as a Source of Epigenetic Change
Epigenetic changes are heritable phenotypic traits that are not caused by changes in the DNA sequence and are of great importance for individual development and disease. The epigenetic changes are preserved when cells divide. In this context, food constituents may act upon several biochemical phenomena at the cell nucleus, thereby affecting gene transcription and modulating gene expression [124]. Nutrients needed for nucleic acid synthesis and for the associated regulating enzymes are especially interesting: essential amino acids, zinc, folate, and vitamins B6 and B12 [125]. In fact, it has been claimed that epigenetic change during individual development to be stochastic and/or determined by environmental factors. Namely, these epigenetic phenomena encompass histone modification or DNA methylation levels, which change over time [115]. Monozygotic twins’ studies may be very helpful for shedding light into this subject, since monozygotic twins are genetically identical [79]. These twins have the same genotype because they are derived from the same zygote. Nevertheless, monozygotic twin siblings may display many phenotypic differences, such as their susceptibility to disease and several anthropomorphic traits. Indeed, there is some evidence that phenotypic discordance between monozygotic twins is partially due to epigenetic factors (Table 1) [124].
For elder twin pairs, differences in gene expression were reported to be four times greater than those in younger twin pairs [115]. There seems to be an age-dependent accumulation of epigenetic differences, thereby suggesting the existence of the so-called epigenetic drift. Particularly, older monozygotic twins displayed remarkable differences in their overall content and genomic distribution of 5-methylcytosine DNA and histone acetylation [115]. It must be remarked that epigenetic changes regulate several genomic effects, including the expression of genes fundamental for normal growth, development, and differentiation, without affecting the DNA sequence, and, differently from the fixed DNA sequence, show significant plasticity [124].
Lower levels of methylation (hypomethylation) were associated with the overexpression of repeated DNA sequences that must be repressed in healthy cells [124]. Hypermethylation typically involves cytosinephosphate- guanine islands in the promoter region and it is linked to gene inactivation. Indeed, hypermethylation is one of the most important epigenetic changes repressing transcription via the promoter regions of tumour suppressor genes [126]. Global hypomethylation is also associated to the development of cancer by different pathways [126]. Diet can both prevent and induce colon carcinogenesis through epigenetic changes, which regulate the homeostasis of the intestinal mucosa [127]. Therefore, the study of epigenetics, such as the DNA methylation profiles in monozygotic twins, is quite important [128]. Recent methodological progress (through methylated DNA immunoprecipitation followed by deep sequencing) has enabled to make significant advances and carry out large cohort twin studies, which may help to explain the mechanisms linking diet and epigenetic changes [129].
Some experimental works [130,131] showed that DNA methylation was influential on the regulation of the glucose transporter 4 and leptin genes during adipocyte differentiation. So, there may be a connection between epigenetic phenomena and obesity. In fact, a study on obesitydiscordant monozygotic twins has shown several differences in the transcription profiles in adipose tissue between twins [132]. The results showed the effects of acquired human obesity, which may be related to epigenetic modulation of the genome.
It has also been reported that epigenetic mechanisms are involved in the etiology of age-related macular degeneration (AMD) and that such mechanisms link specific dietary factors and AMD in monozygotic twins [133]. Indeed, the twin with the less advanced AMD displayed frequently a higher dietary intake of vitamin D, betaine, or methionine [133]. Some study results [134] seem to point to randomness as a cause for epigenetic alterations correlating with discordance for disease among monozygotic twin pairs. However, it is quite possible that dietary differences, even if small, may be a driving force [135]. Hence, the complexity of this scientific field and the intricacies deriving from multiple environmental factors and affecting monozygotic twins entail that diet-epigenetics connections require further study.
Open Issues and Future Perspectives
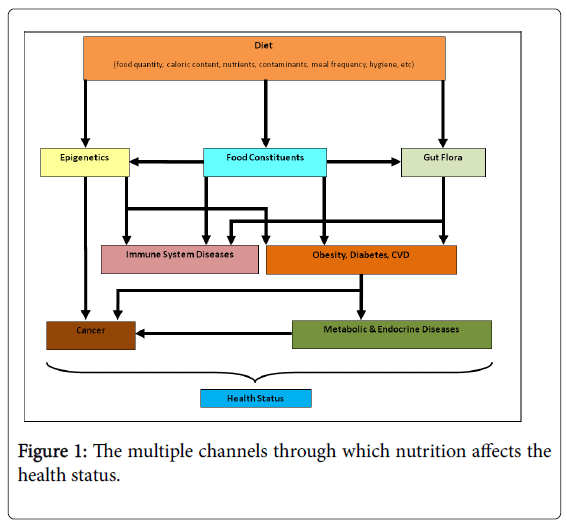
The studies presented in the previous sections provide some insight into the importance of nutrition for the development, ageing, wellbeing, and health status of monozygotic twins (Figure 1). A summary of the more direct and clearly circumscribed links between nutrition and twin health can be seen in Table 2. Nevertheless, there are several issues requiring further study. Namely, a better understanding of the importance of genetics, epigenetics, relation with environmental aspects, and other factors as well as of the synergies between these aspects can be attained from novel monozygotic twin studies. The observed phenotypic differences between genetically identical twins highlight the relationship between genetic determinants and environmental factors [79]. The epigenetic changes bring another level of complexity to the study of interactions between the environment, and in particular diet, and phenotypic traits with special emphasis on disease susceptibility. Twin studies may offer the opportunity to study epigenetic variation across the genome. On the one hand, these studies can improve the understanding of the factors regulating epigenetic variability by assessing the heritability of epigenetic variants [136]. On the other hand, the use of twins in epigenetic research may help to unravel the intricacies associated to human development and disease and make their connections clearer, thereby exposing the role of nutrition.
| Constituent | Food | Health Endpoint | Reference |
|---|---|---|---|
| Bifidobacterium animalis | Fermented milk products | Human fecal metatranscriptome | [27] |
| Lactobacillus delbrueckii | |||
| Lactococcus lactis | |||
| Streptococcus thermophilus | |||
| n-3 Polyunsaturated fatty acids | Fish and other seafood | Cardioprotective high-density lipoprotein 2b | [67] |
| Sugar, fat poor in n-3 polyunsaturated fatty acids | French fries, hamburger, pizza, salty snacks, sweets | Triglycerides and particle size of harmful very low-density lipoprotein | [68] |
| Fat | Butter, margarine, fatty foods | Obesity | [50] |
| Sodium | Salt | Coronary flow reserve | [61] |
| Flavonols | Millet, soybean | Goiter | [76] |
| Cyanogenic glucosides | Cassava | ||
| Glucosinolates | Cruciferous vegetables | ||
| Vitamin D | Fish liver oil, fatty fish, beef liver, egg yolk | Multiple sclerosis | [18] |
| Vitamin D | Fish liver oil, fatty fish, beef liver, egg yolk | Age-related macular degeneration (AMD) | [133] |
| Betaine | Spinach, wheat bran, beet | ||
| Methionine | Seeds, nuts, cereals, eggs, fish, meat | ||
| Monounsaturated fatty acids, dietary fibre, vitamins | Mediterranean diet (cereals, vegetables, fruits, nuts, legumes, fish, olive oil) | Oxidative stress | [13] |
Table 2: Constituents, foods, and health endpoints in monozygotic twins.
A recent study [14] on monozygotic twins found that a Mediterranean diet led to a lower level of oxidative stress, as measured by the glutathione redox pair, thus offering a possible mechanism linking diet and CVD. This is a good example of future randomized controlled trial studies on diet and health outcomes using identical twins as a population where the genetic variability is absent. Furthermore, future scientific work should focus more on age-related traits and diseases and try to link phenotypic differences between monozygotic twins to environmental differences and epigenetic factors [137].
The ageing of populations and the recent great shifts in dietary patterns of large swaths of the world population have decisively contributed for the emergence of new important diseases that were rare one hundred years ago [70,138,139]. Alzheimer disease and other ageing related health conditions belong to this group of emerging diseases and the conduction of trials using identical twin pairs may provide new insights [138]. Other disease group is more directly associated with nutritional factors and encompasses CVD, diabetes, and cancer [139]. The clearer identification of the critical nutritional factors also makes advisable the conduction of more studies on monozygotic twins.
It is highly plausible that over the next decade a full characterization of human genomic, epigenomic, and transcriptomic data will be within the reach of most researchers [136]. This advance may shed much light onto the interplay of genetic determined processes and nutrition effects. It will become much clearer to what extent genes determine human traits and disease and the scope of action of dietary parameters, thereby highlighting how much a given diet may differentiate the nature and fate of monozygotic twins.
Acknowledgments
This work was supported by the following Post-Doctoral Grants: Ref: SFRH/BPD/102689/2014 (“Fundação para a Ciência e a Tecnologia”, FCT) for the author Carlos Cardoso; Ref: SFRH/BPD/ 64951/2009 (FCT) and DIVERSIAQUA (MAR2020) for the author Cláudia Afonso” (FCT).
Conflict of interest
No conflict of interest. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
References
- Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, et al. (2005) Origins and evolution of the Western diet: Health implications for the 21st century. Am J Clin Nutr 81: 341-354.
- Chakravarti A, Little P (2003) Nature nurture and human disease. Nature 421: 412-414.
- Guttmacher AE, Collins FS, Carmona RH (2004) The family history-more important than ever. N Engl J Med 351: 2333-2336.
- Kaplan H, Hill K, Lancaster J, Hurtado M (2000) A theory of human life history evolution: Diet intelligence and longevity. Evol Anthropol Issues News Rev 9: 156-185.
- Swaddling J (1999) The ancient Olympic games. University of Texas Press, Austin, USA.
- Waller JC (2017) Commentary: The birth of the twin study -a commentary on Francis Galton’s ‘The history of twins’. Int J Epidemiol 41: 913-917.
- Rende RD, Plomin R, Vandenberg SG (1990) Who discovered the twin method? Behav Genet 20: 277-285.
- Rozin P, Millman L (1987) Family environment not heredity accounts for family resemblances in food preferences and attitudes: A twin study. Appetite 8: 125-134.
- Thornton LM, Mazzeo SE, Bulik CM (2011) The heritability of eating disorders: Methods and current findings. Curr Top Behav Neurosci 6: 141-156.
- Holland AJ, Hall A, Murray R, Russell GFM, Crisp AH (1984) Anorexia nervosa: A study of 34 twin pairs and one set of triplets. Br J Psych 145: 414-419.
- Pérusse L, Bouchard C (2000) Gene-diet interactions in obesity. Am J Clin Nutr 72: 1285S-1290S.
- Steyn NP, Mann J, Bennett PH, Temple N, Zimmet P, et al. (2004) Diet nutrition and the prevention of type 2 diabetes. Public Health Nutr 7: 147-165.
- Dai J, Jones DP, Goldberg J, Ziegler TR, Bostick RM, et al. (2008) Association between adherence to the Mediterranean diet and oxidative stress. Am J Clin Nutr 88: 1364-1370.
- Hong Y, De Faire U, Heller DA, McClearn GE, Pedersen N (1994) Genetic and environmental influences on blood pressure in elderly twins. Hypertension 24: 663-670.
- Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U (1994) Genetic susceptibility to death from coronary heart disease in a study of twins. N Eng J Med 330: 1041-1046.
- Poulsen P, Vaag A, Kyvik K, Nielsen HB (2001) Genetic versus environmental aetiology of the metabolic syndrome among male and female twins. Diabetologia 44: 537-543.
- North KE, Williams JT, Welty TK, Best LG, Lee ET (2003) Evidence for joint actions of genes on diabetes status and CVD risk factors in American Indians: the Strong Heart Family Study. Int J Obes 27: 491-497.
- Cantorna MT (2006) Vitamin D and its role in immunology: Multiple sclerosis and inflammatory bowel disease. Prog Biophys Mol Biol 92: 60-64.
- Broedbaek K, Ribel-Madsen R, Henriksen T, Weimann A, Petersen M, et al. (2011) Genetic and environmental influences on oxidative damage assessed in elderly Danish twins. Free Radical Biol Med 50: 1488-1491.
- Hoover RN (2000) Cancer-nature nurture or both. N Engl J Med 343: 135-136.
- Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, et al. (2009) Bacterial community variation in human body habitats across space and time. Science 326: 1694-1697.
- Greenblum S, Turnbaugh PJ, Borenstein E (2012) Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease. Proc Nat Acad Sci 109: 594-599.
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, et al. (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464: 59-65.
- Turnbaugh PJ, Ley RE, Hamady M, Liggett CF, Knight R, et al. (2007) The human microbiome project: exploring the microbial part of ourselves in a changing world. Nature 449: 804-810.
- Turnbaugh PJ, Quince C, Faith JJ, McHardy AC, Yatsunenko T, et al. (2010) Organismal genetic and transcriptional variation in the deeply sequenced gut microbiomes of identical twins. Proc Nat Acad Sci 107: 7503-7508.
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, et al. (2009) A core gut microbiome in obese and lean twins. Nature 457: 480-484.
- McNulty NP, Yatsunenko T, Hsiao A, Faith JJ, Muegge BD, et al. (2011) The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci Translational Med 3: 106ra106.
- Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, et al. (2016) A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterol 139: 1844-1854e1.
- Spor A, Koren O, Ley R (2011) Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol 9: 279-290.
- Orholm M, Binder V, Sorensen TIA. (1996) Inflammatory bowel disease in a Danish twin register. Gut 39: A187.
- Satsangi J, Morecroft J, Shah NB, Nimmo E (2003) Genetics of inflammatory bowel disease: scientific and clinical implications. Best Practice Res Clin Gastroenterol 17: 3-18
- Subhani J, Montgomery SM, Pounder RE, Wakefield AJ (1998) Concordance rates of twins and siblings in inflammatory bowel disease (IBD). Gut 42: A40-A48.
- Tysk C, Lindberg E, Järnerot G, Flodérus-Myrhed B. (1988) Ulcerative colitis and Crohn’s disease in an unselected population of monozygotic and dizygotic twins: A study of heritability and the influence of smoking. Gut 29: 990-996.
- Dicksved J, Halfvarson J, Rosenquist M, Jarnerot G, Tysk C (2008) Molecular analysis of the gut microbiota of identical twins with Crohn’s disease. ISME J 2: 716-727.
- Willing B, Halfvarson J, Dicksved J, Rosenquist M, Jarnerot G, et al. (2009) Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn’s disease. Inflamm Bowel Dis 15: 653-660.
- Hanauer SB (2006) Inflammatory bowel disease: Epidemiology pathogenesis and therapeutic opportunities. Inflamm Bowel Dis 12: 2006.
- Krishnan A, Korzenik JR (2002) Inflammatory bowel disease and environmental influences. Gastroenterol Clin North Am 31: 21-39.
- Persson PG, Ahlbom A, Hellers G (1992) Diet and inflammatory bowel disease: a case-control study. Epidemiol 3: 47-52.
- Kelly T, Yang W, Chen CS, Reynolds K, He J (2008) Global burden of obesity in 2005 and projections to 2030. Int J Obes 32: 1431-1437.
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, et al. (2014) Global regional and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 384: 766-781.
- Milagro FI, Mansego ML, De Miguel C, Martínez JA (2013) Dietary factors epigenetic modifications and obesity outcomes: Progresses and perspectives. Mol Aspects Med 34: 782-812.
- Heitmann BL, Harris JR, Lissner L, Pedersen NL (1999) Genetic effects on weight change and food intake in Swedish adult twins. Am J Clin Nutr 69: 597-602.
- Stunkard AJ, Foch TT, Hrubec Z (1986) A twin study of human obesity. JAMA 256: 51-54.
- Wardle J, Carnell S, Haworth CM, Plomin R (2008) Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am J Clin Nutr 87: 398-404.
- Herrera BM, Keildson S, Lindgren CM (2011) Genetics and epigenetics of obesity. Maturitas 69: 41-49.
- Rose KM, Newman B, Mayer-Davis EJ, Selby JV (1998) Genetic and behavioral determinants of waist-hip ratio and waist circumference in women twins. Obesity Res 6: 383-392.
- Bree MVM, Eaves LJ, Dwyer JT (1999) Genetic and environmental influences on eating patterns of twins aged ≥ 50y. Am J Clin Nutr 70: 456-465.
- Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu B, et al. (2011) Changes in diet and lifestyle and long-term weight gain in women and men. New Engl J Med 364: 2392-2404.
- Pimpin L, Ambrosini GL, Llewellyn CH, Johnson L, Jebb SA, et al. (2013) Dietary intake of young twins: Nature or nurture. Am J Clin Nutr 98: 1326-1334.
- Rissanen A, Hakala P, Lissner L, Mattlar CE, Koskenvuo M, et al. (2002) Acquired preference especially for dietary fat and obesity: A study of weight-discordant monozygotic twin pairs. Int J Obes 26: 973-977.
- Silventoinen K, Sarlio-Lähteenkorva S, Koskenvuo M, Lahelma E, Kaprio J (2004) Effect of environmental and genetic factors on education-associated disparities in weight and weight gain: A study of Finnish adult twins. Am J Clin Nutr 80: 815-822.
- Llewellyn CH, van Jaarsveld CHM, Johnson L, Carnell S, Wardle J (2010) Nature and nurture in infant appetite: Analysis of the Gemini twin birth cohort. Am J Clin Nutr 91: 1172-1179.
- Medici F, Hawa M, Ianari A, Pyke DA, Leslie RD (1999) Concordance rate for Type II diabetes mellitus in monozygotic twins: Actuarial analysis. Diabetologia 42: 146-150.
- Beck-Nielsen H, Vaag A, Poulsen P, Gaster M (2003) Metabolic and genetic influence on glucose metabolism in type 2 diabetic subjects-experiences from relatives and twin studies. Best Practice Res Clin Endocrinol Metab 17: 445-467.
- Hales CN, Barker DJ (1992) Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Diabetologia 35: 595-601.
- Stumvoll M, Goldstein BJ, van Haeften TW (2005) Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 365: 1333-1346.
- Hubert HB, Feinleib M, McNamara PM, Castelli WP (1983) Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up of participants in the Framingham heart study. Circulation 67: 968-977.
- Nagata C, Takatsuka N, Shimizu N, Shimizu H (2004) Sodium intake and risk of death from stroke in Japanese men and women. Stroke 35: 1543-1547.
- Kannel WB, McGee DL (1979) Diabetes and glucose tolerance as risk factors for cardiovascular disease: The Framingham study. Diabetes Care 2: 120-126.
- Wienke A, Holm N Skytthe A, Yashin AI (2001) The heritability of mortality due to heart diseases: A correlated frailty model applied to Danish twins. Twin Res 4: 266-274.
- Eufinger SC, Votaw J, Faber T, Ziegler TR, Goldberg J, et al. (2012) Habitual dietary sodium intake is inversely associated with coronary flow reserve in middle-aged male twins. Am J Clin Nutr 95: 572-579.
- Bergvall N, Cnattingius S (2008) Familial (shared environmental and genetic) factors and the foetal origins of cardiovascular diseases and type 2 diabetes: a review of the literature. J Int Med 264: 205-223.
- Barker DJ (1995) Fetal origins of coronary heart disease. BMJ 311: 171-174
- Cook NR, Obarzanek E, Cutler JA, Buring JE, Rexrode KM, et al. (2009) Joint effects of sodium and potassium intake on subsequent cardiovascular disease: The trials of hypertension prevention follow-up study. Arch Internal Med 169: 32-40.
- He FJ, MacGregor GA (2009) A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Human Hypertension 23: 363-384.
- Kho M, Lee JE, Song Y, Lee K, Kim K, et al. (2013) Genetic and environmental influences on sodium intake determined by using half-day urine samples: The Healthy Twin Study. Am J Clin Nutr 98: 1410-1416.
- Bogl LH, Maranghi M, Rissanen A, Kaprio J, Taskinen MR, et al. (2011) Dietary omega-3 polyunsaturated fatty acid intake is related to a protective high-density lipoprotein subspecies profile independent of genetic effects: A monozygotic twin pair study. Atherosclerosis 219: 880-886.
- Bogl LH, Pietiläinen KH, Rissanen A, Kangas AJ, Soininen P, et al. (2013) Association between habitual dietary intake and lipoprotein subclass profile in healthy young adults. Nutr Metab Cardiovasc Dis 23: 1071-1078.
- Scicchitano P, Cameli M, Maiello M, Ciccone MM (2014) Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J Functional Foods 6: 11-32.
- Roche HM, Phillips C, Gibney MJ (2005) The metabolic syndrome: The crossroads of diet and genetics. Proc Nutr Soc 64: 371-377.
- Carmelli D, Cardon LR, Fabsitz R (1994) Clustering of hypertension diabetes and obesity in adult male twins: same genes or same environments? Am J Human Gen 55: 566-573.
- Mayer EJ, Newman B, Austin MA, Zhang D, Quesenberry CP, et al. (1996) Genetic and environmental influences on insulin levels and the insulin resistance syndrome: An analysis of women twins. Am J Epidemiol 143: 323-332.
- Rüst CA, Knechtle B, Rosemann T (2013) Graves’ disease in monozygotic twins – a case report. BMC Endocrine Dis 13: 17-20.
- Brix TH, Kyvik KO, Hegedüs L (1999) Major role of genes in the etiology of simple goiter in females: A population-based twin study. J Clin Endocrinol Metab 84: 3071-3075.
- Malamos B, Koutras DA, Kostamis P, Rigopoulos GA, Zerefos NS, et al. (1967) Endemic goitre in Greece: A study of 379 twin pairs. J Med Gen 4: 16-18.
- Medeiros-Neto G, Camargo RY, Tomimori EK (2012) Approach to and treatment of goiters. Med Clin North Am 96: 351-368.
- Islam T, Gauderman J, Cozen W, Mack TM (2007) Childhood sun exposure influences risk of multiple sclerosis in monozygotic twins. Neurology 69: 381-388.
- Brix TH, Kyvik KO, Hegedüs L (2000) A population-based study of chronic autoimmune hypothyroidism in Danish twins. J Clin Endocrinol Metab 85: 536-539.
- Ballestar E (2010) Epigenetics lessons from twins: Prospects for autoimmune disease. Clin Rev Allergy Immunol 39: 30-41.
- Hayes CE, Cantorna MT, DeLuca HF (1997) Vitamin D and multiple sclerosis. Proc Soc Exp Biol Med 216: 21-27.
- Munger KL, Zhang SM, O’Reilly E, Hernán MA, Olek MJ, et al. (2004) Vitamin D intake and incidence of multiple sclerosis. Neurol 62: 60-65.
- Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A (2006) Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 296: 2832-2838.
- Orton SM, Morris AP, Herrera BM, Ramagopalan SV, Lincoln MR, et al. (2008) Evidence for genetic regulation of vitamin D status in twins with multiple sclerosis. Am J Clin Nutr 88: 441-447.
- Kampman MT, Wilsgaard T, Mellgren SI (2007) Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic Circle. J Neurol 254: 471-477.
- Hawkes CH, Macgregor AJ (2009) Twin studies and the heritability of MS: a conclusion. Multiple Sclerosis 15: 661-667.
- Wordsworth BP, Lanchbury JSS, Sakkas LI, Welsh KI, Panayi GS, et al. (1989) HLA-DR4 subtype frequencies in rheumatoid arthritis indicate that DRB1 is the major susceptibility locus within the HLA class II region. Proc Nat Acad Sci USA 86: 10049-10053.
- Kjeldsen-Kragh J (1999) Rheumatoid arthritis treated with vegetarian diets. Am J Clin Nutr 70: 594S-600S.
- Silman AJ (1991) Is rheumatoid arthritis an infectious disease? BMJ 303: 200-201.
- Järvinen P, Koskenvuo M, Koskimies S, Kotaniemi K, Aho K (1991) Rheumatoid arthritis in identical twins: A clinical and immunogenetic study of eight concordant pairs derived from a nationwide twin panel. Scand J Rheumatol 20: 159-164.
- Pattison DJ, Symmons DPM, Lunt M, Welch A, Luben R, et al. (2004) Dietary risk factors for the development of inflammatory polyarthritis. Arthritis Rheumatism 50: 3804-3812.
- Linos A, Kaklamani VG, Kaklamani E, Koumantaki Y, Giziaki E, et al. (1999) Dietary factors in relation to rheumatoid arthritis: A role for olive oil and cooked vegetables? Am J Clin Nutr 70: 1077-1082.
- Greene DA, Naughton GA (2011) Calcium and vitamin-D supplementation on bone structural properties in peripubertal female identical twins: A randomized controlled study. Osteoporosis Int 22: 489-498.
- Atkinson MA, Eisenbarth GS (2001) Type 1 diabetes: New perspective on disease pathogenesis. Lancet 358: 221-229.
- Barnett AH, Eff C, Leslie RDG, Pyke DA (1981) Diabetes in identical twins A study of 200 pairs. Diabetologia 20: 87-93.
- Virtanen SM, Knip M (2003) Nutritional risk predictors of b cell autoimmunity and type 1 diabetes at a young age. Am J Clin Nutr 78: 1053-1067.
- Vaag AA, Holst JJ, Volund A, Beck-Nielsen HB (1996) Gut incretin hormones in identical twins discordant for non-insulin-dependent diabetes mellitus (NIDDM): Evidence for decreased glucagon-like peptide 1 secretion during oral glucose ingestion in NIDDM twins. Eur J Endocrinol 135: 425-432.
- Knip M, Akerblom HK (1998) Putative environmental factors in Type 1 diabetes. Diabetes/Metabolism Rev 14: 31-67.
- Beyan H, Riese H, Hawa MI, Beretta G, Davidson HW, et al. (2012) Glycotoxin and autoantibodies are additive environmentally determined predictors of type 1 diabetes A twin and population study. Diabetes 61: 1192-1198.
- Mericq V, Piccardo C, Cai W, Chen X, Zhu L, et al. (2010) Maternally transmitted and food-derived glycotoxins: A factor preconditioning the young to diabetes? Diabetes Care 33: 2232-2237.
- Zdraveska N, Kocova M (2012) Hashimoto thyroiditis in childhood Review of the epidemiology genetic susceptibility and clinical aspects of the disease. Macedonian J Med Sci 5: 336-345.
- Uzzaman A, Komarow HD (2008) The immunological basis of non-IgE-mediated reactions In: Food allergy-adverse reactions to foods and food additives (Ed Metcalfe D D Sampson H A & Simon R A) Blackwell Publishing: Malden MA USA, pp: 29-42.
- Braunstahl GJ (2005) The unified immune system: Respiratory tract-nasobronchial interaction mechanisms in allergic airway disease. J Allergy Clin Immunol 115: 142-148.
- Begg CB (2001) The search for cancer risk factors: When can we stop looking? Am J Public Health 91: 360-364.
- Peto J (2001) Cancer epidemiology in the last century and the next decade. Nature 411: 390-395.
- Aggarwal BB, Shishodia S (2006) Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 71: 1397-1421.
- Ruiz RB, Hernández PS (2014) Diet and cancer: Risk factors and epidemiological evidence. Maturitas 77: 202-208.
- Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, et al. (2000) Environmental and heritable factors in the causation of cancer Analyses of cohorts of twins from Sweden Denmark and Finland. N Engl J Med 343: 78-85.
- Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, et al. (1991) Association between infection with Helicobacter pylori and risk of gastric cancer: Evidence from a prospective investigation. BMJ 302: 1302-1305.
- Malaty HM, Graham DY, Isaksson I, Engstrand L, Pedersen NL (1998) Co-twin study of the effect of environment and dietary elements on acquisition of Helicobacter pylori infection. Am J Epidemiol 148: 793-797.
- Alemán JO, Eusebi LH, Ricciardiello L, Patidar K, Sanyal AJ, et al. (2014) Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterol Rev Basic Clin Gastroenterol Hepatol 146: 357-373.
- Strate LL, Erichsen R, Baron JA, Mortensen J, Pedersen JK, et al. (2013) Heritability and familial aggregation of diverticular disease: A population-based study of twins and siblings. Gastroenterol 144: 736-742.
- Zheng Z, Nordenstedt H, Pedersen NL, Lagergren J, Ye W (2007) Lifestyle factors and risk for symptomatic gastroesophageal reflux in monozygotic twins. Gastroenterol 132: 87-95.
- Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, et al. (2012) Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338: 120-123.
- Walsh CJ, Guinane CM, O’Toole PW, Cotter PD (2014) Beneficial modulation of the gut microbiota. FEBS Letters 588: 4120-4130.
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, et al. (2005) Epigenetic diferences arise during the lifetime of monozygotic twins. Proc Nat Acad Sci USA 102: 10604-10609.
- Rando TA (2010) Epigenetics and aging. Exp Gerontol 45: 253-254.
- Almén MS, Nilsson EK, Jacobsson JA, Kalnina I, Klovins, J et al. (2014) Genome-wide analysis reveals DNA methylation markers that vary with both age and obesity. Gene 548: 61-67.
- Campion J, Milagro FI, Martinez JA (2009) Individuality and epigenetics in obesity. Obes Rev 10: 383-392.
- Swerdlow AJ, De Stavola BL, Swanwick MA, P Mangtani P, Maconochie NES (1999) Risk factors for testicular cancer: A case-control study in twins. Br J Cancer 80: 1098-1102.
- Spector LG, Xie Y, Robison LL, Heerema NA, Hilden JM,et al. (2005) Maternal diet and infant leukemia: the DNA topoisomerase II inhibitor hypothesis: A report from the children’s oncology group. Cancer Epidemiol Biomarkers Prev 14: 651-655.
- Greaves M (2006) Infection immune responses and the aetiology of childhood leukaemia. Nat Rev Cancer 6: 193-203.
- Maia AT, van der Velden VH, Harrison CJ, Szczepanski T, Williams MD, et al. (2003) Prenatal origin of hyperdiploid acute lymphoblastic leukemia in identical twins. Leukemia 17: 2202-2206.
- Montesano R, Hall J (2001) Environmental causes of human cancers. Eur J Cancer 37: S67-S87.
- Poulsen P, Esteller M, Vaag A, Fraga MF (2007) The epigenetic basis of twin discordance in age-related diseases. Pediatr Res 61: 38R-42R.
- Davis CD, Uthus EO (2004) DNA methylation cancer susceptibility and nutrient interactions. Exp Biol Med (Maywood) 229: 988-995.
- Wong NC, Craig JM (2011) Epigenetics: A reference manual. Caister Academic Press, Norfolk, UK.
- Nyström M, Mutanen M (2009) Diet and epigenetics in colon cancer. World J Gastroenterol 15: 257-263.
- Kaminsky ZA, Tang T, Wang SC, Ptak C, Oh GH, et al. (2009) DNA methylation profiles in monozygotic and dizygotic twins. Nat Genet 41: 240-245.
- Paul DS, Beck S (2014) Advances in epigenome-wide association studies for common diseases. Trends Mol Med 20: 541-543.
- Yokomori N, Tawata M, Onaya T (1999) A transcriptional repressor regulates mouse GLUT4 gene expression during the differentiation of 3T3-L1 cells. Diabetes 48: 2471-2474.
- Yokomori N, Tawata M, Onaya T (2002) DNA demethylation modulates mouse leptin promoter activity during the differentiation of 3T3-L1 cells. Diabetologia 45: 140-148.
- Pietiläinen KH, Naukkarinen J, Rissanen A, Saharinen J, Ellonen P, et al. (2008) Global transcript profiles of fat in monozygotic twins discordant for BMI: Pathways behind acquired obesity. PLoS Med 5: e51.
- Seddon JM, Reynolds R, Shah HR, Rosner B (2011) Smoking dietary betaine methionine and vitamin D in monozygotic twins with discordant macular degeneration: Epigenetic implications. Ophthalmol 118: 1386-1394.
- Weksberg R, Shuman C, Caluseriu O, Smith AC, Fei YL, et al. (2002) Discordant KCNQ1OT1 imprinting in sets of monozygotic twins discordant for Beckwith-Wiedemann syndrome. Hum Mol Genet 11: 1317-1325.
- Waterland RA (2006) Epigenetic mechanisms and gastrointestinal development. J Pedriatics 149: S137-S142.
- Bell JT, Saffery R (2012) The value of twins in epigenetic epidemiology. Int J Epidemiol 41: 140-150.
- Spector TD, Williams FMK (2006) The UK adult twin registry (TwinsUK). Twin Res Hum Genet 9: 899-906.
- Dosunmu R, Wu J, Basha MR, Zawia NH (2007) Environmental and dietary risk factors in Alzheimer’s disease. Expert Rev Neurotherapeutics 7: 887-900.
- Jew S, AbuMweis SS, Jones PJH (2009) Evolution of the human diet: Linking our ancestral diet to modern functional foods as a means of chronic disease prevention. J Med Food 12: 925-934.
Relevant Topics
- Addiction
- Adolescence
- Children Care
- Communicable Diseases
- Community Occupational Medicine
- Disorders and Treatments
- Education
- Infections
- Mental Health Education
- Mortality Rate
- Nutrition Education
- Occupational Therapy Education
- Population Health
- Prevalence
- Sexual Violence
- Social & Preventive Medicine
- Women's Healthcare
Recommended Journals
Article Tools
Article Usage
- Total views: 4742
- [From(publication date):
June-2017 - Jul 13, 2025] - Breakdown by view type
- HTML page views : 3829
- PDF downloads : 913