The Implications of SFRP2 Gene Hypermethylation in Colorectal Cancer
Received: 24-Dec-2022 / Manuscript No. AOT-22-84613 / Editor assigned: 26-Dec-2022 / PreQC No. AOT-22-84613 (PQ) / Reviewed: 09-Jan-2023 / QC No. AOT-22-84613 / Revised: 29-Mar-2023 / Manuscript No. AOT-22-84613 (R) / Published Date: 06-Apr-2023
Abstract
Background: The Wnt pathway is stimulated by the DNA hypermethylation of SFRP2, which inhibits gene activity, downregulates the gene expression and promotes CRC.
Objectives: This study aims to assess the prognostic impact of hypermethylation of SFRP2 in colorectal cancer.
Methodology: The research used twenty (20) blood samples from patients with colorectal cancer detected at Sher-I-Kashmir institute of medical sciences as well as thirty (30) patients with histologically confirmed colorectal cancer who underwent surgical excision in the department of general surgery. Methylation Specific PCR (MSP) was employed to assess SFRP2 methylation and the results were correlated with several investigated clinicopathological characteristics.
Results: Our results showed that SFRP2 methylation levels are significantly present in tumor tissues and blood samples as compared to normal counterparts.
Conclusion: We infer that the hypermethylation of the SFRP2 promoter in colorectal tumors may contribute to the development of cancer in normal colon and rectum cells.
Keywords: SFRP2; Methylation; Methylation Specific PCR (MSP); Clinicopathological; Hypermethylation
Introduction
Colorectal cancer is a very heterogeneous illness that includes a variety of tumor phenotypes with distinct genetic and morphological characteristics. Proto oncogenes, DNA repair pathway genes and tumor suppressor genes are all mutated in CRC [1]. It is currently the most frequent malignant cancer of the gastrointestinal tract, accounting for 13% of all malignant tumors [2]. It normally grows as a polyp, a tumor projecting into the lumen, in the lining of the colon and rectum. Colorectal cancer is the second most prevalent cancer to cause death in 2020, according to the GLOBOCAN 2020 database, with more than 1.9 million new cases and almost 935,000 fatalities [3].
DNA methylation abnormalities are also known to play a function in carcinogenesis [4]. Promoter hypermethylation is becoming acknowledged playing a critical part in epigenetic gene silencing in human neoplasia, including CRC. One of the primary epigenetic mechanisms known to be involved in carcinogenesis is the methylation of the cytosine residues of CpG rich sequences (CpG islands) located inside the promoter regions of genes directing cell proliferation, death and DNA repair [5]. APC, MLH1, CDKN2A, VIM and CDH1 have aberrant promoter hypermethylation in CRC, which silences their expression and promotes to carcinogenesis [6,7]. CRC is linked to aberrant activation of the Wnt canonical signaling pathway, which also promotes tumor cell growth, proliferation and loss of apoptosis. Adenomatous Polyposis Coli (APC), a tumor suppressor that is highly mutated in colorectal cancers, is a crucial component of the Wnt canonical pathway that is involved in the degradation of β-catenin. One of the main signaling pathways in cancer, Wnt signaling controls cell proliferation, motility and differentiation [8].
Gastrointestinal cancers have high amounts of β-catenin. APC inactivation can be caused by germline and somatic mutations, as well as promoter hypermethylation. CIN can also be caused by changes to other genes in this route, including β-catenin. These mutations are detected in 48% of CRCs that do not have APC. A group of 162 Wnt pathway target genes are activated by β-catenin in a colon cancer cell line [9]. Secreted Frizzled Related Proteins (SFRPs) are a group of five secreted glycoproteins that suppress Wnt signaling both canonically and noncanonically via various ways. 90% of colorectal tumors have aberrant Wnt signaling as an early stage event. As a result, SFRP’s role as a repressive Wnt signaling regulator could be significant in carcinogenesis and its down regulation has been linked to human malignancies [10].
In the N-terminal half of the proteins, SFRPs have a distinctive Cysteine Rich Domain (CRD) that is homologous to the CRD of the Frizzled (Fz) receptor of Wnt. In order to prevent Wnt proteins from attaching to Fz proteins, SFRPs may interact with them or they may create inactive complexes with Fz, to limit Wnt signaling. SFRPs have been shown to possibly block the complete canonical Wnt pathway in colon cancer cells, even in the presence of mutations that activate APC or β-catenin downstream of the Fz receptor [11]. The DNA hypermethylation of SFRP2 , which is situated upstream of the canonical Wnt signaling pathway, causes the Wnt pathway to be activated, downregulate gene expression, block gene function and promote CRC. Additionally, these genes’ DNA hypermethylation may serve as a biomarker for the detection of CRC [12]. So, this study intends to evaluate the prognostic significance of hypermethylation of SFRP2 in colorectal cancer.
Materials and Methods
Sample collection
The study was given ethical clearance by the ethical committee of SKIMS, Srinagar, Jammu and Kashmir, India and all patients provided written consent. Thirty (30) histologically confirmed colorectal cancer people who had surgical excision in the department of general surgery, at Sher-I-Kashmir institute of medical sciences and twenty (20) blood samples from colorectal cancer diagnosed patients were incorporated into this research. Neither of the CRC patients had been given chemotherapy or radiotherapy. Amounting to 500 mg, sterile vials were immediately filled with surgically removed tumor tissue and normal tissue that was nearby and 10 cm away from the tumor and 2 ml of venous blood in EDTA vial (purple topped collection tube) were collected from the CRC patient along with control blood samples collected from healthy individuals The collected tissues and blood were stored at 80°C till further processing. Histopathological reports of the amassed tissues were acquired from the department of pathology, SKIMS.
From a tissue sample, molecular DNA was extracted by the saltingout method, and from the blood, it was done by the phenol-chloroform proteinase-k method. Utilizing an EZ DNA methylation kit, 1 μg-2 μg of extracted genomic DNA was treated to the bisulfite modification (Zymo research, Irvine, California). The primers used for the methylation and unmethylated alleles’ promoter regions are listed in the Table 1. Products that had been methylated and those that hadn’t were both 138 base pairs and 145 base pairs long.
| CpG status | Primers (5’-3’) | Annealing temperature (°C) | Product size (bp) |
|---|---|---|---|
| SFRP2 MF | GGGTCGGAGTTTTTCGGAGTTGCGC | 62°C | 138 bp |
| SFRP2 MR | CCGCTCTCTTCGCTAAATACGACTCG | ||
| SFRP2 UF | TTTTGGGTTGGAGTTTTTTGGAGTTGTGT | 58°C | 145 bp |
| SFRP2 UR | AACCCACTCTCTTCACTAAATACAACTCA | ||
| Note: M=Methylated; U=Unmethylated; R=Forward; R=Reverse. | |||
Table 1: Table is showing primers used for the methylation and unmethylated alleles’ promoter regions.
Statistical analysis
For continuous variables, the independent t-test and paired t-test were used; for discrete variables, Pearson's χ² test, Fisher's exact test or χ² test (trend) were used. Using logistic regression analysis, Odds Ratios (ORs) and 95% Confidence Intervals (CIs) were calculated. On two sided tests, all given P values were based. P<0.05 was used as the significance threshold. The statistical software STATA 16 was used to conduct the tests.
Results
The study involved a total of fifty CRC cases (n=50) which included 29 (58%) males and 21 (42%) females. 15 of 50 (30%) subjects were >50 years and 35 of 50 (70%) with a mean age of ≤ 50 years, 55.32 ± 15.38. Out of 50 cases, 20 (40%) were smokers and 30 (60%) were non-smokers. 14 (28%) had colon cancer and 36 (72%) had rectal cancer. 35 (70%) exhibited stage I or stage II illness and 15 (30%) had stage III or IV illness. On the basis of the grade of differentiation 15 (30%) cases were well differentiated and 21 (42%) were moderate and 14 (28%) were poorly differentiated (Table 2).
| Characteristics | Number and percentage (%) |
|---|---|
| Age | |
| >50 | 15 (30) |
| ≤ 50 | 35 (70) |
| Gender | |
| Male | 29 (58) |
| Female | 21 (42) |
| Dwelling | |
| Rural | 29 (58) |
| Urban | 21 (42) |
| Social class | |
| Low | 15 (30) |
| Middle | 35 (70) |
| Family history | |
| Yes | 12 (24) |
| No | 38 (76) |
| Smoking status | |
| Smoker | 20 (40) |
| Non-smoker | 30 (60) |
| Lifestyle | |
| Active | 38 (76) |
| Sedentary | 12 (24) |
| Body mass | |
| Normal | 29 (58) |
| Obese | 13 (26) |
| Underweight | 08 (16) |
| Salt tea intake | |
| Yes | 46 (92) |
| No | 04 (8) |
| Red meat consumption | |
| Yes | 28 (56) |
| No | 22 (44) |
| Sundried vegetables | |
| Yes | 41 (82) |
| No | 09 (18) |
| Junk food consumption | |
| Yes | 07 (14) |
| No | 43 (86) |
| Pesticide exposure | |
| Yes | 08 (16) |
| No | 42 (84) |
| Site of tumour | |
| Colon | 14 (28) |
| Rectum | 36 (72) |
| Tumour differentiation | |
| Well | 15 (30) |
| Moderate | 21 (42) |
| Poor | 14 (28) |
| TNM stage | |
| T1 | 12 (24) |
| T2 | 18 (36) |
| T3 | 14 (28) |
| T4 | 06 (12) |
| T1+T2 | 30 (60) |
| T3+T4 | 20 (40) |
| Stage | |
| I+II | 35 (70) |
| III+IV | 15 (30) |
| Tumour grade | |
| 1 | 16 (32) |
| 2 | 21 (42) |
| 3 | 13 (26) |
Table 2: This study examined the clinicopathologic and clinicoepidemiological characteristics of patients with colorectal cancer.
Methylation status of SFR2 in tissue samples
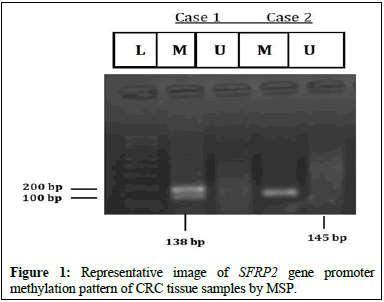
It is showing the Figure 1.
L: 100 bp unmethylated SFRP2 is indicated by the DNA marker U: (145 bp); while methylated SFRP2 is indicated by M: (138 bp).
In tumor tissues, methylation was found to be present in 66.66% (20/30) cases whereas no methylation was found in 33.33% (10/30) of the cases. Out of 20 CRC cases with hypermethylated SFRP2 gene promoters, 20% (4/20) showed both methylated and unmethylated bands whereas 80% (16/20) showed only methylated bands.
In the adjacent normal tissues, only 10% (3/30) showed the presence of methylation. While the remaining normal tissues showed no methylation.
Further SFRP2 gene promoter methylation pattern was correlated with several clinicopathological characteristics such as age, gender, tumor location, tumor grading, tumor staging, smoking status and family history. The connection between the SFRP2 promoter methylation status and other clinicopathological characteristics is summarized in Table 3.
| Characteristics | Methylation present | Methylation absent | Odds ratio (95% CI) | P-value | Chi² |
|---|---|---|---|---|---|
| Age | |||||
| >50 | 3 (50) | 3 (50) | 0.41 (0.06-2.55) | 0.333 | 0.9375 |
| ≤ 50 | 17 (29.17) | 7 (70.83) | |||
| Gender | |||||
| Male | 10 (62.50) | 6 (37.50) | 0.66 (0.14-3.10) | 0.605 | 0.2679 |
| Female | 10 (71.43) | 4 (28.57) | |||
| Dwelling | |||||
| Rural | 9 (64.29) | 5 (35.71) | 0.81 (0.17-3.74) | 0.796 | 0.0670 |
| Urban | 11 (68.75) | 5 (31.25) | |||
| Social class | |||||
| Low | 7 (58.33) | 5 (41.67) | 0.53 (0.11-2.51) | 0.429 | 0.6250 |
| Middle | 13 (66.67) | 5 (33.33) | |||
| Family history | |||||
| Yes | 5 (62.50) | 3 (37.50) | 0.77 (0.14-4.21) | 0.770 | 0.0852 |
| No | 15 (68.18) | 7 (31.82) | |||
| Smoking status | |||||
| Smoker | 8 (66.67) | 4 (33.33) | 1 (0.21-4.70) | 1.000 | 0.000 |
| Non-smoker | 12 (66.67) | 6 (33.33) | |||
| Lifestyle | |||||
| Active | 14 (63.64) | 8 (36.36) | 0.58 (0.09-3.60) | 0.559 | 0.3409 |
| Sedentary | 6 (75) | 2 (25) | |||
| Body mass | |||||
| Normal | 10 (55.56) | 8 (44.44) | 0.138 (0.01-1.33) 9 (0.28-285.5) |
0.157 | 3.7 |
| Obese | 9 (90) | 1 (10) | |||
| Underweight | 1(50) | 1(50) | |||
| Red meat consumption | |||||
| Yes | 9 (64.29) | 5 (35.71) | 0.81 (0.17-3.74) | 0.796 | 0.0670 |
| No | 11 (68.75) | 5 (31.25) | |||
| Sundried vegetables | |||||
| Yes | 17 (65.38) | 9 (34.62) | 5.6 (0.51-62.65) | 0.704 | 0.1442 |
| No | 3 (75) | 1 (25) | |||
| Junk food consumption | |||||
| Yes | 4 (100) | 0 (0) | - | 0.129 | 2.3077 |
| No | 16 (61.54) | 10 (38.46) | |||
| Pesticide exposure | |||||
| Yes | 5 (83.33) | 1 (16.67) | 3 (0.30-29.94) | 0.333 | 0.9375 |
| No | 15 (62.50) | 9 (37.50) | |||
| Site of tumor | |||||
| Colon | 7 (87.50) | 1 (12.50) | 4.84 (0.50-46.49) | 0.144 | 2.1307 |
| Rectum | 13 (59.09) | 9 (40.91) | |||
| Tumor differentiation | |||||
| Well | 9 (75) | 3 (25) | 1.28 (0.19-8.43) 2.33 (0.33-16.18) |
0.490 | 1.4250 |
| Moderate | 7 (70) | 3 (30) | |||
| Poor | 4 (50) | 4 (50) | |||
| TNM stage | |||||
| T1 | 9 (75) | 3 (25) | 5.6 (0.99-32.4) | 0.126 | 8.6011 |
| T2 | 8 (80) | 2 (20) | |||
| T3 | 2 (33.33) | 4 (66.67) | |||
| T4 | 1 (50) | 1(50) | |||
| T1+T2 | 17 (77.27) | 5 (22.73) | 0.75 (0.09-5.6) | ||
| T3+T4 | 3 (37.50) | 5 (62.50) | |||
| Stage | |||||
| I+II | 16 (72.73) | 6 (27.27) | 2.66 (0.50-14.21) | 0.243 | 1.3636 |
| III+IV | 4 (50) | 4 (50) | |||
| Tumour grade | |||||
| 1 | 9 (75) | 3 (25) | 1.28 (0.19-8.43) 2.33 (0.33-16.18) |
0.490 | 1.4250 |
| 2 | 7 (70) | 3 (30) | |||
| 3 | 4 (50) | 4 (50) | |||
Table 3: Correlation between the SFRP2 gene’s methylation pattern and clinicopathological traits in tissue samples from CRC patients.
We found that the SFRP2 gene promoter methylation pattern did not significantly correlate with any of the examined clinicopathological characteristics.
Methylation status of SFRP2 in blood samples
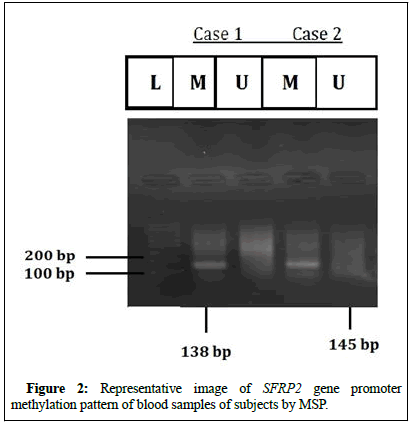
Figure 2 shows representative outcomes for MSP analysis seen in blood samples of subjects.
L: 100 bp unmethylated SFRP2 is indicated by the DNA marker U: (145 bp); while methylated SFRP2 is indicated by M: (138 bp).
In blood, the SFRP2 gene promoter methylation pattern was assessed in 20 CRC cases and also 20 controls taken from healthy individuals, using MSP. In CRC, methylation was found to be present in 60% (12/20) cases whereas 40% (8/20) cases showed the absence of methylation. Out of 12 cases in which methylation were present only 8.3% (1/12) showed both methylated and unmethylated bands whereas 91.6% (11/12) showed only methylated bands. In the normal blood samples, no methylation was found.
Correlation between the SFRP2 gene’s methylation pattern and clinicopathological traits in blood samples from CRC patients
SFRP2 gene promoter methylation pattern was correlated with several clinicopathological characteristics such as age, gender, tumor location, tumor grading, tumor staging, smoking status and family history. The correlation between the SFRP2 promoter methylation status and several clinicopathological characteristics is summarized in Table 4.
| Characteristics | Methylation present | Methylation absent | Odds ratio (95% CI) | P-value | Chi² |
|---|---|---|---|---|---|
| Age | |||||
| >50 | 5 (55.56) | 4 (44.44) | 0.714 (0.11-4.31) | 0.714 | 0.1347 |
| ≤ 50 | 7 (63.64) | 4 (36.36) | |||
| Gender | |||||
| Male | 9 (69.23) | 4 (30.77) | 3 (0.446-20.153) | 0.251 | 1.3187 |
| Female | 3 (42.86) | 4 (57.14) | |||
| Dwelling | |||||
| Rural | 9 (60) | 6 40) | 1 (0.126-7.893) | 1.000 | 0.000 |
| Urban | 3 (60) | 2 (40) | |||
| Social class | |||||
| Low | 2 (66.67) | 1 (33.33) | 1.4 (0.105-18.61) | 0.798 | 0.0654 |
| Middle | 10 (58.82) | 7 (41.18) | |||
| Family history | |||||
| Yes | 4 (100) | 0 (0) | ---------- | 0.068 | 3.33 |
| No | 8 (50) | 8 (50) | |||
| Smoking status | |||||
| Smoker | 7 (87.50) | 1 (12.50) | 9.8 (0.89-106.8) | 0.04 | 5.20 |
| Non-smoker | 5 (41.67) | 7 (58.33) | |||
| Lifestyle | |||||
| Active | 9 (56.25) | 7 (43.75) | 0.4 (0.03-5.06) | 0.494 | 0.468 |
| Sedentary | 3 (75) | 1 (25) | |||
| Body mass | |||||
| Normal | 4 (36.36) | 7 (63.64) | ------------ | 0.05 | 5.92 |
| Obese | 3 (100) | 0 (0) | |||
| Underweight | 5 (83.33) | 1 (16.67) | |||
| Salt tea intake | |||||
| Yes | 11 (61.11) | 7 (38.89) | 1.57 (0.08-29.4) | 0.761 | 0.092 |
| No | 1 (50) | 1 (50) | |||
| Red meat consumption | |||||
| Yes | 9 (64.29) | 5 (35.41) | 1.8 (0.25-12.5) | 0.550 | 0.357 |
| No | 3 (50) | 3 (50) | |||
| Sundried vegetables | |||||
| Yes | 9 (60) | 6 (40) | 1 (0.126-7.893) | 1 | 0.0000 |
| No | 3 (60) | 2 (40) | |||
| Junk food consumption | |||||
| Yes | 1 (33.33) | 2 (66.67) | 0.27 (0.02-3.66) | 0.306 | 1.045 |
| No | 11 (64.71) | 6 (35.29) | |||
| Pesticide exposure | |||||
| Yes | 2 (100) | 0 (0) | --------------- | 0.224 | 1.48 |
| No | 10 (55.56) | 8 (44.44) | |||
| Site of tumour | |||||
| Colon | 7 (77.78) | 2 (22.22) | 0.77 (0.08-6.98) | 0.0822 | 0.0505 |
| Rectum | 9 (81.82) | 2 (18.18) | |||
| Tumour differentiation | |||||
| Well | 3 (75.0) | 1 (25.0) | 0.66 (0.04-10.25) 6.75 (0.64-71.17) |
0.232 | 2.922 |
| Moderate | 9 (81.82) | 2 (18.18) | |||
| Poor | 2 (40) | 3 (60) | |||
| TNM stage | |||||
| T1 | 2 (66.67) | 1 (33.33) | |||
| T2 | 7 (87.50) | 1 (12.50) | |||
| T3 | 6 (85.71) | 1 (14.29) | 0.28 (0.11-6.91) 0.56 (0.04-7.44) |
0.926 | 1.379 |
| T4 | 2 (100) | 0 (0) | |||
| T1+T2 | 9 (81.82) | 2 (18.18) | |||
| T3+T4 | 8 (88.89) | 1 (11.11) | |||
| Stage | |||||
| I+II | 12 (85.71) | 2 (14.29) | -------------- | 0.329 | 0.9524 |
| III+IV | 6 (100) | 0 (0) | |||
| Tumour grade | |||||
| 1 | 3 (75.0) | 1 (25.0) | 0.66 (0.04-10.25) 6.75 (0.64-71.17) |
0.232 | 2.922 |
| 2 | 9 (81.82) | 2 (18.18) | |||
| 3 | 2 (40) | 3 (60) | |||
Table 4: Hypermethylation of the SFRP2 gene in colorectal cancer blood and clinicopathological factors are correlated.
We observed that smoking status showed a significant association with SFRP2 gene promoter methylation (p-value <0.05).
DISCUSSION
CRC is a frequent malignancy that develops from benign neoplasms and progresses from adenomas or hyperplastic polyps/serrated adenomas into adenocarcinomas in a stepwise histological progression sequence. Genetic changes have been linked to particular stages in this adenoma-carcinoma sequence and it is thought that these changes are what propel CRC histopathological advancement [13,14]. The presence of epigenetic changes, particularly DNA methylation (hyper or hypomethylation), in colorectal polyps, adenomas and CRCs has been demonstrated. The onset and progression of colorectal polyps and adenomas to CRC appear to be influenced by aberrant gene methylation in conjunction with genetic changes. Another epigenetic method used by colorectal cancer to silence tumor suppressor genes is aberrant gene methylation. Changes in DNA methylation have been identified as a major mechanism of colorectal cancer development [15]. It has been discovered that the main mechanism in the inactivation of a number of tumor suppressor genes is aberrant hypermethylation of CpG islands in gene promoters. The presence of gene silence caused by hypermethylation is a crucial aspect of colorectal tumors, though [16].
A family of secreted glycoproteins known as Secreted Frizzled Related Proteins (SFRPs). In the N-terminal half of the proteins, SFRPs have a distinctive Cysteine Rich Domain (CRD) that is homologous to the CRD of the Frizzled (Fz) receptor of Wnt. As a result, SFRPs may inhibit Wnt signaling by creating inactive complexes with Fz or by interacting with Wnt proteins to prevent them from binding to Fz proteins. It has been noted that SFRPs may be able to block the complete canonical Wnt pathway in colon cancer cells, even in the presence of mutations that activate APC or β-catenin downstream of the Fz receptor. Therefore, it may be necessary for the abnormal activation of the Wnt canonical pathway in the development of colorectal tumors for the SFRP genes to be silenced [17]. One SFRP gene family member involved in the wingless/Wnt signaling pathway is SFRP2. The genes are crucial for cell proliferation, apoptosis and the control of cell differentiation. In human malignancies such as prostate, hepatocellular and CRC, it is frequently methylated. Tumor development and Wnt signaling activity are tightly related to its downregulation. A growing body of evidence suggests that SFRP2, a crucial member of the SFRP family, inhibits the oncogenic Wnt pathway by competing with frizzled membrane bound receptors. Epigenetic inactivation of SFRP2 by promoter hypermethylation is typically found in a wide variety of cancers, including CRC. Numerous malignancies, including colorectal, ovarian, breast, gastric and liver cancers, have regularly shown loss of SFRP2 expression due to promoter hypermethylation [18].
In the present study, we have explored the methylation pattern of the SFRP2 gene promoter region in colorectal cancers by MSP as a possible mechanism for the downregulation of SFRP2 and also correlated results with several clinico pathological characteristics.
When colorectal tumor tissues were analyzed it was observed that 20 out of 30 (66.66%) CRC tissues had methylation present i.e. were hypermethylated and 10 out of 30 (33.33%) had no methylation present. In contrast, in colorectal cancer blood samples, it was observed that 12 out of 20 (60%) CRC blood samples had methylation present i.e. were also hypermethylated and 8 out of 20 (40%) had no methylation present. In our study majority of colorectal cancer tissues, 66.66% and 60% of CRC blood samples were hypermethylated. The hypermethylation in CRC tissues and CRC blood might be a mechanism underlying decreased expression of the SFRP2 gene in colorectal cancers which may be an important event in colon carcinogenesis. Similar results have also been observed by Tang, et al., where they found SFRP2 gene promoters to be hypermethylated in colon cancers [19]. Another study revealed that SFRP2 mRNA is considerably downregulated in melanoma and this downregulation of SFRP2 may be caused by promoter methylation. When the SFRP2 gene was demethylated by 5-aza-dCyd in melanoma cell lines, SFRP2 expression was restored at both the mRNA and protein levels, which prevented cell invasion [20]. Canonical Wnt signaling controls a number of processes involved in embryogenesis and adult homeostasis, according to research by, Bian et al. At the base of the crypt in the adult intestine, the Wnt pathway is turned on to preserve intestinal stem cell compartments. But the disruption of the Wnt pathway is primarily responsible for colon cancer cells’ expansion, invasion, and survival [21]. According to Sui, et al., in CRC, DNA hypermethylation of the SFRP2 gene has been found in a variety of studies using tissue, feces and blood detection. SFRP2 methylation occurred at the same time as the CRC's histological evolution phase.
All of these findings lend credence to our study’s finding that the SFRP2 gene promoter hypermethylation is a key element in the development of colon cancer. These findings imply that SFRP2 methylation is intricately connected to the beginning and development of CRC carcinogenesis. In order to identify CRC before it manifests itself fully, it may be helpful to check the SFRP2 methylation status. In our study, we observed that smoking status showed a significant association with SFRP2 gene promoter methylation (p value<0.05) in CRC blood samples, SFRP2 methylation status, however, did not correlate with any of the other factors that were investigated, such as age, gender, tumor size, lymph node metastasis or TNM stage.
In CRC tumor samples, there was no discernible relationship between SFRP2 gene promoter hypermethylation and any clinicopathological factor such as age, tumor differentiation or stage. However, the study’s sample size was extremely tiny, which might account for the lack of statistical significance.
No significant study in the past has correlated hypermethylation of the SFRP2 gene with clinicopathological characteristics whereas Li, et al., correlated the expression of the SFRP2 gene with several clinicopathological characteristics and found a statistically significant association with early stage disease.
Conclusion
We investigated the significance of SFRP2 gene hypermethylation in colorectal cancer, as a possible underlying mechanism affecting its expression. Our study is an attempt to accentuate the diagnostic and prognostic importance of SFRP2 gene hypermethylation in CRC. Hypermethylation was evident in both CRC tissues and blood samples. This research encourages further investigation into the diagnostic and prognostic value of the methylation status of the Secreted Frizzled Related Protein 2 (SFRP2) in Colorectal Cancer (CRC).
References
- Testa U, Castelli G, Pelosi E (2020) Genetic alterations of metastatic colorectal cancer. Biomedicines 8: 414.
[Crossref] [Google Scholar] [PubMed]
- Peluso G, Incollingo P, Calogero A, Tammaro V, Rupealta N, et al. (2017) Current tissue molecular markers in colorectal cancer: A literature review. Biomed Res Int 2017: 1-8.
[Crossref] [Google Scholar] [PubMed]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71: 209-249.
[Crossref] [Google Scholar] [PubMed]
- Pehlivan S, Artac M, Sever T, Bozcuk H, Kilincarslan C, Pehlivan M. Gene methylation of SFRP2, P16, DAPK1, HIC1, and MGMT and KRAS mutations in sporadic colorectal cancer. Cancer Genetics Cytogenet 201: 128-132.
[Crossref] [Google Scholar] [PubMed]
- Huang Z, Li L, Wang J (2007) Hypermethylation of SFRP2 as a potential marker for stool based detection of colorectal cancer and precancerous lesions. Dig Dis Sci 52: 2287-2291.
[Crossref] [Google Scholar] [PubMed]
- Jung G, Hernandez-Illan E, Moreira L, Balaguer F, Goel A (2020) Epigenetics of colorectal cancer: Biomarker and therapeutic potential. Nat Rev Gastroenterol Hepatol 17: 111-130.
[Crossref] [Google Scholar] [PubMed]
- Ng C, Li H, Wu WK, Wong SH, Yu J (2019) Genomics and metagenomics of colorectal cancer. J Gastrointest Oncol 10: 1164-1170.
[Crossref] [Google Scholar] [PubMed]
- Qi J (2006) Hypermethylation and expression regulation of secreted frizzled related protein genes in colorectal tumor. WJG 12: 7113-7117.
[Crossref] [Google Scholar] [PubMed]
- Tariq K, Ghias K. Colorectal cancer carcinogenesis: A review of mechanisms. Cancer Biol Med 13: 120.
[Crossref] [Google Scholar] [PubMed]
- Marley AR, Nan H (2016) Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet 7: 105-114.
[Google Scholar] [PubMed]
- Liu X, Fu J, Bi H, Ge A, Xia T, et al. (2019) DNA methylation of SFRP1, SFRP2, and WIF1 and prognosis of postoperative colorectal cancer patients. BMC Cancer 19: 1212.
[Crossref] [Google Scholar] [PubMed]
- Jass JR (2004) Hyperplastic polyps and colorectal cancer: Is there a link?. Clin Gastroenterol Hepatol 2: 1-8.
[Crossref] [Google Scholar] [PubMed]
- Souglakos J (2007) Genetic alterations in sporadic and hereditary colorectal cancer: Implementations for screening and follow-up. Dig Dis 25: 9-19.
[Crossref] [Google Scholar] [PubMed]
- Wang DR, Tang D (2008) Hypermethylated SFRP2 gene in fecal DNA is a high potential biomarker for colorectal cancer noninvasive screening. World J Gastroenterol 14: 524-531.
[Crossref] [Google Scholar] [PubMed]
- Qi J, Zhu YQ, Luo J, Tao WH, Zhang JM (2007) Hypermethylation and regulation of expression of secreted frizzled related protein genes in colorectal tumor. Zhonghua Zhong Liu Za Zhi 29: 842-845.
[Google Scholar] [PubMed]
- Li H, Wang Z, Zhao G, Ma Y, Chen Y, et al. (2019) Performance of a methylight assay for methylated SFRP2 DNA detection in colorectal cancer tissue and serum. Int J Biol Markers 34: 54-59.
[Crossref] [Google Scholar] [PubMed]
- Takagi H, Sasaki S, Suzuki H, Toyota M, Maruyama R, et al. (2008) Frequent epigenetic inactivation of SFRP genes in hepatocellular carcinoma. J Gastroenterol 43: 378-389.
[Crossref] [Google Scholar] [PubMed]
- Tang D, Liu J, Wang D, Yu H, Li Y, et al. (2011) Diagnostic and prognostic value of the methylation status of secreted frizzled related protein 2 in colorectal cancer. Clin Invest Med 34: 88-95.
[Crossref] [Google Scholar] [PubMed]
- Luo X, Wei B, Chen A, Zhao H, Huang K, et al. (2016) Methylation mediated loss of SFRP2 enhances melanoma cell invasion via Wnt signaling. Am J Transl Res 8: 1502.
[Google Scholar] [PubMed]
- Bian J, Dannappel M, Wan C, Firestein R (2020) Transcriptional regulation of Wnt/β-catenin pathway in colorectal cancer. Cells 9: 2125.
[Crossref] [Google Scholar] [PubMed]
- Sui C, Ma J, Chen Q, Yang Y (2016) The variation trends of SFRP2 methylation of tissue, feces and blood detection in colorectal cancer development. Eur J Cancer Prev 25: 288-298.
[Crossref] [Google Scholar] [PubMed]
Citation: Ahmad S, Rashid G, Niyaz M, Nisar S, Mudassar S (2023) The Implications of SFRP2 Gene Hypermethylation in Colorectal Cancer. J Oncol Res Treat 8: 222.
Copyright: © 2023 Ahmad S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 1236
- [From(publication date): 0-2022 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 1008
- PDF downloads: 228