Research Article Open Access
The Immunoregulatory Role of Cytokines in Congestive Heart Failure
Chen O1*, Patel J2, Mohamed E1, Greene M1, Moskovits N1and Shani J11Department of Cardiology, Maimonides Medical Center, Brooklyn, New York, USA
2Department of Internal Medicine, Maimonides Medical Center, Brooklyn, New York, USA
- *Corresponding Author:
- On Chen
Maimonides Medical Center
Department of Cardiology
Brooklyn, New York, USA
Tel: 631-2910908
Fax: 718-6357436
E-mail: onn.chen@yahoo.com
Received date: August 14, 2014; Accepted date: September 23, 2014; Published date: September 25, 2014
Citation: Chen O, Patel J, Mohamed E, Greene M, Moskovits N, et al. (2014) The Immunoregulatory Role of Cytokines in Congestive Heart Failure. Microinflammation 1:111. doi: 10.4172/2381-8727.1000111
Copyright: © 2014 Chen O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at International Journal of Inflammation, Cancer and Integrative Therapy
Abstract
Background: Cytokines have been shown to be associated with heart failure and to have multiple biological effects that can lead to left ventricular dysfunction, pulmonaryedema, and left ventricular remodeling. However, the effects of different cytokines, separately and in combination have not been fully determined. We aim to evaluate the levels of both pro-inflammatory and anti-inflammatory cytokines in patients with congestive heart failure (CHF), as well as in patients with coronary artery disease (CAD) and compare them to levels in normal controls (individuals without CHF or CAD) as to suggest their possible etiologic role.
Methods and results: In our study, the serum levels of IL-6, IL-10, IL-1β, IL-2 and TNF-α were measured in 19 patients with CHF, 18 patients with CAD without CHF and 8 controls, using immunoassays. There was a significant increase in the serum levels of IL-6, IL1-β, IL-10 and TNFα in the CHF group, and of IL-6, IL-1β and TNFα in the CAD group, compared with controls. Patients with CHF had significantly higher levels of anti-inflammatory cytokines IL-6 and IL-10 and lower levels of pro-inflammatory cytokines IL-2 compared when compared patients with CAD, emphasizing the different pathophysiologic mechanisms for these conditions.
Conclusion: Our studies suggest that patients with CHF had higher levels of Th2-type cytokines than patients with CAD, suggesting their role in the development of heart failure. Further studies are required to elucidate the role of cytokines in heart failure, and to understand the biological effects of cytokines and their role in the development of CHF. This may lead to development of new treatments modalities targeting their effects.
Introduction
The quest to find the biologic mechanisms that explain the development and the progression of heart failure has evolved throughout the decades. Several models have been proposed to explain the pathophysiology of congestive heart failure including the cardio-renal model, the circulatory/hemodynamic model and the neuro-hormonal model [1]. More recent studies have demonstrated the significance of immuno-senescence in heart failure patients compared to age matched controls, and a novel immunological-inflammatory model is gaining increased attention explaining the development and progression of heart failure [2].
Various inflammatory cytokines produce a myriad of effects and cause imbalance in cardiac homeostasis. These effects include pulmonary edema, left ventricular dysfunction, left ventricular remodeling, cardiomyopathy and abnormalities in myocardial metabolism [3-8]. It is the particular balance of pro-inflammatory and anti-inflammatory cytokines that influence cardiac homeostasis.
Pro-inflammatory Th1 cytokines have been detected in patients with heart disease including Interleukin-2 (IL-2) and Tumor Necrosis Factor-α (TNF-α) [9-12]. IL-2 further stimulates downstream production of other pro-inflammatory cytokines Interferon-g (IFN-g) and Interleukin-4 (IL-4). TNF-α, produced by activated lymphocytes and macrophages, plays an important role in the regulation of nitric oxide metabolism in leukocytes and vascular endothelial cells [13,14]. Additionally, other pro-inflammatory cytokines like IL-1α, IL-2 and interferon-γ can induce TNFα production [15]. TNF-α has negative inotropic effects, and has been implicated in systolic dysfunction, ventricular remodeling, interstitial fibrosis and myocyte apoptosis [16-19].
Other pro-inflammatory cytokines include Interleukin-1α(IL-1α) and Interleukine-1β(IL-1β). They have similar biologic activities and exert their pro-inflammatory activities through binding with Type I receptors. It was reported that IL-1β and TNF-α could separately and synergistically depress human myocardial function (in vitro) [20]. IL-1-receptor antagonists might have a protective effect on the heart by attenuating this inflammatory process [21]. This was shown by IL-1 blockade with Anakinra, which improved myocardial relaxation properties and aerobic exercise tolerance in patients with HFpEF [22].
Anti-inflammatory Th2 cytokines have not been as frequently studied as Type –I cytokines. Interleukin-10 (IL-10), has many anti-inflammatory effects including inhibition of proliferation and synthesis of IFN-gamma and IL-2 by Th1 lymphocytes; IL-4 and IL-5 by Th2 lymphocytes; IL-1β, IL-6, IL-8, IL-12 and TNF-α by mononuclear phagocytes; and IFN-g and TNFα by NK cells [23,24]. It has been reported that deficiency of IL-10 exacerbates myocardial injury after ischemia-reperfusion injury and enhances the infiltration of neutrophils into the myocardium [25].
Interleukin-6 (IL-6) is an inflammatory marker that has the ability to mediate T-cell growth and differentiation, induce macrophage maturation and stimulate acute phase proteins synthesis in the liver. This cytokine has both pro- and anti-inflammatory activities. However, elevated TNFα and IL-6 levels appears to portend severity of congestive heart failure and has been correlated with plaque instability, predicting a more severe index of an acute coronary syndrome with increased 6 and 12 month mortality, independent of cardiac Troponins [26-32].
This introduction has provided evidence for the role of both pro- and anti-inflammatory cytokines in the pathophysiology of cardiac disease. It is not clear, however, if the pathologic changes seen in congestive heart failure and coronary artery disease are caused by the activated components of the immune system to the same level across a population. In this study, we have determined the levels of IL-6, IL-10, IL-1β, IL-2, and TNF-α in congestive heart failure patients and compared those with the levels in coronary artery disease patients without heart failure. The exact role of these immune system components in the pathogenesis heart failure is needed before deciding whether they should be used as targets for treatment, prognostic factors or as follow-up markers to determine optimal therapy.
Methods
Population
Our study population consisted of 45 patients, of which 19 had presented with acute exacerbation of congestive heart failure, 18 with coronary artery disease and 8 healthy subjects (individuals without coronary artery disease or heart failure) who served as controls. Patients in the heart failure group were enrolled during hospital admission for decompensated heart failure and the blood samples were drawn during their hospital stays at our institution.
Patients with coronary artery disease were admitted to the hospital for elective cardiac catheterization. For these patients, blood samples were drawn prior to cardiac catheterization. Demographics and baseline characteristics were obtained by chart review. Immunoassay for cytokines. Peripheral blood samples were drawn from congestive heart failure patients (n=19), coronary artery disease patients (n=18) and controls (n=8), after informed consent was obtained. Blood samples were collected in non-heparinized red-top monojet tubes (Sherwood Medical, St. Louis, MO) and were allowed to clot for 30 minutes at room temperature. Sera were obtained by spinning the samples at 1000×g for 10 minutes and were aliquoted and stored at –20°F. The serum levels of IL-10, TNF-α, and IL-1β assays were measured using Biosource immunoassay commercial kit (Biosource International Inc, Camarillo, CA). IL-6 was measured using an immunoassay kit from Endogen (Endogen Inc, Woburn, MA) and IL-2 was measured by using R&D system commercial kit (R&D Systems, Minneapolis, MN). The sensitivities of the ELISA kits were as follows. For TNF-α 1.7 pg/ml, for IL-10<5 pg/ml, for IL-1-β<1 pg/ml, for IL-6<1 pg/ml, and for IL-2<7 pg/ml. Serum levels of all cytokines were measured by employing solid phase sandwich ELISA methods according to manufacturer’s instructions. Briefly, the commercial plates are coated with a monoclonal antibody specific for the cytokine. Samples (serum) and standards are pipetted into the wells and the immobilized antibody binds any cytokine present. After washing away any unbound proteins, an enzyme linked polyclonal antibody specific for cytokine is added to the wells to sandwich the cytokine immobilized during the first incubation. Following a wash to remove any unbound antibody-enzyme reagent, a substrate solution is added to the wells and a color reaction develops in proportion to the amount of cytokine bound in the initial step. The color development is stopped and the intensity of the color is measured. A curve is prepared, plotting the optical density versus the concentration of cytokine in the standard wells. By comparing the optical density of samples to this standard curve, the concentrations of the cytokines in the samples were measured, according to the manufacturer instructions.
Statistical analysis
Data in text and tables are expressed as mean ± standard deviation (SD) for continuous variables or number (%) for categorical variables. To compare the levels of the cytokines measured between the study and control groups, Student t test was used whenever normal distribution was present, otherwise the Mann Whitney test was used.
Results
There was no significant difference between the heart failure patients and the coronary artery disease patients in regards to their age, female ratio, serum creatinine, white blood cell count, beta-blocker or statin use. More patients in the heart failure group were on angiotensin converting enzyme inhibitors and digoxin (Table 1). The heart failure patients were in NYHA classes II-IV with most of the patients in class III. The etiologies for heart failure were mainly ischemic heart disease and/or hypertensive heart disease. One patient had heart failure secondary to severe mitral regurgitation. The results of cytokine levels, including both pro-inflammatory and anti-inflammatory cytokines were listed in Table 2.
| Variable | CHF | CAD |
|---|---|---|
| Age (mean±SD) | 70.9(±13.1) | 69.3(±12.7) |
| Female ratio | 9/19(47%) | 9/18(50%) |
| Serum creatinine (mean±SD) | 1.2(±0.4) | 1.2(±0.7) |
| WBC count (mean±SD) | 9.6(±3.8) | 9.3(±3.8) |
| Beta-blocker n (%) | 18/19(95%) | 16/18(88%) |
| ACE-inhibitor or ARB n (%) | 19/19(100%) | 8/18(44%) |
| Digoxin n (%) | 9/19(47%) | 0/18(0%) |
| Statin n (%) | 7/19(37%) | 6/18(33%) |
Table 1: Data are expressed as mean ± standard deviation (SD) or n (%). ACEInhibitor=angiotensin-converting-enzyme inhibitor, ARB= angiotensin receptor blocker, CAD=coronary artery disease, CHF=congestive heart failure.
| Interleukin | CHF (mean±sem) | CAD (mean±sem) | Controls (mean±sem) | p value* | p value† | p valueπ |
|---|---|---|---|---|---|---|
| IL-6 | 18.9(±2.5) | 11.6(±2.3) | 3.7(±0.4) | <0.0001 | <0.05 | <0.005 |
| IL-1β | 3.70(±0.5) | 2.55(±0.3) | 0.74(±0.2) | <0.0001 | 0.12 | 0.0001 |
| IL-10 | 9.7(±3.9) | 6.12(±1.6) | 5.3(±1.2) | 0.005φ | <0.01φ | 0.69 |
| IL-2 | 0.87(±0.1) | 1.52(±0.3) | 1.34(±0.2) | 0.07 | 0.001φ | 0.6 |
| TNFα | 10.9(±2.4) | 12.9(±1.8) | 9.26(±0.8) | <0.01φ | 0.5 | <0.01φ |
| IL6/IL10 | 5.23(±1.2) | 1.95(±0.3) | 0.85(±0.1) | <0.01 | <0.05 | <0.01 |
| TNFα/IL10 | 1.75(±0.3) | 2.21(±0.4) | 2.38(±0.6) | 0.3 | 0.4 | 0.8 |
Table 2: Data are expressed as mean ± standard deviation (SD), *CHF>controls, †CHF>CAD, πCAD>controls, φdetermined using Mann Whitney Test CAD>CHF, CHF=congestive heart failure, CAD=coronary artery disease.
Pro-inflammatory cytokines
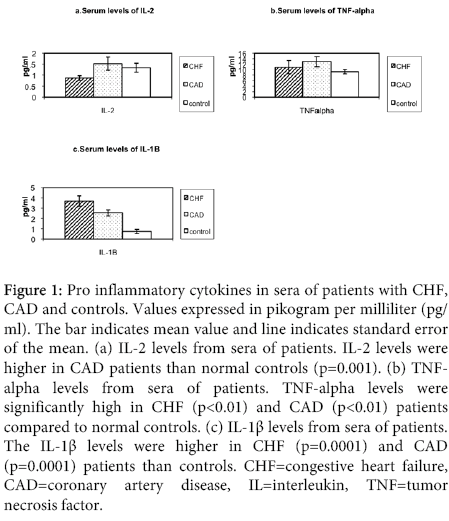
The comparison of pro-inflammatory cytokine levels in congestive heart failure and coronary artery disease group patients are shown in Figure 1. IL-2: The levels of IL-2 were significantly higher in coronary artery disease patients than in controls (p=0.001). There was no significant elevation of IL-2 levels in congestive heart failure patients than controls.
Figure 1: Pro inflammatory cytokines in sera of patients with CHF, CAD and controls. Values expressed in pikogram per milliliter (pg/ ml). The bar indicates mean value and line indicates standard error of the mean. (a) IL-2 levels from sera of patients. IL-2 levels were higher in CAD patients than normal controls (p=0.001). (b) TNFalpha levels from sera of patients. TNF-alpha levels were significantly high in CHF (p<0.01) and CAD (p<0.01) patients compared to normal controls. (c) IL-1β levels from sera of patients. The IL-1β levels were higher in CHF (p=0.0001) and CAD (p=0.0001) patients than controls. CHF=congestive heart failure, CAD=coronary artery disease, IL=interleukin, TNF=tumor necrosis factor.
TNFα: The serum levels of TNFα were significantly higher in patients with congestive heart failure than normal controls (<0.01). The serum levels of TNFα were also significantly higher in coronary artery disease patients compared to controls (p<0.01). There was no difference in levels of TNFα between congestive heart failure and coronary artery disease groups.
IL-1β: The serum levels of IL1-β were higher in patients with congestive heart failure compared with controls (p<0.0001). The serum levels of IL-1 β were significantly elevated in patients with coronary artery disease compared to controls (p=0.0001). There were no significant differences in the levels of IL-1 β in coronary artery disease patients and congestive heart failure patients.
Anti-inflammatory cytokines
The following anti-inflammatory cytokine levels were compared between congestive heart failure and coronary artery disease patients in Figure 2.
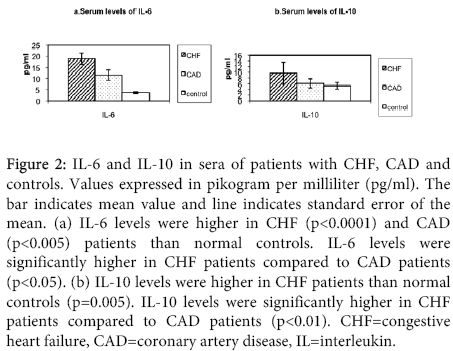
Figure 2: IL-6 and IL-10 in sera of patients with CHF, CAD and controls. Values expressed in pikogram per milliliter (pg/ml). The bar indicates mean value and line indicates standard error of the mean. (a) IL-6 levels were higher in CHF (p<0.0001) and CAD (p<0.005) patients than normal controls. IL-6 levels were significantly higher in CHF patients compared to CAD patients (p<0.05). (b) IL-10 levels were higher in CHF patients than normal controls (p=0.005). IL-10 levels were significantly higher in CHF patients compared to CAD patients (p<0.01). CHF=congestive heart failure, CAD=coronary artery disease, IL=interleukin.
IL-10: The serum levels of IL-10 were significantly elevated in patients with congestive heart failure compared to controls (p=0.005). There was no difference in IL-10 levels in coronary artery disease patients and controls. The serum levels of IL-10 were significantly higher in congestive heart failure patients than coronary artery disease patients (p<0.01).
IL-6: The serum levels of IL-6 were significantly higher in patients with congestive heart failure compared with controls (p<0.0001). The serum levels of IL-6 were significantly higher in coronary artery disease patients as compared to normal controls (p<0.005). Congestive heart failure patients had significantly higher levels of IL-6 than coronary artery disease patients (p<0.05).
IL6/IL10 ratio: The IL6/IL10 ratio was significantly higher in patients with congestive heart failure (mean-5.23) as compared to controls (mean-0.85) (p<0.01). The ratio was significantly elevated in patients with coronary artery disease (mean-1.95) as compared to normal controls (<0.01). The ratio was significantly higher in congestive heart failure patients than in coronary artery disease patients (<0.05).
TNF/IL10 ratio: The TNF/IL10 ratio was not significant in patients with congestive heart failure (mean-1.75) as compared to controls (mean-2.38). The ratio was not significant in patients with coronary artery disease (mean-2.21) as compared to controls. The ratio is not significant between patients with congestive heart failure and coronary artery disease.
Discussion
The results of this study show that the immune system is activated in heart failure patients as well as patients with coronary artery disease without heart failure. The serum levels of IL-6, IL-10 and IL-6/IL-10 ratio are significantly higher in congestive heart failure patients than in coronary artery disease patients. This may indicate that IL-6 and IL-10 play a bigger role in the pathophysiology of congestive heart failure compared to other forms of heart disease. There have been several hypotheses with respect to the source of production of pro-inflammatory cytokines in different forms of heart diseases. The first hypothesis suggests that activation of the immune system in response to some kind of tissue injury is responsible for cytokine production [33-35]. The second hypothesis suggests that pro-inflammatory cytokines are activated in stressed myocardial tissue and that the elevated levels in the peripheral circulation represent a spillover from the cytokines produced locally in the myocardium [36-38]. The third hypothesis is that pro-inflammatory cytokines are produced by the peripheral tissues as a result of under perfusion [39]. The etiology for the increased levels of IL-6 in heart failure patients as compared to patients with coronary disease is not known. In a study from MESA (Multi-Ethnic Study of Atherosclerosis) a strong correlation between increased IL-6 levels and regional left ventricular dysfunction by MRI tagging in asymptomatic men and women was shown, suggesting an underlying pathogenic link between inflammation and left ventricular dysfunction [40]. It was reported that the trans-cardiac IL-6 gradient was higher in patients with acute coronary syndromes but not in heart failure patients compared to controls [41]. This suggests that the underlying patho-physiology and mechanism of IL-6 activation is different in both conditions. In our study, patients with coronary artery disease diagnosed on elective coronary angiography did not have significantly elevated IL-6 levels. However, in a study with ST elevation myocardial infarction (STEMI) patients, Serum levels of IL-6>20 pg/ml in the first 24 hours after a STEMI was significantly associated with higher frequency of poor in-hospital outcomes such as arrhythmias and death [42]. It has also been reported that individuals with asymptomatic left ventricular dysfunction have elevated levels of IL-6, and that IL-6 levels also increase with increasing NYHA class [40-42]. These two findings may or may not represent a continuum.
IL-6 was originally identified as a B-cell differentiation factor that stimulated terminal differentiation/maturation of B cells into antibody producing plasma cells [15]. A a result there may be an excessive production of antibodies against various cardiac muscle components, e.g. cardiac myosin, which may play a role in the pathogenesis of this syndrome. One study showed that therapy with intravenous immunoglobulin (IVIG) decreased TNFα and IL-1β, increased IL-10, IL-1α, soluble tumor necrosis factor receptor (sTNFR) levels and left ventricular ejection fraction after 26 weeks of treatment [43]. It was demonstrated that the use of immunoglobulin adsorption in patients with idiopathic dilated cardiomyopathy resulted in a significant increase in left ventricular ejection fraction after 1 year of follow-up [44]. Therefore the mechanism by which the immune system is involved in heart failure could involve both the humoral immunity as well as the cellular immunity.
Although IL-6 and IL-10 are elevated in heart failure patients, the IL-6/IL-10 ratio is significantly higher in this group. These ratios represent a pro-inflammatory to anti-inflammatory ratio. This suggests that the degree of elevation of IL-10 is smaller relative to degree of IL-6 elevation. The mechanism for the elevation of IL-10 serum levels is unclear. It may be a response to the overall immune system activation. The TNF/IL-10 ratio was not significantly higher in congestive heart failure patients. This points to the fact that the various cytokines, except for IL-6, are activated to a similar degree. In a recent study it was reported that the addition of IL-10 profoundly inhibits lipid polysaccharide (LPS) stimulated TNF release from peripheral blood mononuclear cells isolated from patients with chronic heart failure [45]. It is unclear however whether IL-10 has the same effect on the production of IL-6. Against its conventional value of being anti-inflammatory in one study elevated IL-10 levels failed to show protective effects in patients with heart failure and in association with increased TNFα, it actually suggested significantly higher mortality [46]. IL-10 may also indirectly enhance antibody production due to inhibition of IL-2 and INF-g by Th1 cells thereby favoring Th2 response. This also supports the hypothesis that antibody production may play a big role in heart failure.
The levels of IL-1β and TNFα were significantly elevated in both groups of patients, compared to normal controls. There was no significant difference between heart failure patients and patients with coronary artery disease. Unlike these cytokines, the levels of IL-6 were significantly higher in congestive heart failure than in coronary artery disease, which indicates a special role of IL-6 in pathogenesis of congestive heart failure.
Interestingly, IL-2 was higher in coronary artery disease patients compared to congestive heart failure patients. Its elevation in coronary artery disease patients indicates a more vigorous inflammatory mechanism in this subset. There was no significant IL-2 elevation in heart failure patients compared to normal controls. Increased levels of IL-2 was demonstrated in patients with coronary artery disease and those with coronary artery calcification suggesting its intricate role in promoting atherosclerosis in coronary system [47]. IL-2 is a pro-inflammatory cytokine with the ability to activate all types of acquired immune response. Paradoxically, IL-2 and IL-2 receptor β appears to play an important role in limiting such responses and eliminating auto-reactive T cells through promoting apoptosis. Furthermore mutations that inactivate IL-2 and IL-2 receptor β lead to excessive T-cell proliferation and autoimmunity [48-49]. Independent of T cell receptor activation, IL-2 can directly activate human invariant natural killer (iNK) T cells, which are thought to be important regulators of autoimmunity [50].
The exact role cytokines play in the pathogenesis of heart failure remains unclear. Some therapies known to decrease the mortality in heart failure patients such as carvedilol and candesartan were shown to decrease IL-6 levels in heart failure patients [51-52]. Therapy with infliximab, a monoclonal antibody against TNFα, did not improve the clinical status of patients with moderate to severe heart failure [53-55]. However, recent experimental data suggest that ablation of the gene for TNF receptor 1 blunts heart failure, whereas ablation of the gene for TNF receptor 2 exacerbates heart failure. This could explain the failure of anti-TNF therapy and suggests that the benefit in anti-cytokine therapy lies in the balance between receptor activities rather than the antagonistic effect on one specific receptor [56]. Therapy directed against autoantibodies has shown some promising outcomes. Two studies using intravenous immunoglobulin and immunoglobulin adsorption produced significant increases in left ventricular ejection fraction at 6 months and 12 months, respectively [44-45].
Our study shows that more of Th2 types of cytokines were elevated in heart failure patients compared to coronary artery disease patents pointing towards different pathophysiologic mechanisms of immune activation in these conditions. Specific roles of these immunologic inflammatory pathways in coronary artery disease and heart failure need to be better elucidated which in turn will help targeting them for treatment of these conditions more efficiently in the future. The main limitation to our study is the small number of patients. Further studies are needed to confirm our findings.
References
- Packer M (1993) How should physicians view heart failure? The philosophical and physiological evolution of three conceptual models of the disease. Am J Cardiol 71: 3C-11C.
- Moro-García MA, Echeverría A, Galán-Artímez MC, Suárez-García FM, Solano-Jaurrieta JJ, et al. (2014) Immunosenescence and inflammation characterize chronic heart failure patients with more advanced disease. Int J Cardiol 174: 590-599.
- Packer M (1992) The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am CollCardiol 20: 248-254.
- Millar AB, Foley NM, Singer M, Johnson NM, Meager A, et al. (1989) Tumour necrosis factor in bronchopulmonary secretions of patients with adult respiratory distress syndrome. Lancet 2: 712-714.
- Suffredini AF, Fromm RE, Parker MM, Brenner M, Kovacs JA, et al. (1989) The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med 321: 280-287.
- Pagani FD, Baker LS, Hsi C, Knox M, Fink MP, et al. (1992) Left ventricular systolic and diastolic dysfunction after infusion of tumor necrosis factor-alpha in conscious dogs. J Clin Invest 90: 389-398.
- Natanson C, Eichenholz PW, Danner RL, Eichacker PQ, Hoffman WD, et al. (1989) Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J Exp Med 169: 823-832.
- Hegewisch S, Weh HJ, Hossfeld DK (1990) TNF-induced cardiomyopathy. Lancet 335: 294-295.
- Semb H, Peterson J, Tavernier J, Olivecrona T (1987) Multiple effects of tumor necrosis factor on lipoprotein lipase in vivo. J BiolChem 262: 8390-8394.
- Smith KA (1980) T-cell growth factor.Immunol Rev 51: 337-357.
- Robb RJ, Munck A, Smith KA (1981) T cell growth factor receptors. Quantitation, specificity, and biological relevance.J Exp Med 154: 1455-1474.
- Schwartz RH (1990) A cell culture model for T lymphocyte clonal anergy.Science 248: 1349-1356.
- Uchiyama T, Nelson DL, Fleisher TA, Waldmann TA (1981) A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. II. Expression of Tac antigen on activated cytotoxic killer T cells, suppressor cells, and on one of two types of helper T cells.J Immunol 126: 1398-1403.
- Teresa krakauer, Vilcek A, Joost J (1999) Oppenheim.Proinflammatory cytokines. Fundamental Immunology. Fourth edition, Chapter 22, Lippincott-Raven Publishers.
- Yoshizumi M, Perrella MA, Burnett JC Jr, Lee ME (1993) Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life.Circ Res 73: 205-209.
- Pulkki KJ (1997) Cytokines and cardiomyocyte death.Ann Med 29: 339-343.
- Yokoyama T, Vaca L, Rossen RD, Durante W, Hazarika P, et al. (1993) Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart.J Clin Invest 92: 2303-2312.
- Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, et al. (1997) Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha.Circ Res 81: 627-635.
- Bradham WS, Bozkurt B, Gunasinghe H, Mann D, Spinale FG (2002) Tumor necrosis factor-alpha and myocardial remodeling in progression of heart failure: a current perspective.Cardiovasc Res 53: 822-830.
- Aikawa R, Nitta-Komatsubara Y, Kudoh S, Takano H, Nagai T, et al. (2002) Reactive oxygen species induce cardiomyocyte apoptosis partly through TNF-alpha.Cytokine 18: 179-183.
- Cain BS, Meldrum DR, Dinarello CA, Meng X, Joo KS, et al. (1999) Tumor necrosis factor-alpha and interleukin-1beta synergistically depress human myocardial function.Crit Care Med 27: 1309-1318.
- Van Tassell BW, Arena R, Biondi-Zoccai G, McNair Canada J, Oddi C, et al. (2014) Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study).Am J Cardiol 113: 321-327.
- Suzuki K, Murtuza B, Smolenski RT, et al. (2001) over-expression of interleukin-1 receptor antagonist provides cardioprotection against ishemia-reperfusion injury associated with reduction of apoptosis. Circulation 104: 1308-1313.
- Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR (1993) Interleukin-10.Annu Rev Immunol 11: 165-190.
- Ho AS, Liu Y, Khan TA, Hsu DH, Bazan JF, et al. (1993) A receptor for interleukin 10 is related to interferon receptors.ProcNatlAcadSci U S A 90: 11267-11271.
- Gearing DP, Comeau MR, Friend DJ, Gimpel SD, Thut CJ, et al. (1992) The IL-6 signal transducer, gp130: an oncostatin M receptor and affinity converter for the LIF receptor.Science 255: 1434-1437.
- Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, et al. (1986) Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin.Nature 324: 73-76.
- Levine B, Kalman J, Mayer L, Fillit HM, Packer M (1990) Elevated circulating levels of tumor necrosis factor in severe chronic heart failure.N Engl J Med 323: 236-241.
- Dutka DP, Elborn JS, Delamere F, Shale DJ, Morris GK (1993) Tumour necrosis factor alpha in severe congestive cardiac failure.Br Heart J 70: 141-143.
- McMurray J, Abdullah I, Dargie HJ, Shapiro D (1991) Increased concentrations of tumour necrosis factor in "cachectic" patients with severe chronic heart failure.Br Heart J 66: 356-358.
- Ansari AA, Wang YC, Danner DJ, Gravanis MB, Mayne A, et al. (1991) Abnormal expression of histocompatibility and mitochondrial antigens by cardiac tissue from patients with myocarditis and dilated cardiomyopathy.Am J Pathol 139: 337-354.
- Rordorf R, Savastano S, Sanzo A, Spazzolini C, De Amici M, et al. (2014) Tumor Necrosis Factor-α Predicts Response to Cardiac Resynchronization Therapy in Patients With Chronic Heart Failure.Circ J 78: 2232-2239.
- Gerli R, Rambotti P, Spinozzi F, Bertotto A, Chiodini V, et al. (1986) Immunologic studies of peripheral blood from patients with idiopathic dilated cardiomyopathy.Am Heart J 112: 350-355.
- Hwang S, Harris TJ, Wilson NW, Maisel AS (1993) Immune function in patients with chronic stable congestive heart failure.Am Heart J 125: 1651-1658.
- Giroir BP, Johnson JH, Brown T, Allen GL, Beutler B (1992) The tissue distribution of tumor necrosis factor biosynthesis during endotoxemia.J Clin Invest 90: 693-698.
- Kapadia S, Lee J, Torre-Amione G, Birdsall HH, Ma TS, et al. (1995) Tumor necrosis factor-alpha gene and protein expression in adult feline myocardium after endotoxin administration.J Clin Invest 96: 1042-1052.
- Kapadia SR, Oral H, Lee J, Nakano M, Taffet GE, et al. (1997) Hemodynamic regulation of tumor necrosis factor-alpha gene and protein expression in adult feline myocardium.Circ Res 81: 187-195.
- Sindhwani R, Yuen J, Hirsh H, et al. Reversal of low flow state attenuates immune activation in severe decompensated congestive heart failure.
- Deliargyris EN, Raymond RJ, Theoharides TC, Boucher WS, Tate DA, et al. (2000) Sites of interleukin-6 release in patients with acute coronary syndromes and in patients with congestive heart failure.Am J Cardiol 86: 913-918.
- Sakamoto A, Ishizaka N, Imai Y, Ando J, Nagai R, et al. (2014) Association of serum IgG4 and soluble interleukin-2 receptor levels with epicardial adipose tissue and coronary artery calcification.ClinChimActa 428: 63-69.
- Raymond RJ, Dehmer GJ, Theoharides TC, Deliargyris EN (2001) Elevated interleukin-6 levels in patients with asymptomatic left ventricular systolic dysfunction.Am Heart J 141: 435-438.
- Offer Amir MD, OriRogowski MD, Miriam David PhD, NitzaLahat PhD, Rafael Wolff MD et al. (2010) Circulating Interleukin-10: Association with Higher Mortality in Systolic Heart Failure Patients with Elevated Tumor Necrosis Factor-Alpha 12: 158-162.
- Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, et al. (1996) Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD).J Am CollCardiol 27: 1201-1206.
- Gullestad L, Aass H, Fjeld JG, Wikeby L, Andreassen AK, et al. (2001) Immunomodulating therapy with intravenous immunoglobulin in patients with chronic heart failure.Circulation 103: 220-225.
- Müller J, Wallukat G, Dandel M, Bieda H, Brandes K, et al. (2000) Immunoglobulin adsorption in patients with idiopathic dilated cardiomyopathy.Circulation 101: 385-391.
- Tamariz L, Hare JM (2010) Inflammatory cytokines in heart failure: roles in aetiology and utility as biomarkers.Eur Heart J 31: 768-770.
- Armstrong EJ, Morrow DA, Sabatine MS (2006) Inflammatory biomarkers in acute coronary syndromes: part I: introduction and cytokines.Circulation 113: e72-75.
- Bolger AP, Sharma R, von Haehling S, Doehner W, Oliver B, et al. (2002) Effect of interleukin-10 on the production of tumor necrosis factor-alpha by peripheral blood mononuclear cells from patients with chronic heart failure.Am J Cardiol 90: 384-389.
- Kondo M1, Scherer DC, Miyamoto T, King AG, Akashi K, et al. (2000) Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines.Nature 407: 383-386.
- Suzuki H, Kündig TM, Furlonger C, Wakeham A, Timms E, et al. (1995) Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta.Science 268: 1472-1476.
- Hou R, Goloubeva O, Neuberg DS, Strominger JL, Wilson SB (2003) Interleukin-12 and interleukin-2-induced invariant natural killer T-cell cytokine secretion and perforin expression independent of T-cell receptor activation.Immunology 110: 30-37.
- Matsumura T, Tsushima K, Ohtaki E, Misu K, Tohbaru T, et al. (2002) Effects of carvedilol on plasma levels of interleukin-6 and tumor necrosis factor-alpha in nine patients with dilated cardiomyopathy.J Cardiol 39: 253-257.
- Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, et al. (2000) Angiotensin II type 1 receptor antagonist decreases plasma levels of tumor necrosis factor alpha, interleukin-6 and soluble adhesion molecules in patients with chronic heart failure.J Am CollCardiol 35: 714-721.
- Andrew T. Yan, Raymond T. Yan, Mary Cushman, Alban Redheuil et al. (2010) Relationship of interleukin-6 with regional and global left-ventricular function in asymptomatic individuals without clinical cardiovascular disease: insights from the Multi-Ethnic Study of Atherosclerosis, European Heart Journal 31: 875–882.
- Borrayo-Sánchez G, Pacheco-Bouthillier A, Mendoza-Valdez L, Isordia-Salas I et al. Prognostic value of serum levels of interleukin-6 in patients with ST-segment elevation acute myocardial infarction, Cirugia y cirujanos 78: 25-30.
- Chung ES, Packer M, Lo KH, et al. (2003) Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. Epub107: 3133-3140.
Relevant Topics
Recommended Journals
- Journal of Lung Cancer Diagnosis & Treatment
- Advances in Cancer Prevention
- Breast Cancer: Current Research
- Cancer Surgery
- Immunology: Current Research
- Current Trend in Gynecologic Oncology
- Journal of Cancer Diagnosis
- Journal of Gastrointestinal Cancer and Stromal Tumors
- Cervical Cancer: Open Access
- Journal of Mucosal Immunology Research
- Journal of Oncology Research and Treatment
- Journal of Orthopedic Oncology
- Journal of Prostate Cancer
- Research and Reviews on Pathogens
Article Tools
Article Usage
- Total views: 15009
- [From(publication date):
September-2014 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 10492
- PDF downloads : 4517