The Immunomodulation and Myelin Sheath Protection of Bilobalide in Experimental Autoimmune Encephalomyelitis
Received: 08-Dec-2020 / Accepted Date: 22-Dec-2020 / Published Date: 29-Dec-2020 DOI: 10.4172/jceni.1000121
Abstract
Bilobalide (BB), a sesquiterpene isolated from Ginkgo biloba extract, has attracted great interest as a potential therapeutic agent for several neurological diseases. Multiple Sclerosis (MS) is a chronic immune-mediated inflammatory and neurodegenerative disease of the Central Nervous System (CNS), which results in demyelination and axonal degeneration. Experimental Autoimmune Encephalomyelitis (EAE), characterized by infiltration of T cells and macrophages, neuroinflammation and severe demyelination, is the best imitation and extensively used to study MS. The recent paper, “The therapeutic potential of bilobalide on Experimental Autoimmune Encephalomyelitis (EAE) mice”, provides evidence that BB protected myelin sheath by immunomodulatory, anti-inflammation and antiapoptosis. In this review, we discussed the findings of BB treatment on EAE.
Keywords: Bilobalide; Neuroprotection; Immunomodulatory; Anti-inflammation; Anti-apoptosis
Background
Multiple Sclerosis (MS) is a chronic immune-mediated inflammatory demyelinating disease of the Central Nervous System (CNS). T cells appear early in lesion formation, and the disease is considered to be initiated by aberrant responses against CNS autoantigens, the precise pathogenesis, however, remains enigmatic. Autoreactive lymphocytes penetrate the Blood-Brain Barrier (BBB) and infiltrate into CNS to initiate inflammation, followed by gliosis, apoptosis of oligodendrocytes and demyelination [1]. Experimental Autoimmune Encephalomyelitis (EAE) is the most commonly used experimental model for MS, characterized by infiltration of T cells and macrophages, neuroinflammation and severe demyelination, is the best imitation and extensively used to study MS. Along with immune cell infiltration, neuroinflammation can inhibit axonal transport and is closely associated with microglia activation and the presence of macrophage-like cells [2]. Thus, EAE has become a powerful tool for studying disease pathogenesis as well as potential therapeutic interventions by targeting inflammation, demyelination and neurodegeneration.
Therapeutic Potential of BB Treatment Based on CPZ-Induced Demyelination
Cuprizone (CPZ), a selective copper-chelating agent, can selectively chelate the copper ion of the mitochondrial complex IV and trigger energy metabolic disorders of mature oligodendrocytes leading to demyelination, particularly in the Corpus Callosum (CC). Remarkably, CPZ model reflects several histopathologies of demyelination found in human progressive MS. The apoptosis of primary oligodendrocytes and the activation of glial cell are the major histopathological features of the CPZ model [3]. CPZ feeding also induces behavioral alterations, such as motor skills, anxiety and cognition, as observed in MS patients [4]. In recent years, BB has gained great interest as a potential therapeutic agent for several neurological diseases, such as Middle Cerebral Artery Occlusion (MCAO), focal cerebral ischemia, Alzheimer's Disease (AD) and others. In the previous study, we observed that the protective and therapeutic potential of BB in CPZ-induced demyelination [5]. The results showed that CPZ feeding results in extensive demyelination, accompanied by the enrichment of microglia and astrocytes around myelin sheath as well as, damage of Blood-Brain Barrier (BBB) and the infiltration of CD4+ T cells and CD68+ macrophages into the brain, which were effectively inhibited by the administration of BB. Surprisingly, autoantibody against MOG35-55 was detectable in the serum, but markedly inhibited by BB treatment. Subsequently, the data from flow cytometry showed the infiltration of CD4+IFN-g+ and CD4+IL-17+ T cells in the brain, revealing that the demyelination mediated by CPZ feeding can cause the infiltration of Th1 and Th17 T cells. As expected, the level of IFN-γ and IL-17 also elevated in the extract of the brain [6]. These results, on one hand, indicate that BB may protect myelin, and on the other hand, demonstrate that BB may have the potential to regulate peripheral immunity because of a peripheral immune response existing in CPZ-induced demyelination. Therefore, we speculate that BB should be able to treat EAE.
Multiple Biological Effects of BB Treatment Based on Anti- Inflammation, Immunomodulation and Anti-Apoptosis in EAE
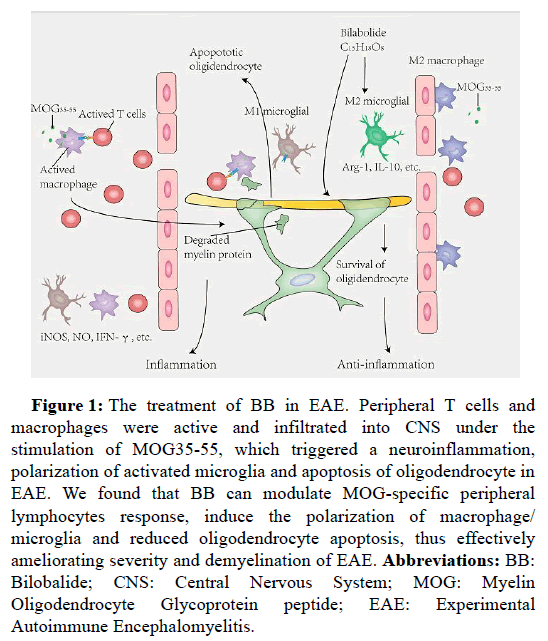
Firstly, our results also indicated that BB treatment ameliorated the severity and delayed the onset of EAE, accompanied by suppressing inflammation, immunomodulation and demyelination (Figure 1), implicating the role of BB in treating EAE [7].
Figure 1: The treatment of BB in EAE. Peripheral T cells and macrophages were active and infiltrated into CNS under the stimulation of MOG35-55, which triggered a neuroinflammation, polarization of activated microglia and apoptosis of oligodendrocyte in EAE. We found that BB can modulate MOG-specific peripheral lymphocytes response, induce the polarization of macrophage/ microglia and reduced oligodendrocyte apoptosis, thus effectively ameliorating severity and demyelination of EAE. Abbreviations: BB: Bilobalide; CNS: Central Nervous System; MOG: Myelin Oligodendrocyte Glycoprotein peptide; EAE: Experimental Autoimmune Encephalomyelitis.
MS is an autoimmune inflammatory disorder caused by the recruitment of self-reactive lymphocytes in the CNS, mainly CD4+ T cells [1,8]. Indeed, the infiltration of T helper (Th) cells and the secretion of the related cytokines and chemokines have been found in CNS lesions and in Cerebrospinal Fluid (CSF) of MS patients, thus contributing to the breakdown of the BBB, the activation of resident microglia and astrocytes, and finally the outcome of neuroinflammation [9]. For EAE, under the selective injection of emulsified MOG peptides, lymphocytes recognize them as antigens to infringing outer, resulting in pathogenic self-reactive T cells migrate to CNS, and then cause a series of pathological damages [2]. Obviously, the effectiveness of BB in the treatment of EAE should depend on whether it can inhibit the activity of peripheral lymphocytes and reduce the infiltration of peripheral lymphocytes into the CNS, so as to reduce the activation and amplification of central neuroinflammation, and finally alleviate demyelination and neurodegeneration. In our study, BB treatment effectively prevented the infiltration of CD4+ T cells and CD68+ macrophages in the spinal cord, accompanied by the down-regulation of inflammatory molecules iNOS, IFN-γ, TNF-α, IL-6 and IL-17, indicating that the therapeutic effect of BB should be related to reduced infiltration and neuroinflammation mediated by peripheral immune cells in EAE. To support our hypothesis, on the 9th day after immunization, splenocytes of mice were stimulated with antigen MOG35-55 in vitro to detect whether BB could inhibit antigen-specific T cell response. Our results showed that BB inhibited CD4+IFN-γ+ T cells, especially decreased the production of inflammatory cytokines. These results explained that BB hindered the invasion of T cells into the spinal cord and seemed to mediate the tolerance of MOG-specific T cells possibly by decreasing the production of inflammatory cytokines, therefore improving the EAE.
Secondly, our study revealed that BB contributed to the polarization of microglia from M1 to M2 phenotype. Microglia are the innate immune cells of the CNS, and considered to exacerbate neuroinflammation in MS and EAE. During the induction phase of MS and EAE, activated microglia modulate their function as Antigen- Presenting Cells (APC) to initiate naive T cells activation toward Th1 and Th17 cells [10,11]. More importantly, the inflammatory cytokines, such as TNF-γ, IL-6 and IL-1β, are mainly derived from the activated microglia, accompanied by the activation of classic M1 phenotype [10,12]. By using mouse EAE model, our data showed that BB promoted the transformation of inflammatory M1 toward the antiinflammatory M2 phenotype, accompanied by the inhibition of inflammatory cytokines and the increase of anti-inflammatory cytokines. Taken together, BB exhibits an anti-inflammatory effect also revealed that BB resulted in a marked downregulation of Toll-like receptor 4 (TLR4), MyD88 and p-NF-κB/p65 in EAE mice, which is related to the suppression of inflammation. Lipopolysaccharide (LPS) can active the intracellular signaling cascade through ligand-receptor binding to TLR4, which induces the inflammatory response and microglia M1 responses [13]. To clarify the effects of BB on the inflammatory response of microglia, we further carried out in vitro experiments with BV2 microglia stimulated by LPS. As expected, LPS stimulated the proliferation and polarization of M1 microglia (CD16/32+ and IL-12+) as well as the inflammatory response of BV2 cells. However, BB treatment inhibited neuroinflammation, induced the polarization of anti-inflammatory M2 microglia (CD206+ and IL-10+) and increased the anti-inflammatory cytokine IL-10. These results support the idea that BB treatment converted the activated microglial cells from M1 to M2 phenotype, contributing to the suppression of inflammation response.
Finally, our data provide evidence that BB could antagonize the apoptosis of oligodendrocytes in EAE (Figure 1). Previous studies have shown that oligodendrocyte loss and tissue damage are involved in the pathological process in MS and EAE, which should be related to neuroinflammation and immune imbalance. Like neurons, oligodendrocytes are highly sensitive to different stimuli of injury including inflammatory response, oxidative stress and infection [14,15]. It has been demonstrated that the apoptosis of oligodendrocytes and the destruction of myelin sheaths are simultaneously derived from reactive T cells and specific autoantibody [16,17]. Meanwhile, repeated immune attacks further attended to the death of the surviving oligodendrocytes. Besides, in recent years, the targeted therapy of oligodendrocytes in MS and EAE has attracted extensive attention. At present, most of the therapies for MS focus on immune regulation and disease-modification, so the disease progression and functional disability can’t be controlled ultimately [18]. The apoptotic oligodendrocytes are observed in new acute MS lesions, with the absence of T cells and activated macrophages [19]. One possible explanation is that the demyelination is not improved by immunomodulation therapy and disease-modification in MS patients. TNF-α can directly induce oligodendrocyte death, thereby impairing OPC differentiation because of the presence of the TNF receptor in oligodendrocyte lineage [20]. IL-17A inhibits the maturation of oligodendrocyte lineage cells (OPCs) and exacerbates the TNF-α- induced oligodendrocyte apoptosis [21]. However, the causes of oligodendrocyte apoptosis remain unclear. Our study described that BB contributed to the re-balance of pro-and anti-apoptotic proteins including the down-regulation of apoptosis proteins (i.e., Cleave- Caspase-3 and Bax) and up-regulation of the Bcl-2 protein. More importantly, the administration of BB resulted in a fall of apoptotic cleaved Caspase-3-positive oligodendrocytes, explaining that BB protects oligodendrocytes from apoptosis. Besides, BB did not enhance the expression of NG2, a marker of oligodendrocyte precursor cells. Moreover, anti-apoptosis of BB was linked with the decline of inflammatory cytokines. These results supported the antiapoptotic effect of BB may be correlated with the decreased TNF-α, IFN-γ and IL-17.
Conclusion
In summary, our study provided evidence that BB can effectively alleviate clinical symptoms and reduce myelin loss in EAE, which should be related to anti-inflammation, immunoregulation and antiapoptosis. However, the precise cellular and molecular mechanism of BB for promoting myelin regeneration remain to be further explored and confirmed, so, BB can be used for targeting myelin therapy in clinical MS patients in the future.
Acknowledgments
Qiang Miao is supervised by Prof. Cun-gen Ma and financially supported by a grant from the National Natural Science Foundation of China (81473577).
Author ’s Contribution
Qiang Miao and Qing Wang conceived and wrote the manuscript. Cun-Gen Ma and Bao-Guo Xiao conceived and discussed the commentary.
Compliance with Ethical Standards
Conflict of interest none of the authors has any potential financial and non-financial conflicts of interest related to this manuscript.
References
- Dargahi N, Katsara M, Tselios T, Androutsou ME, de Courten M, et al. (2017) Multiple sclerosis: Immunopathology and treatment update. Brain Sci. 7(7):78.
- Lassmann H, Bradl M (2017)Â Multiple sclerosis: experimental models and reality. Acta Neuropathol. 133(2):223-244.
- Fresegna D, Bullitta S, Musella A, Rizzo FR, De Vito F, et al. (2020) Re-Examining the Role of TNF in MS Pathogenesis and Therapy. Cells. 9(10):2290.
- Sen MK, Mahns DA, Coorssen JR, Shortland PJ (2019)Â Behavioural phenotypes in the cuprizone model of central nervous system demyelination. Neurosci Biobehav Rev. 107:23-46.
- Sui RX, Miao Q, Wang J, Wang Q, Song LJ, et al. (2019) Protective and therapeutic role of Bilobalide in cuprizone-induced demyelination. Int Immunopharmacol.; 66:69-81.
- An J, Yin JJ, He Y, Sui RX, Miao Q, et al. (2020)Â Temporal and spatial dynamics of astroglial reaction and immune response in cuprizone-induced demyelination. Neurotox Res. 37(3):587-601.
- Miao Q, Zhang XX, Han QX, Ren SS, Sui RX, et al. (2020) The therapeutic potential of bilobalide on experimental autoimmune encephalomyelitis (EAE) mice. Metab Brain Dis. 35(5):793-807.
- Dendrou CA, Fugger L, Friese MA (2015) Immunopathology of multiple sclerosis. Nat Rev Immunol. 15(9):545-58.
- Kunkl M, Frascolla S, Amormino C, Volpe E, Tuosto L (2020)Â T Helper cells: The modulators of inflammation in multiple sclerosis. Cells. 9(2):482.
- Jiang Z, Jiang JX, Zhang GX. (2014)Â Macrophages: A double-edged sword in experimental autoimmune encephalomyelitis. Immunol Lett. 160(1):17-22.
- Gao Z, Tsirka SE (2011) Animal models of MS reveal multiple roles of microglia in disease pathogenesis. Neurol Res Int. 383087.
- Nally FK, De Santi C, McCoy CE (2019)Â Nanomodulation of macrophages in multiple sclerosis. Cells. 8(6):543.
- Zheng C, Chen J, Chu F, Zhu J, Jin T (2020)Â Inflammatory role of TLR-MyD88 signaling in multiple sclerosis. Front Mol Neurosci. 12:314.
- Lucchinetti CF, Popescu BF, Bunyan RF, Moll NM, Roemer SF, et al. (2011) Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 365(23):2188-97.
- Jana A, Pahan K (2007)Â Oxidative stress kills human primary oligodendrocytes via neutral sphingomyelinase: implications for multiple sclerosis. J Neuroimmune Pharmacol. 2(2):184-93.
- Lopes Pinheiro MA, Kooij G, Mizee MR, Kamermans A, Enzmann G, et al. (2016) Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochim Biophys Acta. 1862(3):461-71.
- Cannella B, Gaupp S, Omari KM, Raine CS. (2007) Multiple sclerosis: Death receptor expression and oligodendrocyte apoptosis in established lesions. J Neuroimmunol. 188(1-2):128-37.
- Piehl F. Current and emerging disease modulatory therapies and treatment targets for multiple sclerosis (2020) J Intern Med. 1.
- Cudrici C, Niculescu T, Niculescu F, Shin ML, Rus H (2006) Oligodendrocyte cell death in pathogenesis of multiple sclerosis: Protection of oligodendrocytes from apoptosis by complement. J Rehabil Res Dev. 43(1):123-32.
- Taoufik E, Tseveleki V, Euagelidou M, Emmanouil M, Voulgari-Kokota A, et al. (2008) Positive and negative implications of tumor necrosis factor neutralization for the pathogenesis of multiple sclerosis. Neurodegener Dis. 5(1):32-7.
- Chen J, Liu X, Zhong Y (2020) Interleukin-17A: The key cytokine in neurodegenerative diseases. Front Aging Neurosci. 12:566922.
Citation: Miao Q, Wang Q, Xiao BG, Ma CG (2021) The Immunomodulation and Myelin Sheath Protection of Bilobalide in Experimental Autoimmune Encephalomyelitis. J Clin Exp Neuroimmunol 6: 121 DOI: 10.4172/jceni.1000121
Copyright: © 2020 Miao Q, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2016
- [From(publication date): 0-2021 - Dec 19, 2024]
- Breakdown by view type
- HTML page views: 1407
- PDF downloads: 609