Review Article Open Access
The Great Significance of Research into the Physiological Relev ance between AQP4 and Vasopressin for Studying Alzheimers Disease
Yu-Long Lan1,2, Tonghui Ma2, Jie Zhao2,3, Shao Li2*
1Department of Neurosurgery, the First Affiliated Hospital of Dalian Medical University, Dalian, 116011, China
2Department of Physiology, Dalian Medical University, Dalian, 116044, China
3Liaoning Engineering Technology Centre of Target-based Nature Products for Prevention and Treatment of Ageing-related Neurodegeneration, Dalian, 116044, China
- Corresponding Author:
- Shao Li
Department of Physiology
Dalian Medical University
Dalian 116044, China
Tel: +86-411-8611-0352
E-mail: lishao89@hotmail.com
Received date: August 22, 2015; Accepted date: September 14, 2015; Published date: September 21, 2015
Citation: Lan YL, Ma T, Zhao J, Li S (2015) The Great Significance of Research into the Physiological Relevance between AQP4 and Vasopressin for Studying Alzheimer’s Disease. J Alzheimers Dis Parkinsonism 5:192.doi:10.4172/2161-0460.1000192
Copyright: © 2015 Lan YL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Aquaporin 4 (AQP4), which is the predominant aquaporins (AQPs) isoform in the adult brain, is specifically localized to astrocytes. Current findings regarding AQP4 and various neurological diseases have initiated our interest in unraveling the mystery of AQP4 function in Alzheimer’s disease (AD), which is a progressive neurodegenerative disorder characterized by the loss of memory and cognitive disturbances; however, far less is known regarding the precise mechanisms. Vasopressin plays an important role in the regulation of central nervous functions, including learning and memory. Research into vasopressin might contribute to clarification of the neuroprotective effect of AQP4 against AD. Currently the research regarding the functional interaction of AQP4 and vasopressin has demonstrated to be of great significance for studying AD. Here we review the interaction of AQP4 and vasopressin in astrocyte that might have a pivotal role in the regulation of distinct cellular responses directed to neuropretection against AD, as experimental results strongly emphasize the importance of this topic for future investigations.
Keywords
Alzheimer’s disease; AQP4; Vasopressin; Astrocyte
Abbreviations
AD: Alzheimer’s Disease; AQPs: Aquaporins; NMO: Neuromyelitis Optica; MS: Multiple Sclerosis; LTP: Long- Term Potentiation; LTD: Long-Term Depression; LRP1: Lipoprotein Receptor-Related Protein-1; OGD: Oxygen-Glucose Deprivation
Introduction
Since Alzheimer’s disease (AD) prevalence is age-related and the aging population is progressively growing up, a dramatic increase of the disease is expected in the coming decades [1]. Research has indicated that 115.4 million people may be living with dementia by 2050. The pathogenesis of AD is a complex process involving both genetic and environmental factors [2]. Despite these complexities, extensive research has laid the foundation of current understanding of the etiology and pathogenesis of AD [3,4], and many hypotheses have been put forward for AD pathogenesis, including cholinergic hypothesis, tau hypothesis and amyloid cascade hypothesis [2]. Current therapies may ease symptoms by providing temporary improvement and reducing the rate of cognitive decline. More significant research efforts should be directed toward clarifying the etiology and pathogenesis of AD as well as more adequate therapies against AD. Research into the development of drugs aimed at the treatment of AD via various targets has great potential for success [5]. Aquaporins (AQPs) are water-channel proteins on the plasma membrane that play critical roles in the control of cellular water content. Aquaporin 4 (AQP4) is the predominant AQP isoform in the adult brain, which is previously demonstrated to be associated with demyelination and neuroinflammation in chronic and acute brain diseases [6-12]. The possible link between neuroinflammation and AQP4 was first suggested in Neuromyelitis Optica (NMO) [13]. And it has been suggested that the pathogenesis of many clinical diseases, such as NMO, Multiple Sclerosis (MS) and brain injuries, is related to the regulation of AQP4 expression [14]. Current evidence has indicated that brain AQP4 is involved in various astrocytic functions related to neurological diseases, including brain fluid and ion homeostasis [15-17], potassium uptake and release by astrocytes [18], astrocyte migration and glial scarring [19,20], neural signal transduction [21], pro-inflammatory factor secretion [22], astrocyte-to-astrocyte cell communication [23] and synaptic plasticity [24]. There is growing evidence that glia play a role In Long-Term Potentiation (LTP) [25-29], which could subsequently be influenced by AQP4, for that AQP4, is specifically localized to astrocytes. Recent studies have examined LTP, Long-Term Depression (LTD), and the behavior in AQP4 knockout and wild-type mice to gain additional insights into its potential roles. Thus AQP4 could be the promising target for AD treatments [30]; however, far less is known regarding the precise molecular mechanisms. It’s known that the water balance and neurohormone release in the neurohypophysis are processes that are closely interconnected. Vasopressin is a nonapeptide and neurotransmitter or neuromodulator; it plays an important role in the regulation of central nervous functions, including learning and memory. Numerous studies have shown that vasopressin and its analogs can improve learning and memory-related performance in experimental animals [31]. Both AQP4 and vasopressin could play roles in preventing the impairment of cognitive function in AD patients. Recently various studies have shown functional interaction of AQP4 and vasopressin in astrocyte that might have a pivotal role in the regulation of distinct cellular responses directed to neuronal preservation and neuropretection against AD, thus a deeper research into the functional interaction of AQP4 and vasopressin could have promising significance for clearing AD pathogenesis and exploring potential target for AD treatments. Furthermore, AQP4 could be considered a molecular target for Aβ metabolism and clearance in AD [30]; more efforts directed toward clarifying their physiological relevance may help clear the promising neuroprotective effect of AQP4 and vasopressin against AD, and clarify the closely interconnected processes of water balance and neurohormone release in anti-AD neuroprotective mechanisms.
AQP4 Is associated with alzheimer’s disease
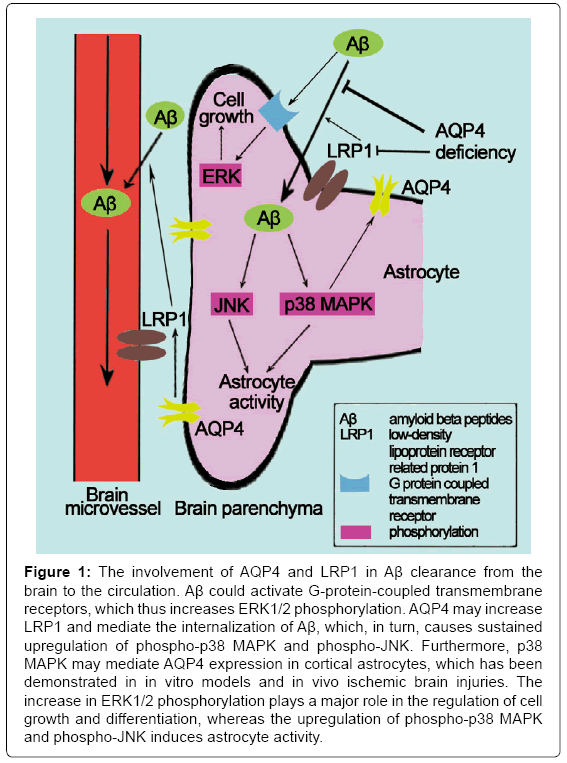
LTP is a persistent strengthening of synapses based on recent patterns of activity. Impaired LTP have a role in AD. Skucas et al. [24] have investigated hippocampal synaptic plasticity and spatial memory function in mice with a deletion of the astrocyte-specific channel AQP4, and it was the first to demonstrate that LTP changed in AQP4 knockout mice using electrophysiology. The study of Scharfman et al. [32] was the first study to demonstrate the direct effect of AQP4 on specific forms of activity-dependent plasticity. Thereafter, studies that have investigated the effect of AQP4 on AD have gradually increased. AQP4 may mediate the clearance of amyloid beta peptides (Aβ) and exert neuroprotection against AD [33]. Low-density Lipoprotein Receptor-Related Protein-1 (LRP1) is expressed in the perivascular end feet of astrocytes and brain microvascular endothelial cells; it mediates a continuous efflux of brain Aβ into the circulation [34]. MAPKs are a family of serine/threonine kinases that comprise 3 major subgroups: ERK, JNK and p38 MAPK. The ERK pathway plays a major role in the regulation of cell growth and differentiation, whereas the JNK and p38 MAPK cascades are most frequently associated with astrocyte activity [35]. Aβ has been demonstrated to activate G-protein-coupled transmembrane receptors, which induces a transient increase in the phosphorylation of ERK1/2 [36]. Moreover, the internalization of Aβ results in mitochondrial dysfunction, which induces the generation of reactive oxygen species and, in turn, causes a sustained upregulation of phospho-p38 MAPK and phospho- JNK [37,38]. Intriguingly, several studies have demonstrated that the expression of AQP4 is regulated by MAPKs in response to changes in osmolality [39-41]. Hyperosmotic stress has also been reported to increase AQP4 through a p38 MAPK-dependent pathway in cultured rat astrocytes [40]. Nito et al. [42] examined the role of MAPK pathways in AQP4 regulation in rat primary astrocytes using Oxygen-Glucose Deprivation (OGD) injury and the immunoreactivity of p38 MAPK and AQP4 in brain edema formation; the authors further hypothesized that MAPK pathways, particularly p38 MAPK, mediate AQP4 expression in cortical astrocytes after in vitro and in vivo ischemic brain injuries. Yang et al. [33] demonstrated that AQP4 deficiency decreases LRP1 upregulation and Aβ uptake, which thus attenuates changes in MAPK signaling pathways and ultimately reduces astrocyte activity. Therefore, AQP4 may be significantly important in the upregulation of LRP1 and the clearance of Aβ (Figure 1). Thus, AQP4 is a molecular target for AD, and it is significant to explore the novel roles of AQP4 in the pathogenesis of neurological disorders.
Figure 1: The involvement of AQP4 and LRP1 in Aβ clearance from the brain to the circulation. Aβ could activate G-protein-coupled transmembrane receptors, which thus increases ERK1/2 phosphorylation. AQP4 may increase LRP1 and mediate the internalization of Aβ, which, in turn, causes sustained upregulation of phospho-p38 MAPK and phospho-JNK. Furthermore, p38 MAPK may mediate AQP4 expression in cortical astrocytes, which has been demonstrated in in vitro models and in vivo ischemic brain injuries. The increase in ERK1/2 phosphorylation plays a major role in the regulation of cell growth and differentiation, whereas the upregulation of phospho-p38 MAPK and phospho-JNK induces astrocyte activity.
In addition, AQP4 may influence potassium (K+) and calcium (Ca2+) ion transport which plays decisive roles in the pathogenesis of AD [30,43]. AQP4 deficiency may impair learning and memory, in part, through glutamate transporter-1 (GLT-1) [44-46]. Furthermore, AQP4 knockout is involved in neuroinflammation and interferes with AD [47-50]. Ample evidence has indicated that the regulation of astrocyte functions via AQP4 may offer a new therapeutic option for AD [51].
Relationship of vasopressin and alzheimer’s disease
Aβ is crucially involved in AD as the main component of the amyloid plaques found in the brains of Alzheimer patients. It is interesting that vasopressin and its receptors are present in the same brain regions as Aβ deposits. There are several studies that have demonstrated vasopressin and its analogues, in contrast to Aβ, may function as memory-facilitating peptides and can improve learning and memory-related performance in experimental animals [31]. For example, in the 1990s, [Arg8]-vasopressin (AVP) administered systemically or centrally was demonstrated to facilitate the consolidation and retrieval processes of active [52], working and reference memory in the radial maze [53]. With respect to the electrophysiological mechanism of AVP in the improvement of memory function, one of the most important research techniques focuses on central synaptic plasticity, such as hippocampal LTP. Although inconclusive, many experiments support a facilitatory action of AVP on LTP [54,55]. Jing et al. [56] indicated for the first time that AVP, as a memory-facilitating peptide, could effectively protect against Aβ- induced impairment of LTP via the upregulation of synaptic plasticity in the hippocampal CA1 region. The authors suggested that pretreatment with various concentrations of AVP dose-dependently prevented the Aβ- induced suppression of LTP and enhanced high frequency stimulation (HFS)-induced LTP in the hippocampal CA1 region instead of affecting baseline synaptic transmission. These results are supported by the study of Pan et al. [57] who demonstrated that centrally administered AVP protects against Aβ-induced memory decline in the Morris water maze test. Intriguingly, a recent study conducted by Varga et al. [58] identified increased levels of AD-related markers; memory deficits were only observable in vasopressin-deficient animals. Furthermore, the tissue samples were obtained from the parietal cortex, in which dysfunction is an important characteristic of early AD [59]. Thus, the study by Varga is a canonical paper in support of the beneficial effect of central AVP in the prevention and treatment of AD.
Vasopressin might be one factor in the explanation of the neuroprotective mechanisms of AQP4 against alzheimer’s disease
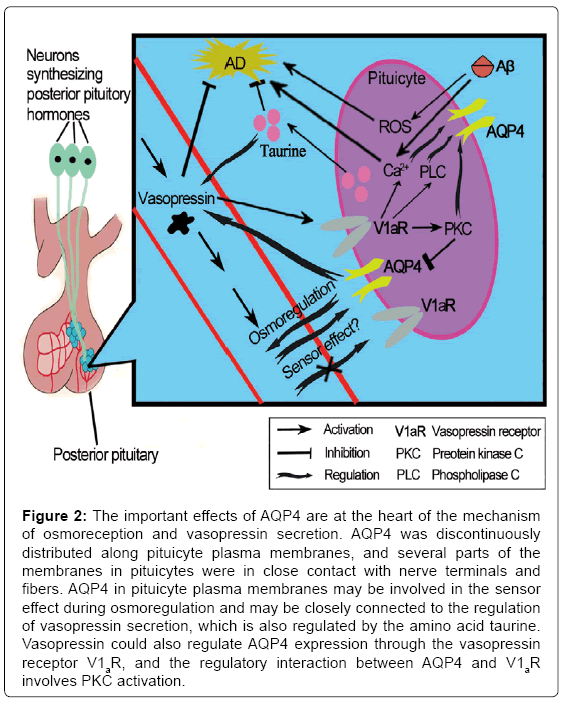
Although the main function of AVP is antidiuresis in the kidney, it plays a key role in stress-related psychiatric diseases, such as depression [60], which is a marked symptom in preclinical AD as previously discussed. A study conducted by Mesbah-Benmessaoud et al. [61] was the first study to describe the distribution of AQP4 throughout the neural lobe of the adult mouse hypophysis, and they demonstrated that AQP4 is abundant in the mouse hypophysis, mainly in the neural lobe, which was recently described in the rat pituitary gland [62,63]. AQP4 was discontinuously distributed along plasma membranes of pituicyte, which is one kind of astrocyte. Some parts of the pituicyte membranes were in close contact with nerve terminals and fibers. After salt loading, the staining was more intense. This finding implicates AQP4 water channels in neurohypophyseal neuroglial interactions that affect water homeostasis during pathologies, such as brain edema, as well as physiological conditions [61]. Furthermore, pituicytes appear to be key elements in the osmoregulation process [64]; these cells are sensitive to osmolar changes and have been recently described as osmotic sensors [65]. In addition, they could modulate neurohormone output [65] that may be locally controlled by the amino acid taurine, which is produced by pituicytes [66]. Taurine is a naturally occurring β-amino acid in the brain, which has been demonstrated to have neuroprotective properties. Pretreatment with taurine significantly attenuated Aβ-induced neuronal death [67]; similarly, taurine reversed mitochondrial function in the presence of Aβ. Moreover, taurine attenuated the intracellular Ca2+ and ROS generation induced by Aβ. And the effective maintenance of intracellular Ca2+ homeostasis and ROS generation during exposure to neurotoxic insults are considered to be mechanistic components of neuroprotection against AD [68,69]. As a ubiquitous osmolyte involved in the regulation of cell volume, taurine is also a regulator of vasopressin release in the hypothalamo-neurohypophyseal system [70]. Thus, water balance and neurohormone release in the neurohypophysis are processes that are closely interconnected. Vasopressin exerts its effects via a family of G protein-coupled receptors. The most prominently expressed are the V1a and the V2 type (V1aR and V2R) [71]. The V1aR is found in a number of tissues including brain; it has been detected in neurons, glial cells, and endothelial cells of the blood-brain barrier [72], while The V2R has a more restricted distribution, and is predominantly expressed in the kidney [73]. Interestingly, the most recent published data demonstrated that V1aR and V2R respond directly to vasopressin exposure, but they do not have an ability to act as osmo- or volume sensors when exposed to an osmotic gradient in the absence or presence of vasopressin [74], although regrettably this study was exerted in Xenopus oocytes or in mammalian cells. Therefore, AQP4 in pituicyte plasma membranes may be involved in this sensor effect during osmoregulation instead [61] and may also be closely connected to the regulation of vasopressin secretion. This research indicates that the movement of water regulated by AQP4 may lie at the heart of the mechanism of osmoreception and AVP secretion (Figure 2). However, because the absence or presence of AVP did not influence the levels of AD-related markers, the clearance of Aβ or other aggregated agents of AD might not be a factor in the explanation of the neuroprotective mechanisms of AQP4 via AVP.
Figure 2: The important effects of AQP4 are at the heart of the mechanism of osmoreception and vasopressin secretion. AQP4 was discontinuously distributed along pituicyte plasma membranes, and several parts of the membranes in pituicytes were in close contact with nerve terminals and fibers. AQP4 in pituicyte plasma membranes may be involved in the sensor effect during osmoregulation and may be closely connected to the regulation of vasopressin secretion, which is also regulated by the amino acid taurine. Vasopressin could also regulate AQP4 expression through the vasopressin receptor V1aR, and the regulatory interaction between AQP4 and V1aR involves PKC activation.
The neuroprotective effects of vasopressin against alzheimer’s disease could be partly through regulating AQP4 expression via binding to v1ar
The effects of AVP regulation on AQP4 have also been investigated by Moeller et al. [75]. First, they demonstrated the co-expression of AQP4 and the vasopressin receptor V1aR. Furthermore, they demonstrated that the regulatory interaction between AQP4 and V1aR involves protein kinase C (PKC) activation. A PKC-dependent reduction of the water permeability of AQP4 has previously been identified in oocytes [76] and mammalian cells [77]; however, the mechanism must be elucidated. Furthermore, Ser180 (numbering from the M1 isoform) is a strong PKC consensus site in AQP4 [78] and is involved in the PKC-dependent down regulation of AQP4, as previously reported [77], via direct phosphorylation or an unknown regulatory protein. The binding of AVP to V1aR leads to the increased generation of inositol trisphosphate (IP3), the activation of phospholipase C (PLC) and the release of Ca2+ and PKC [78,79], which could play important roles in the regulation of AQP4 expression. Furthermore, V1aR antagonism led to the upregulation of AQP4 and attenuated water content, injury, and cerebral edema [80]. Therefore, it can be hypothesized that the down regulation of AVP may upregulate AQP4 via the upregulation of PKC activation. Importantly, the future manipulation of AQP4 expression through AVP receptor antagonism may serve as an important therapeutic target for neurotoxicity and ischemia-evoked cytotoxic cerebral edema. It has been previously demonstrated that the V1aRmediated down regulation of AQP4 membrane expression levels and proposed that this down regulation might be beneficial during periods of dehydration in an attempt to limit the loss of water from the brain [75]. Vasopressin-dependent short-term down regulation of AQP4 may play a role in normal and pathophysiological conditions that induce AD or other neurological disorders. Future studies on mammalian cells and whole brains will identify the extent of this functional interaction and its physiological relevance.
Conclusion and Perspectives
Recent findings have evidenced the presence the interaction of AQP4 with vasopressin involved in neuronal maintenance and neuropretection against AD. There is a general agreement that AQP4 at the plasma membrane, can induce preservation against Aβ toxicity and, furthermore, this water channel has been shown to influence vasopressin function. And the neuroprotective effects of AQP4 regulation against AD could also be regulated by vasopressin. Besides pituicyte, more efforts should be directed toward clarifying this phenomenon in other kinds of astocytes in certain cognitive-related functional brain regions. It is clinically important to further explore the pivotal role of the physiological relevance between AQP4 and vasopressin in the regulation of distinct cellular responses directed to neuropretection against AD, and further the precise mechanisms of the neuroprotection effect of AQP4 and vasopressin against AD.
Conflict Of Interest
The authors declare no competing financial interests.
Acknowledgement
The work was supported by the National Natural Science Foundation of China (81371223 and 30871006), the Research Fund for the Doctoral Program of Higher Education of China (20122105110010) and the Science and Technology Research Funds of Ministry of Education of Liaoning Province (L2014343).
References
- Puzzo D, Gulisano W, Arancio O, Palmeri A (2015) The keystone of Alzheimer pathogenesis might be sought in Aß physiology. Neuroscience 307: 26-36.
- Barage SH, Sonawane KD (2015) Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer's disease. Neuropeptides 52: 1-18.
- Tanzi RE (1999) Caspases land on APP: One small step for apoptosis, one giant leap for amyloidosis? Nat Neurosci 2: 585-586.
- Tanzi RE, Bertram L (2001) New frontiers in Alzheimer's disease genetics. Neuron 32: 181-184.
- Lan YL, Zhao J, Li S (2015) Update on the neuroprotective effect of estrogen receptor alpha against alzheimer’s disease. J Alzheimers Dis 43: 1137-1148.
- Tourdias T, Mori N, Dragonu I, Cassagno N, Boiziau C, et al. (2011) Differential aquaporin 4 expression during edema build-up and resolution phases of brain inflammation. J Neuroinflammation 8: 143.
- Verkman AS (2012) Aquaporins in clinical medicine. Annu Rev Med 63: 303-316.
- Badaut J, Copin JC, Fukuda AM, Gasche Y, Schaller K, et al. (2012) Increase of arginase activity in old apolipoprotein-E deficient mice under Western diet associated with changes in neurovascular unit. J Neuroinflammation 9: 132.
- Chi Y, Fan Y, He L, Liu W, Wen X, et al. (2011) Novel role of aquaporin-4 in CD4? CD25? T regulatory cell development and severity of Parkinson’s disease. Aging Cell 10: 368-382.
- Hinson SR, Romero MF, Popescu BF, Lucchinetti CF, Fryer JP, et al.(2012) Molecular outcomes of neuromyelitisoptica (NMO)-IgG binding to aquaporin-4 in astrocytes. ProcNatlAcadSci USA 109: 1245-1250.
- Kinoshita M, Nakatsuji Y, Kimura T, Moriya M, Takata K, et al. (2010) Anti-aquaporin-4 antibody induces astrocytic cytotoxicity in the absence of CNS antigen-specific T cells. BiochemBiophys Res Commun 4: 205-210.
- Pham H, Doerrbecker J, Ramp AA, D’Souza CS, Gorasia DG, et al. (2011) Experimental autoimmune encephalomyelitis (EAE) IN C57Bl/6 mice is not associated with astrogliosis. J Neuroimmunol 2: 51-62.
- Nishibe M, Barbay S, Guggenmos D, Nudo RJ (2010) Reorganization of motor cortex after controlled cortical impact in rats and implications for functional recovery. J Neurotrauma 27: 2221-2232.
- Lan YL, Fang DY, Zhao J, Ma TH, Li S (2015) A research update on the potential roles of aquaporin 4 in neuroinflammation. ActaNeurolBelg 2015 Aug 11. [Epub ahead of print].
- Thrane AS, Rappold PM, Fujita T, Torres A, Bekar LK, et al. (2011) Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. ProcNatlAcadSci U S A 108: 846-851.
- Kong H, Fan Y, Xie J, Ding J, Sha L, et al. (2008) AQP4 knockout impairs proliferation, migration and neuronal differentiation of adult neural stem cells. J Cell Sci 121: 4029-4036.
- Li X, Gao J, Ding J, Hu G, Xiao M (2013) Aquaporin-4 expression contributes to decreases in brain water content during mouse postnatal development. Brain Res Bull 94: 49-55.
- MacAulay N, Zeuthen T (2010) Water transport between CNS compartments: contributions of aquaporins and cotransporters. Neuroscience 168: 941-956.
- Auguste KI, Jin S, Uchida K, Yan D, Manley GT, et al. (2007) Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J 21: 108-116.
- Papadopoulos MC, Saadoun S, Verkman AS (2008) Aquaporins and cell migration. Pflugers Arch 456: 693-700
- Fan Y, Zhang J, Sun XL, Gao L, Zeng XN, et al. (2005) Sex- and region-specific alterations of basal amino acid and monoamine metabolism in the brain of aquaporin-4 knockout mice. J Neurosci Res 82: 458-464.
- Li L, Zhang H, Varrin-Doyer M, Zamvil SS, Verkman AS (2011) Pro-inflammatory role of aquaporin-4 in autoimmune neuroinflammation. FASEB J 25: 1556-1566
- Benfenati V, Ferroni S (2010) Water transport between CNS compartments: Functional and molecular interactions between aquaporins and ion channels. Neuroscience 168: 926-940.
- Skucas VA, Mathews IB, Yang J, Cheng Q, Treister A, et al. (2011)Impairment of select forms of spatial memory and neurotrophin-dependent synaptic plasticity by deletion of glial aquaporin-4. J Neurosci 31: 6392-6397.
- Filosa A, Paixao S, Honsek SD, Carmona MA, et al. (2009) Neuron-glia communication via EphA4/ephrin-A3 modulates LTP through glial glutamate transport. Nature neuroscience 12: 1285-1292.
- Haydon PG, Blendy J, Moss SJ, Rob Jackson F (2009) Astrocytic control of synaptic transmission and plasticity: A target for drugs of abuse? Neuropharmacology 56 Suppl 1: 83-90.
- Bains JS, Oliet SH (2007) Glia: they make your memories stick! Trends in neurosciences 30: 417-424.
- Perea G, Navarrete M, Araque A (2009) Tripartite synapses: Astrocytes process and control synaptic information. Trends in neurosciences 32: 421-431.
- Stevens B (2008) Neuron-astrocyte signaling in the development and plasticity of neural circuits. Neuro-Signals 16: 278-288.
- Xiao M, Hu G (2014) Involvement of Aquaporin 4 in Astrocyte Function and Neuropsychiatric Disorders. CNS NeurosciTher 20: 385-90.
- Pan YF, Chen XR, Wu MN, Ma CG, Qi JS (2010) Arginine vasopressin prevents against Aß25–35-induced impairment of spatial learning and memory in rats. Hormones and Behavior 57: 448-454.
- Scharfman HE, Binder DK (2013) Aquaporin-4 water channels and synaptic plasticity in the hippocampus. Neurochemistry international 63: 702-711.
- Yang W, Wu Q, Yuan C, Gao J, Xiao M, et al. (2012) Aquaporin-4 mediates astrocyte response to beta-amyloid. Molecular and cellular neurosciences 49: 406-414.
- Koistinaho M, Lin S, Wu X, Esterman M, Koger D, et al. (2004) Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med 10:719-726.
- Origlia N, Arancio O, Domenici L, Yan SS. MAPK (2009) beta-amyloid and synaptic dysfunction: The role of RAGE. Expert review of neurotherapeutics 9: 1635-1645
- Matos M, Augusto E, Oliveira CR, Agostinho P (2008) Amyloid-beta peptide decreases glutamate uptake in cultured astrocytes: Involvement of oxidative stress and mitogen-activated protein kinase cascades. Neuroscience 156: 898-910.
- Bartov O, Sultana R, Butterfield DA, Atlas D (2006) Low molecular weight thiol amides attenuate MAPK activity and protect primary neurons from Abeta(1-42) toxicity. Brain research 1069: 198-206.
- Jin Y, Yan EZ, Fan Y, Zong ZH, Qi ZM, et al.(2005) Sodium ferulate prevents amyloid-beta-induced neurotoxicity through suppression of p38 MAPK and upregulation of ERK-1/2 and Akt/protein kinase B in rat hippocampus. ActapharmacologicaSinica 26: 943-951.
- Rao KV, Jayakumar AR, Reddy PV, Tong X, Curtis KM, et al. (2010) Aquaporin-4 in manganese-treated cultured astrocytes. Glia 58: 1490-1499.
- Arima H, Yamamoto N, Sobue K, Umenishi F, Tada T, et al. (2003) Hyperosmolar mannitol simulates expression of aquaporins 4 and 9 through a p38 mitogen-activated protein kinase-dependent pathway in rat astrocytes. The Journal of biological chemistry 278: 44525-44534.
- St Hillaire C, Vargas D, Pardo CA, Gincel D, Mann J, et al. (2005) Aquaporin 4 is increased in association with human immunodeficiency virus dementia: implications for disease pathogenesis. Journal of neurovirology 11: 535-543.
- Nito C, Kamada H, Endo H, Narasimhan P, Lee YS, et al. (2012) Involvement of mitogen-activated protein kinase pathways in expression of the water channel protein aquaporin-4 after ischemia in rat cortical astrocytes. Journal of neurotrauma 29: 2404-2412.
- Xiao M, Hu G (2014) Involvement of aquaporin 4 in astrocyte function and neuropsychiatric disorders. CNS Neuroscience Therapy 20: 85-90.
- Zeng XN, Sun XL, Gao L, Fan Y, Ding JH, et al. (2007) Aquaporin-4 deficiency down-regulates glutamate uptake and GLT-1 expression in astrocytes. Mol Cell Neurosci 34:34-39.
- Li YK, Wang F, Wang W, Luo Y, Wu PF, et al. (2012) Aquaporin-4 deficiency impairs synaptic plasticity and associative fear memory in the lateral amygdala: Involvement of downregulation of glutamate transporter-1 expression. Neuropsychopharmacology 37:1867-1878.
- Yang J, Li MX, Luo Y, Chen T, Liu J, et al. (2013) Chronic ceftriaxone treatment rescues hippocampal memory deficit in AQP4 knockout mice via activation of GLT-1. Neuropharmacology 75:213-222.
- Li L, Zhang H, Varrin-Doyer M, Zamvil SS, Verkman AS (2011) Pro-inflammatory role of aquaporin-4 in autoimmune neuroinflammation. FASEB J 25:1556-1566.
- Tomás-Camardiel M, Venero JL, de Pablos RM, Rite I, Machado A, et al. (2011) In vivo expression of aquaporin-4 by reactive microglia. J Neurochem 91:891-899.
- Tourdias T, Mori N, Dragonu I, Cassagno N, Boiziau C, et al. (2011) Differential aquaporin 4 expression during edema build-up and resolution phases of brain inflammation. J Neuroinflammation 8:143.
- Fukuda AM, Badaut J (2012) Aquaporin 4: A player in cerebral edema and neuroinflammation. J Neuroinflammation 9:279.
- Verkman AS (2009) Aquaporins: Translating bench research to human disease. The Journal of experimental biology 212: 1707-1715.
- Metzger D, Alescio-Lautier B, Bosler O, Devigne C, Soumireu-Mourat B (1993) Effect of changes in the intrahippocampal vasopressin on memory retrieval and relearning. Behavioral and neural biology 59: 29-48.
- Dietrich A, Allen JD (1997) Vasopressin and memory. I. The vasopressin analogue AVP4-9 enhances working memory as well as reference memory in the radial arm maze. Behavioural brain research 87: 195-200.
- Chepkova AN, French P, De Wied D, Ontskul AH, Ramakers GM, et al. (1995) Long-lasting enhancement of synaptic excitability of CA1/subiculum neurons of the rat ventral hippocampus by vasopressin and vasopressin(4-8). Brain research 701: 255-266.
- Dubrovsky B, Tatarinov A, Gijsbers K, Harris J, Tsiodras A (2003) Effects of arginine-vasopressin (AVP) on long-term potentiation in intact anesthetized rats. Brain research bulletin 59: 467-472.
- Jing W, Guo F, Cheng L, Zhang JF, Qi JS (2009) Arginine vasopressin prevents amyloid beta protein-induced impairment of long-term potentiation in rat hippocampus in vivo. Neuroscience letters 450: 306-310.
- Pan YF, Jia XT, Wang XH, Chen XR, Li QS, et al. (2013) Arginine vasopressin remolds the spontaneous discharges disturbed by amyloid beta protein in hippocampal CA1 region of rats. Regulatory peptides 183C: 7-12.
- Varga J, Klausz B, Domokos A, Kalman S, Pakaski M, et al. (2014) Increase in Alzheimer's related markers preceeds memory disturbances: studies in vasopressin-deficient Brattleboro rat. Brain research bulletin 100: 6-13.
- Leuba G, Vernay A, Zimmermann V, Saini K, Kraftsik R, et al. (2009) Differential damage in the frontal cortex with aging, sporadic and familial Alzheimer's disease. Brain research bulletin 80: 196-202.
- Landgraf R, Neumann ID (2004) Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in neuroendocrinology 25: 150-176.
- Mesbah-Benmessaoud O, Benabdesselam R, Hardin-Pouzet H, Dorbani-Mamine L, Grange-Messent V (2011) Cellular and subcellular aquaporin-4 distribution in the mouse neurohypophysis and the effects of osmotic stimulation. The journal of histochemistry and cytochemistry: Official journal of the Histochemistry Society 59: 88-97.
- Kuwahara S, Maeda S, Ardiles Y, Jun JG, Tanaka K, et al. (2010) Immunohistochemical localization of aquaporin-4 in the rat pituitary gland. The Journal of veterinary medical science / the Japanese Society of Veterinary Science 72: 1307-1312.
- Pocsai K, Bagyura Z, Kalman M (2010) Components of the basal lamina and dystrophin-dystroglycan complex in the neurointermediate lobe of rat pituitary gland: different localizations of beta-dystroglycan, dystrobrevins, alpha1-syntrophin, and aquaporin-4. The journal of histochemistry and cytochemistry: Official journal of the Histochemistry Society 58: 463-479.
- Hussy N (2002) Glial cells in the hypothalamo-neurohypophysial system: Key elements of the regulation of neuronal electrical and secretory activity. Progress in brain research 139: 95-112.
- Rosso L, Mienville JM (2009) Pituicyte modulation of neurohormone output. Glia 57: 235-243.
- Hussy N, Bres V, Rochette M, Duvoid A, Alonso G, et al.(2001) Osmoregulation of vasopressin secretion via activation of neurohypophysial nerve terminals glycine receptors by glial taurine. The Journal of neuroscience: the official journal of the Society for Neuroscience 21: 7110-7116.
- Sun Q, Hu H, Wang W, Jin H, Feng G, et al. (2014) Taurine attenuates amyloid ß 1-42-induced mitochondrial dysfunction by activating of SIRT1 in SK-N-SH cells. Biochemical and biophysical research communications 447: 485-489.
- Nilsen J, Brinton RD (2004) Mitochondria as therapeutic targets of estrogen action in the central nervous system. Curr Drug Targets CNS NeurolDisord 3: 297-313.
- Rodrigues R, Petersen RB, Perry G (2014) Parallels between Major Depressive Disorder and Alzheimer ’s disease: Role of Oxidative Stress and Genetic Vulnerability. Cell MolNeurobiol 34: 925-949.
- Rosso L, Peteri-Brunback B, Poujeol P, Hussy N, Mienville JM (2004) Vasopressin-induced taurine efflux from rat pituicytes: A potential negative feedback for hormone secretion. The Journal of physiology 554: 731-742.
- Lolait SJ, O’Carroll AM, Brownstein MJ (1995) Molecular biology of vasopressin receptors. Ann NY AcadSci 771: 273-292.
- Szmydynger-Chodobska J, Chung I, Kozniewska E, Tran B, Harrington FJ, et al. (2004) Increased expression of vasopressin v1a receptors after traumatic brain injury. J Neurotrauma 21: 1090-1102.
- Lolait SJ, O’Carroll AM, McBride OW, Konig M, Morel A, et al. (1992) Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature 357: 336-339.
- Lykke K, Assentoft M, Fenton RA, Rosenkilde MM, MacAulay N (2015) Vasopressin receptors V1a and V2 are not osmosensors. Physiol Rep 3: e12519
- Moeller HB, Fenton RA, Zeuthen T, Macaulay N (2009) Vasopressin-dependent short-term regulation of aquaporin 4 expressed in Xenopus oocytes. Neuroscience 164: 1674-1684.
- Han Z, Wax MB, Patil RV (1998) Regulation of aquaporin-4 water channels by phorbol ester-dependent protein phosphorylation. The Journal of biological chemistry 273: 6001-6004.
- Zelenina M, Zelenin S, Bondar AA, Brismar H, Aperia A (2002) Water permeability of aquaporin-4 is decreased by protein kinase C and dopamine. American journal of physiology. Renal physiology 283: F309-318.
- Gunnarson E, Zelenina M, Aperia A (2004) Regulation of brain aquaporins. Neuroscience 129: 947-955.
- Zhao L, Brinton RD (2003) Vasopressin-induced cytoplasmic and nuclear calcium signaling in embryonic cortical astrocytes: Dynamics of calcium and calciumdependent kinase translocation. J Neurosci 23: 4228-4239.
- Liu X, Nakayama S, Amiry-Moghaddam M, Ottersen OP, Bhardwaj A (2010) Arginine-vasopressin V1 but not V2 receptor antagonism modulates infarct volume, brain water content, and aquaporin-4 expression following experimental stroke. Neurocritical care 12: 124-131.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 19518
- [From(publication date):
September-2015 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 14838
- PDF downloads : 4680