The Epidemiology of Infectious Disease and Networks
Received: 01-May-2023 / Manuscript No. ECR-23-95395 / PreQC No. ECR-23-95395 / Reviewed: 04-May-2023 / QC No. ECR-23-95395 / Revised: 22-May-2023 / Manuscript No. ECR-23-95395 / Published Date: 29-May-2023
Abstract
Research into the dynamics of interacting elements has been completely transformed by the science of networks. One may argue that more than any other field, epidemiology has embraced the possibilities of network theory. Here, we discuss the growing body of research on how infectious diseases spread on networks with an emphasis on how network theory and epidemiology interact. The review is divided into four main sections that look at different types of networks that are relevant to epidemiology, various ways to characterise these networks, statistical methods. It is impossible to do a thorough analysis of all the work because of the variety of topics covered and the growing quantity of publications. Instead, we offer a tailored review of the network epidemiology subfields that have advanced the most recently or have the most potential to offer new insights. As a result, analytical techniques and statistical methods—two fast developing fields—are given a lot of weight. In this review, epidemiological concerns are the only ones we focus on.
Keywords
Epidemiology; Infectious disease; Molecular epidemiology; Viruses
Introduction
Research into the dynamics of interacting elements has been transformed by network science. Numerous domains, including computer science, neurology, social science, and statistical physics, have benefited greatly from the associated methodologies. It may be claimed, nevertheless, that epidemiology has embraced network theory's promise the most out of all disciplines. Since the middle of the 1980s, there has been a very close connection between network theory and epidemiology. This is due to the fact that an infectious disease's ability to spread through links between people (or groups of people) automatically defines a network, and the network that is created sheds light on the epidemiological dynamics [1].
Understanding the transmission network's structure in particular enables us to simulate the entire dynamics of the system and enhance forecasts of the infection's likely distribution and early growth (after invasion). The relationship between networks and epidemiology, however, goes further; because the network identifies potential pathways for disease transmission, understanding its organisation can help with disease prevention. By treating or containing their contacts, for instance [2], contact tracing seeks to discover possible transmission network linkages from known infected individuals, so halting the spread of infection. Contact tracing is a very efficient public health measure because it targets control efforts based on the underlying dynamics of transmission rather than on an in-depth knowledge of the aetiology of the virus. Therefore, it is obvious that studying networks and how they relate to the spread of infectious diseases is an essential tool for comprehending disease progression and, consequently, guiding disease control [3].
In this article, we cover the expanding corpus of literature on the transmission of infectious illnesses on networks with a particular emphasis on how network theory and epidemiology interact. The paper is divided into four main sections that look at the different types of networks that are relevant to epidemiology, the various ways that these networks can be described, the statistical methods that can be used to infer either the epidemiological parameters on a realised network or the likely network structure, and finally simulation and analytical methods to determine epidemic dynamics on a given network. It is impossible to do a thorough analysis of all the work because of the range of topics covered and the growing number of publications (nearly 7,000 articles have been published about infectious illnesses and networks). Instead, we offer a tailored review of the network epidemiology subfields that have advanced the most recently or have the most potential to offer new insights. As a result, analytical methodologies and statistical methods— two fast developing fields—are given significant weight. We observe that a variety of other network-based processes (such as the spread of ideas or panic) can be modelled similarly to how an infection spreads; however, in these contexts, the transmission process is much less clear, so we focus only on epidemiological issues in this paper [4].
While traditional data sources remain important for guiding outbreak interventions, the landscape of infectious disease epidemiology is being revolutionized by high-throughput and near-real-time pathogen genome sequencing. This advancement in genomic technologies is enhancing our ability to understand and control infectious diseases with greater precision, both at the individual and population levels. This integrated approach, termed 'precision epidemiology', leverages the power of genomic data to inform the development of more effective intervention strategies [5].
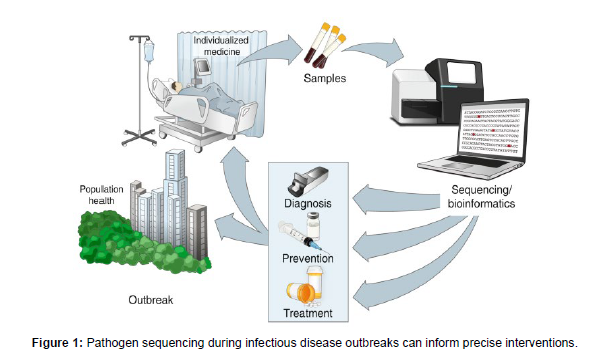
In this context, we will provide a brief overview of how genomic technologies are driving precision epidemiology and facilitating the design of targeted interventions for both individual patients and affected populations as a whole. By harnessing the scale and resolution of genomic sequencing, researchers can gain valuable insights into the genetic makeup of pathogens, their transmission dynamics, and the host-pathogen interactions that influence disease outcomes [6] (Figure 1).
At the individual level, precision epidemiology utilizes genomic information to tailor interventions to the specific characteristics and susceptibilities of patients. By analyzing the genetic sequence of pathogens, healthcare providers can identify specific virulence factors, drug resistance markers, or genetic variants associated with disease severity. This knowledge enables the selection of appropriate treatment regimens and the implementation of personalized prevention strategies, ultimately improving patient outcomes [7].
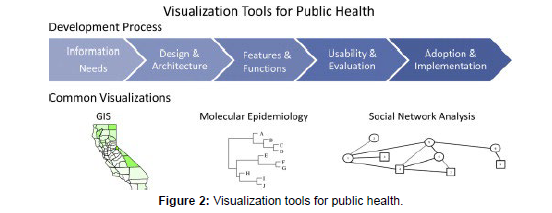
On a broader scale, precision epidemiology also contributes to the development of targeted interventions for affected populations. By examining the genomic data of pathogens circulating within a community, researchers can identify transmission clusters, track the spread of specific strains, and determine the origins of outbreaks. This information is invaluable for implementing timely and effective public health interventions, such as targeted vaccination campaigns, enhanced surveillance measures, and targeted infection control strategies [8] (Figure 2).
Materials and Methods
Identify relevant data sources to gather information on the epidemiology of infectious diseases and networks. These may include national surveillance systems, disease registries, public health databases, research studies, and published literature. Additionally, data from international organizations such as the World Health Organization (WHO) or the Centers for Disease Control and Prevention (CDC) can provide valuable insights into global infectious disease trends. Determine the appropriate study design based on the research objectives. Depending on the specific research question, observational studies (e.g., cohort studies, case-control studies), ecological studies, or modelling approaches may be employed to investigate the epidemiology of infectious diseases and networks [9].
Define the study population based on the research question and available data. This may include individuals at risk of infection, patients with specific infectious diseases, or populations affected by outbreaks or epidemics. Consider the target population and the availability of relevant data from the identified data sources. Identify the variables of interest for the study, such as demographic characteristics, clinical data, network connections, or environmental factors. Determine the appropriate data collection methods, which may involve reviewing medical records, conducting surveys, utilizing laboratory test results, or analyzing existing datasets. Consider the ethical implications of data collection and ensure compliance with relevant privacy regulations [10].
If investigating the role of networks in infectious disease epidemiology, network analysis methods can be applied. This may involve constructing contact networks, social networks, or genetic networks to understand the patterns of disease transmission and identify key nodes or clusters within the network. Network metrics such as centrality, connectivity, or clustering coefficients can be calculated to assess network characteristics. Analyze the collected data using appropriate statistical methods [11].
Descriptive statistics can be used to summarize demographic characteristics, disease prevalence, or network properties. Analytical methods, such as regression models, survival analysis, or network modelling techniques, can be applied to explore associations between variables, assess risk factors, or predict disease transmission dynamics. Ensure compliance with ethical guidelines and obtain necessary approvals from relevant ethical review boards. Safeguard participant privacy and confidentiality, and obtain informed consent when required. Adhere to data protection regulations and anonymized data when reporting results [12].
Discussion
Clearly, a fast expanding area in epidemiology is the utilisation of networks. Researchers are better able to comprehend the observed distribution of infection and develop more accurate predictive models of future prevalence by evaluating (and quantifying) the probable transmission pathways between individuals in a community. We have demonstrated the quantification of a number of structural elements in widely utilised contact networks and the growing knowledge of how these features affect infection spread. Many difficulties still exist, nevertheless [13].
If networks are to continue to have an impact on predictive epidemiology, there are still a number of outstanding issues. The majority of problems are caused by how challenging it is to find accurate transmission networks for a variety of infections. Although considerable research has been done to clarify the interrelated structure of sexual encounters (and, consequently, the network of sexual transmission), it is still rather small-scale in comparison to the population size and is subject to a variety of potential biases. The less precise description of a potential encounter makes it far more difficult to identify comparable networks for airborne illnesses [14].
Therefore, whether new methods can be created that enable contact networks to be evaluated remotely is a practical concern. One option would be proximity loggers, like those employed by Hamede and colleagues, but this would require technology to become sufficiently reliable, transportable, and affordable that a very substantial fraction of the population could be persuaded to carry one at all times. There is the possibility to use mobile phones to gather network information for many human populations when their use is sufficiently broad. However, it is important to recognise the difficulties in creating suitably general software. There would still be some doubt about the nature of each communication even if these remotely sensed networks would offer unmatched information that could be retrieved with the least amount of effort [15].
There are already an increasing number of diary-based studies that have sought to track the personal interactions of many people; POLYMOD is currently the most thorough of these. It is unclear how such egocentric data should be put together, despite the fact that it certainly provides considerable information on individual conduct due to the anonymity of such surveys. An alternative to the setup approach of randomly connecting half-links is needed, however, and would ideally incorporate and specify clustering, spatially localised connections, and assortativity between degree distributions[16].
In order to define such networks in a way that offers valuable insights on the kinds of epidemiological dynamics that could be realized, there is a need to establish realistic contact networks for entire populations. Such a characterization would enable for the epidemiologically important comparison of diverse networks (from various times or locations), as well as the creation of artificial networks that matched specific known network properties. This obviously depends on both current network structure metrics (as described in Section 3) and a solid comprehension of how such properties affect transitory epidemic dynamics (as outlined in Section 4.2). It won't likely be possible to have such a broad understanding of all network aspects for many years. Understanding how local network structure (clustering, cliques, and spatially localised linkages) affects epidemic dynamics is a more pressing task [17].
The vast bulk of research to date on the spread of diseases through networks has been on static networks, where all linkages are of similar strength and hence have the same fundamental rate of transmission. However, it is evident that contact networks evolve with time (both on a short-term scale as in who we encounter each day and on a longer-term one as in who our primary job and social connections are), and that ties have varying weights (such that some contacts are much more likely to lead to the transmission of infection than others). Although it is possible to simulate an infection on such weighted time-varying networks, it is not obvious how the literature on analytical methodologies or the sets of network attributes now in use can be expanded to such higherdimensional networks [18-23].
It is critical to have efficient data collection procedures in place as well as the statistical tools necessary to assess the data for any approach to be of real use in the field. Here, three topics are possibly the most important ones. First, there is generally always a shortage of resources for data collection. Therefore, rather than relying on data augmentation approaches to address issues with ad hoc datasets, well-constructed randomised sample schemata should be used to maximise the power of statistical techniques used to examine data [24]. When working with network data produced from population samples, this component is especially crucial. Second, a thorough investigation of model fit should be used to support any inference made using both network and infectious disease models. Epidemic model diagnostics are now in their infancy compared to techniques in other areas of statistics, despite recent developments in statistical epidemiology giving us an unmatched ability to quantify population/disease dynamics based on publicly accessible field data. As a result, it is anticipated that considerable research effort will be needed in developing such approach as network models for evaluating disease spread become more and more common [25].
Conclusions
We have emphasised the critical role that contact network analysis plays in epidemiology and the amount of resources it offers for comprehending and forecasting the spread of a variety of illnesses. As we have stated above, there are still many obstacles to overcome, but with rising interest in this extremely interdisciplinary topic and everimproving mathematical, statistical, and remote-sensing techniques, these issues may soon be resolved. Therefore, we draw the conclusion that the current state of network epidemiology research is promising since many practical obstacles have been overcome and theoretical ideas have been transformed into practical outcomes that are crucial for infection prevention and public health.
Acknowledgment
None
Conflict of Interest
None
References
- Pastor-Satorras R, Vespignani A (2001) Epidemic spreading in scale-free networks. Phys Rev Lett 86:3200-3203.
- Sharkey KJ (2008) Deterministic epidemiological models at the individual level. J Math Biol 57:311-331.
- Brinton LA (2015) Prediagnostic sex steroid hormones in relation to male breast cancer risk. J Clin Oncol 33:18.
- Thomas DB, Jimenez LM, McTiernan A (1992) Breast cancer in men: risk factors with hormonal implications. Am J Epidemiol 135:734-748.
- Mavraki E, Gray IC, Bishop DT, Spurr NK (1997) Germline BRCA2 mutations in men with breast cancer. Br J Cancer 76:1428-1431.
- Haraldsson K, Loman N, Zhang QX, Johannsson O, Olsson H, et al. (1998) BRCA2 germ-line mutations are frequent in male breast cancer patients without a family history of the disease. Cancer Res 58:1367-1371.
- Csokay B, Udvarhelyi N, Sulyok Z (1999) High frequency of germ-line BRCA2 mutations among Hungarian male breast cancer patients without family history. Cancer Res 59:995-998.
- Pages S, Caux V, Stoppa-Lyonnet D, Tosi M (2001) Screening of male breast cancer and of breast-ovarian cancer families for BRCA2 mutations using large bifluorescent amplicons. Br J Cancer 84:482-488.
- Jedy-Agba E, Curado MP, Ogunbiyi O (2012) Cancer incidence in Nigeria: a report from population-based cancer registries. Cancer Epidemiol 36:271-278.
- Tamimi AF, Tamimi I, Abdelaziz M (2015) Epidemiology of malignant and non-malignant primary brain tumors in Jordan. Neuroepidemiology 45:100-108.
- Chen Z, Xu L, Shi W (2020) Trends of female and male breast cancer incidence at the global, regional, and national levels. Breast Cancer Res Treat 180:481-490.
- Agrawal A, Ayantunde AA, Rampaul R, Robertson JFR (2007) Male breast cancer: a review of clinical management. Breast Cancer Res Treat 103:11-21.
- Rosenblatt KA, Thomas DB, McTiernan A (1991) Breast cancer in men: aspects of familial aggregation. J Natl Cancer Inst 83:849-854.
- Boyd J, Rhei E, Federici MG (1999) Male breast cancer in the hereditary nonpolyposis colorectal cancer syndrome. Breast Cancer Res Treat 53:87-91.
- Hultborn R, Hanson C, Kopf I, Verbiene I, Warnhammar E, et al. (1997) Prevalence of Klinefelter’s syndrome in male breast cancer patients. Anticancer Res 17:4293-4297.
- Beygi S, Saadat S, Jazayeri SB, Rahimi-Movaghar V (2013) Epidemiology of pediatric primary malignant central nervous system tumors in Iran. Cancer Epidemiol 37:396-401.
- Fisher JL, Schwartzbaum JA, Wrensch M, Wiemels JL (2007) Epidemiology of brain tumors. Neurol Clin 25:867-890.
- Mantovani A, Allavena P, Sica A (2004) Tumour-associated macrophages as a prototypic type II polarised phagocyte population: role in tumour progression. Eur J Cancer 40:1660-1667.
- Yu MC, Mack TM, Hanisch R, Cicioni C, Henderson BE, et al. (1986) Cigarette smoking, obesity, diuretic use, and coffee consumption as risk factors for renal cell carcinoma. J Natl Cancer Inst 77:351-356.
- Novick AC (2004) Laparoscopic and partial nephrectomy. Clin Cancer Res 10:6322-6327.
- Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK (2006) Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst 98:1331-1334.
- Miller DC, Saigal CS, Banerjee M, Hanley J, Litwin MS, et al. (2008) Diffusion of surgical innovation among patients with kidney cancer. Cancer 112:1708-1717.
- Diez Roux AV, Merkin SS, Arnett D (2001) Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 345:99-106.
- Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47:1245-1251.
- Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613-619.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Citation: John J (2023) The Epidemiology of Infectious Disease and Networks.Epidemiol Sci, 13: 497.
Copyright: © 2023 John J. This is an open-access article distributed under theterms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2175
- [From(publication date): 0-2023 - Nov 19, 2025]
- Breakdown by view type
- HTML page views: 1844
- PDF downloads: 331