Research Article Open Access
The Emerging Questions from the Current Epidemiology of Visceral Leishmaniasis in South Sudan: A Cross-sectional Study Design
Kofi Boateng1, Abdulmunini Usman1*, Jane Pita1, Ajak CD Akik2, Allan Mpairwe1, Dieu-Donne Bimpa1, Evans Lyosi1, Sylvester Maleghemi1, Mabourok M Leron2, Rumunu John2, and Richard LL Loro21World Health Organization Country Office, South Sudan
2Ministry of Health, Ministry Complex, Juba, Republic of South Sudan
- *Corresponding Author:
- Abdulmunini Usman
World Health Organization Country Office
Juba, South Sudan
Tel: +211 953 333 842
E-mail: usmanaeri@yahoo.com
Received date: November 23, 2016; Accepted date: January 03, 2017; Published date: January 14, 2017
Citation: Boateng K, Usman A, Pita J, Akik ACD, Mpairwe A, et al. (2017) The Emerging Questions from the Current Epidemiology of Visceral Leishmaniasis in South Sudan: A Cross-sectional Study Design. J Emerg Infect Dis 2:121. doi:10.4172/2472-4998.1000121
Copyright: © 2017 Boateng K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Disease and Pathology
Abstract
Leishmaniasis is endemic in South Sudan with at least two outbreaks reported since 1984. A major impediment to effective control is the paucity of epidemiological data to inform prevention and control efforts. The intent of this study is to describe the epidemiology of the disease to inform prevention and control interventions.
An analytical cross-sectional design was applied to the National Leishmaniasis Surveillance database from 2009 to 2013 to determine trends and influences on risk of death. Leishmaniasis cases were defined according to national disease surveillance protocols. Point estimates at 95% C.I. and regression estimates of odds and R2 of risks outcomes were determined.
The prevalence (cases per 10,000) of visceral leishmaniasis (VL) in the endemic areas varied from 0.14 in 2009 to 5.13 in 2011 and 0.2 in 2013. During this period, 2,466 cases were reported of which 89.5% (95% C.I. 88.2-90.6) had primary visceral leishmaniasis while 7.1% (95% C.I 6.2-8.2) were relapse cases. Following treatment, 90.7% (95% C.I. 89.4-91.8) of cases were cured, with a case fatality of 3.7% (95% C.I. 3.0-4.5). Age (R2=0.02, p <0.001), duration of illness (R2=0.01, p<0.001), and treatment regimen (R2=0.039, p<0.001) significantly explained the risk of death even though marginal. Primary Visceral leishmaniasis were 2.5 times (OR=2.5 [95% C.I. 1.4, 4.6]; p<0.002) more likely to die as compared to other categories. There are epidemic foci in North Eastern counties of Ayod, Fangak, Baliet and Canal Khorfulus. Further studies, both ecological and behavioral are required to understand the risk of exposure to VL.
Keywords
Leishmaniasis; Endemic; Prevention and Control; Risk; Outcomes; South Sudan; World Health Organization
Introduction
Among the factors constituting impediments to MDG’s poverty alleviation are the neglected tropical diseases which elimination and eradication remain largely distant [1]. The Sustainable Development Goals (SDGs) extend beyond the Millennium Development Goals (MDGs) targeting the end of epidemics of neglected tropical diseases (NTDs) by 2030 while ensuring equity [2]. Leishmaniasis, one of the world’s neglected tropical diseases, threatens an estimated 350 million people of all ages causing high mortality and disability among the poorest of persons in 88 countries in South America, South East Asia and Africa [2,3]. The disease is caused by 20 pathogenic protozoa belonging to the genus leishmania and transmitted through a bite by an infected female phlebotomine sandfly [3]. Control efforts have been challenged by the multiplicity and complex nature of factors associated with the disease [4]. Reservoirs for the causative organism have been identified in several sources including humans, dogs, and cattle; risk factors are also varied [3,5]. The World Health Assembly’s approved Resolution 60.13 in 2007 has brought in global efforts for the control of leishmaniasis. Following this, major scientific interventions including improved diagnosis, treatment, and prevention of the diseases as well as reduction in prices of key medicines have contributed to the control agenda [3]. Also, challenges of resources mobilization in low-income settings have called for the application of integrated approaches [6]. Yet, there remain limited epidemiological information in understanding the contextual factors contributing to low impact of control measures on the burden of leishmaniasis.
South Sudan is endemic to visceral leishmaniasis. An epidemic of VL that occurred from 1984 to 1994 in the then Southern Sudan (Western Upper Nile) highlighted that the epidemic was characterized by high mortality and high infection rates associated with population movement and malnutrition as major factors for the exaggerated risk of infection [7]. The study also predicted that the disease is likely to become endemic in the area.
In 2009 an outbreak of VL was reported in Jonglei and was considered a humanitarian emergency leading to an outbreak response by different partners through a coordinated effort between the Ministry of Health (MoH), United Nations agencies, and Non-Governmental Organizations (NGOs). VL reporting relies on the national surveillance system, and control uses the standard operating procedures but reporting occurs mainly in four states of South Sudan: Upper Nile, Jonglei, Unity and Eastern Equatoria. Control measures include diagnostics screening, nutritional supplements and food rationing of cases, and distribution of Long Lasting Impregnated Nets (LLINs) for vector control.
Methods
We used an analytical cross-sectional design to assess the primary outcomes of the management of leishmaniasis cases in South Sudan from 2009-2013. We examined the burden of the disease in respect of demographic and geographic characteristics as well as clinical assessment regarding diagnosis, type of treatment and treatment outcomes. The extent of reduction of risk of death due to specific demographic and clinical management outcomes was also determined to explore variables that may be of significance in designing public health interventions for the purposes of leishmaniasis control. We used secondary data sources from an annual compilation of trends for the disease from passive surveillance systems, while the electronic line-listed data from treatment sites were used for analyzing primary outcomes of interest. For the purpose of our study, we adopted the country protocols for the definition of cases.
Relevant protocols for reporting sites
Reporting sites: The reporting sites vary from year to year based on a number of factors ranging from the security situation, inaccessibility and the seasonality of the disease. There were 18 reporting sites in 2009, 13 in 2010, 24 in 2011, 25 in 2012, 23 in 2013, 21 in 2014, 25 in 2015 and 12 in 2016. The reporting sites are mainly from the two foci (southern focus - composed of former Jonglei, upper Nile, and Unity states) and the northern focus in eastern Equatoria State (Greater Kapoeta). Due to non-availability of line list data from 2014 to date, we relied on the dataset reported from 2009-2013.
Case management
Cases definition: Suspect VL: History of prolonged fever (more than 2 weeks) AND splenomegaly or wasting (weight loss). Confirmed VL Any person, serologically positive with (Rapid diagnostic Test (rK39): or Direct Agglutination test (DAT)) or positive parasitological test (Lymph node aspirate/ spleen/Bone marrow) is a visceral leishmaniasis case.
Post KA dermal leishmaniasis (PKDL): This is a rash that starts on the face occurring in many forms (Macules and papules) and sometimes spread to the whole body within 6 months of having VL.
Relapse: A patient with confirmed VL treated for at least 17 to 30 days with an appropriate amount of antimonial either as single or combined and discharged with a negative parasitological test and then returns within 6 months with a positive parasitological test.
Clinical diagnosis: A patient was defined as a clinical suspect of VL if she/he presents with a history of prolonged fever (2 weeks or more) associated with clinical splenomegaly or wasting (weight loss) after excluding malaria through the rapid test and/or treatment. For a patient who is severely ill, both tests (Malaria and VL) are done at the same time in order not to delay the VL treatment.
Laboratory diagnosis: Primary VL was confirmed using Rapid diagnostic test (rK39) using Inbios® and DiaMed-IT LEISH® tests that detect specific antibodies against the kinesin-related antigen that is present in Leishmania donovani or by direct agglutination test (DAT). This procedure uses freeze-dried antigen (FDA) (most preferred) and the positive cut-off titer is 1:3200 (well 7). If the DAT is borderline (1: 400, 1:800 and 1:1600), the test is repeated within one month or alternatively a parasitological test (lymph node/bone marrow/spleen aspiration) is performed in the absence of contraindication. Since DAT and the parasitological test are conducted by few health facilities, we relied on clinical diagnosis and rK39 test as a confirmation of cases of VL in the dataset.
Data collection and analysis: Reporting sites compiled weekly summaries from the laboratory and admission registers onto a standard form that is submitted to higher administrative levels and finally stored in the VL database at the national level at the MoH and WHO. Aggregate data per month existed over the years but lacked further details. However, treatment sites submitted monthly line list secured by the MoH/WHO. We, therefore, extracted collated line list for the period 2009-2013 and validated it with individual treatment sites lines list submitted via email over the same period. Case records without one or more of the variables of interest were deleted. In all out of the total of 2472 cases, 2466 were analyzed.
The cleaned data in Ms Excel format were converted into MDB and dta formats for further analysis in Epi Info 7 and Statistical Package for Social Sciences (SPSS) version 20.0 software respectively. We conducted univariate analysis of key demographic, clinical, diagnostic and treatment outcomes by representing point estimates using confidence interval (C.I) of 95%. Prevalence estimates were also computed using census-based population estimates from 2009-2013. Cumulative curves for age were presented in the graph in addition to spot map to demonstrate case distribution per year. An unconditional regression analysis was conduct to assess the extent of reduced risk of death to kala-azar influenced by age, sex, diagnosis, and treatment regimen. Apart from age, that was linearly regressed, logistics regression was used for all other variables. Thus, independent variables were binary coded as “0” and “1” and analyzed separately with all others categories as referenced. We computed for Odds, Pseudo R square, 95% C.I and p-values (Table 1).
| Year | No. of Cases | National | Endemic Area (Jonglei, Upper Nile, and Kapoeta) | Relative Risk | ||
|---|---|---|---|---|---|---|
| Popn. | Prevalence (per 10,000popn) | Popn. | Prevalence (per 10000popn) | |||
| 2009 | 32 | 8990000 | 0.04 | 2247500 | 0.14 | 3.5 |
| 2010 | 660 | 9268690 | 0.71 | 2317173 | 2.85 | 4 |
| 2011 | 1225 | 9556019 | 1.28 | 2389005 | 5.13 | 4 |
| 2012 | 557 | 9852256 | 0.57 | 2463064 | 2.26 | 3.9 |
| 2013 | 52 | 10157676 | 0.05 | 2539419 | 0.2 | 4 |
Table 1: Prevalence of Kala-azar at National and Endemic areas, South Sudan 2009-2013.
Limitations
Due to the inconsistency in the dataset, we could not assess the Body Mass Index and its relationship with clinical and treatment outcomes. In addition, we were unable to provide ecological or extensive behavioral factors such as exposure to bites of sandflies, dogs, and cattle, by the clients that could inform extents of exposure to the diseases, and responses to clinical interventions [3-5].
Results
The national prevalence of VL was 0.04/10,000 population in 2009 but peaked to 1.28/10,000 population in 2011 and declined to 0.05/10,000 population in 2013. The same trend was observed in endemic areas of Jonglei, Upper Nile states and Kapoeta areas in Eastern Equatoria state. In the endemic areas, the prevalence was 0.14, 5.13 and 0.20 for 2009, 2011 and 2013 respectively. The relative risk of the diseases to persons living in endemic areas ranged from 3.5 to 4.0.
Of the total of 2,466 cases of VL, 89.5% (95% C.I. 88.2-90.6) were diagnosed as Primary VL, while 7.1% (95% C.I 6.2-8.2) were a relapse of which 95.4% (169/177) were primary and the rest secondary. In addition, multiple Tuberculosis and HIV co-infections were observed among 0.5% of the cases.
The cases were treated with Sodium Stibogluconate in 53.2% (95% C.I. 51.1-55.2); Sodium Stibogluconate and Paromomycin in 37.4% (95% C.I. 35.4-39.3); and Ambisome (liposomal amphotericin B) in 7.5% (95% C.I. 6.5-8.3) as detailed in Table 2 below.
| Variable | % (N=2466) | 95% C.I. |
|---|---|---|
| Duration of Sickness | ||
| - 1–7days | 64.8 | 62.8, 66.7 |
| - 8–14days | 2.6 | 2.0, 3.3 |
| - 15–21 days | 27.7 | 25.9, 29.6 |
| - 21 days and above | 4.8 | 3.9, 5.8 |
| Diagnosis/Patient Classification | ||
| - Primary Kala- azar | 89.5 | 88.1, 90.3 |
| - Primary Kala- azar + HIV | 0.3 | 0.1, 0.6 |
| - Primary Kala- azar + TB | 0.2 | 0.1, 0.5 |
| - Post Kala- azar dermal | 2.8 | 2.2, 3.5 |
| - Post Kala- azar dermal + Relapse | 0.1 | 0.0, 0.3 |
| - Relapse | 7.1 | 6.2, 8.2 |
| Treatment regimen | ||
| - Ambisome* | 7.5 | 6.5, 8.3 |
| - Ambisome + Meltefosine | 0.2 | 0.1, 0.5 |
| - Meltifosine | 0.8 | 0.4, 1.5 |
| - Glucantime | 0.5 | 0.3, 0.9 |
| - PM**+SSG+Ambisome | 0.1 | 0.0, 0.3 |
| - Sodium Stibogluconate (SSG) | 53.2 | 51.1, 55.2 |
| - SSG + Ambisome | 0.3 | 0.2, 0.6 |
| - SSG + Paramomycin | 37.4 | 35.4, 39.3 |
| Treatment Outcomes | ||
| - Cured | 90.7 | 89.4, 91.8 |
| - Defaulted | 4.4 | 3.6, 5.3 |
| - Died | 3.7 | 3.0, 4.5 |
| - Transferred | 1.1 | 0.7, 1.6 |
| *Liposomal amphotericin B **PM=Paromomycine |
||
Table 2: Proportional distribution of patients according to the duration of sickness, patient classification, treatment regimen, and disease outcome.
A higher percentage (64.8%; 95% C.I. 62.8-66.7) of the patients reported to the treatment centers within 1-7 days. The median duration of sickness was 2 days with a range of 7-19 days and an interquartile range of 28 days.
Treatment outcomes analysis showed that 90.7% (95%.C.I. 89.4- 91.8) of cases were cured, while case fatality of 3.7% (95% C.I. 3.0-4.5) was recorded. Defaulters represented 4.4% (95% C.I. 3.6-5.3).
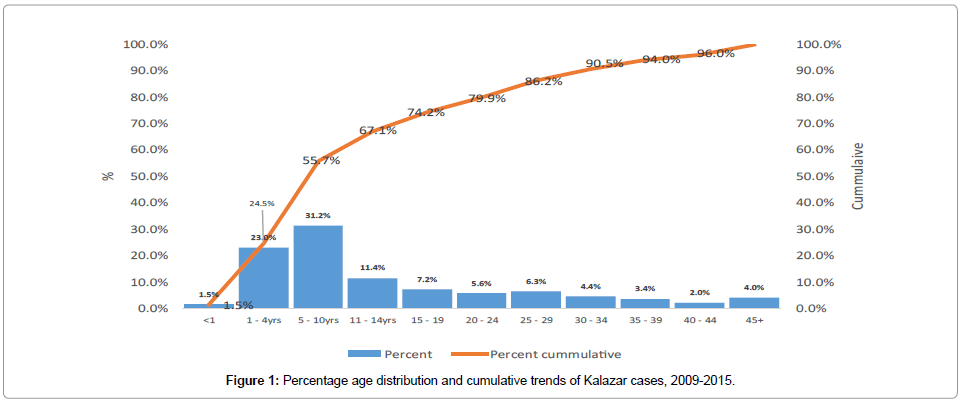
The median age of the VL patients was 9 years with an interquartile range of 16 years. The ages ranged from 2 months-70 years. Out of the total of 2,466 cases the major age groups affected were 5-10 years representing 31.2% (95% C.I. 29.3, 33.1), 1-4 years forming 22.9% (95% C.I. 21.3 24.5) all other age groups represented less than 10% including children less than one year representing 1.5% (95% C.I. 1.1-2.1). The proportion of the disease in the 5-10 years age group is significantly higher than all the age groups. Cumulative representation showed that over half (55.7%) of the cases were below 10 years and 86.2% below 30years as shown in Figure 1. Of the total of 2,466 cases, 57.4% (95% C.I. 55.4-59.6) were males and 42.6% (95% C.I. 40.6-44.5) were females.
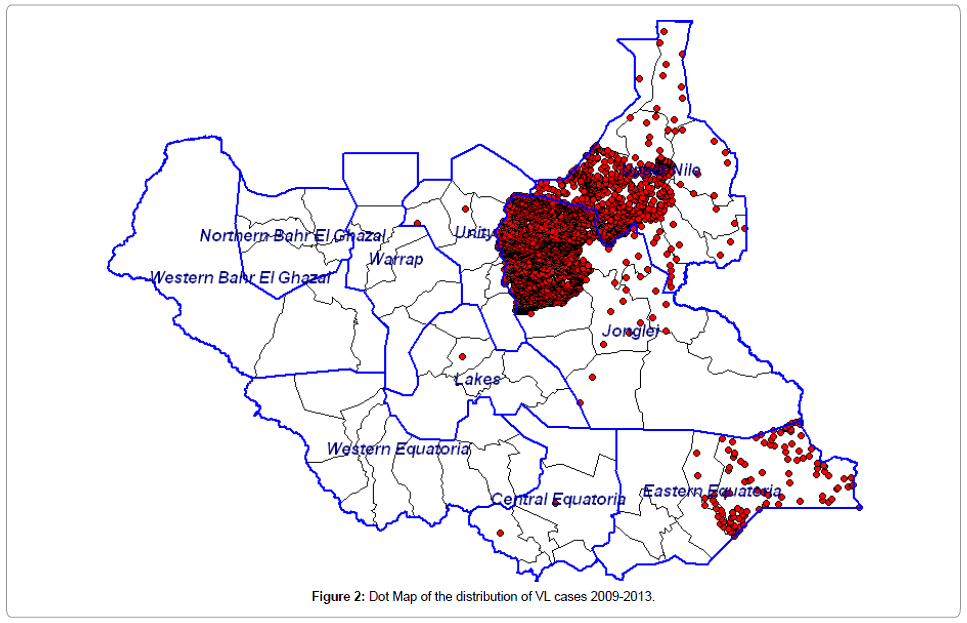
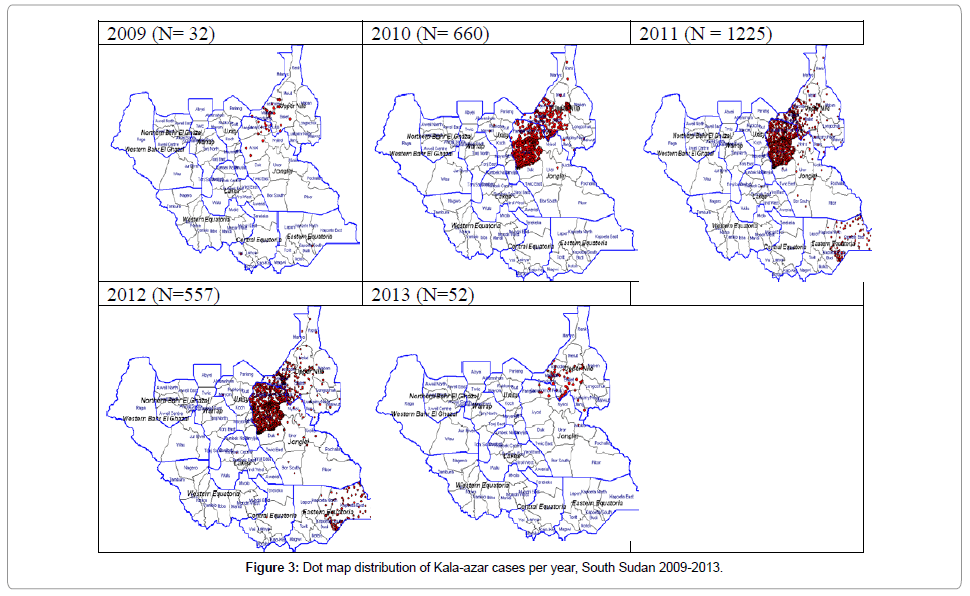
Among the 10 states of South Sudan, VL cases are distributed in Jonglei 65.2% (95% C.I 63.2-67.0), Upper Nile 30.0% (95% C.I. 28.2- 31.8), and Eastern Equatoria 4.6% (95% C.I. 3.8-5.5). At the second administrative level (counties) the distribution of cases shows Ayod, Fangak, Baliet and Canal (Khor fulus) accounting for 39.7%, 16.4%, 11.8% and 10.1% respectively. The dot map showing the distribution of cases is shown in Figures 2 and 3.
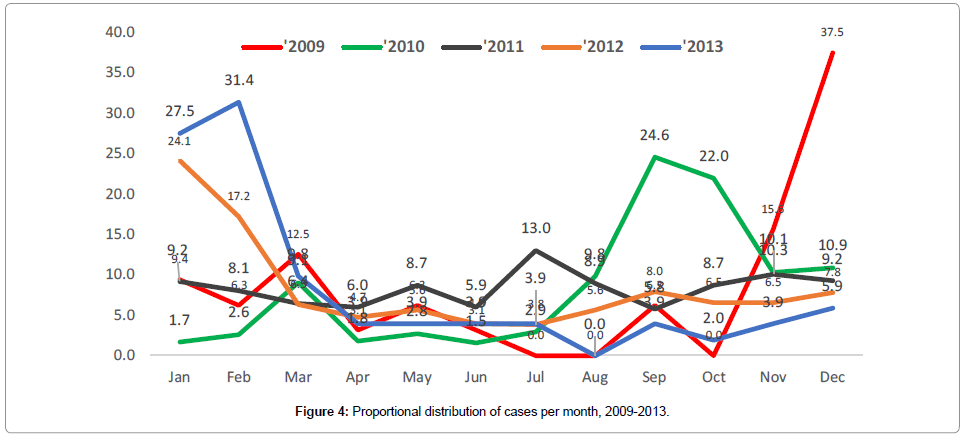
The monthly distribution of cases per year as shown in Figure 4 depicts multiple peaks without any seasonal predictions.
Age and duration of illness on the risk of death in the course of treatment of VL showed that 2% is attributed to a unit change of age (R2=0.02, p <0.001) and 1% by the unit change of duration of illness before treatment (R2=0.01, p<0.001) as shown in Table 3. A logistic regression of diagnosis against death outcome showed that the type of diagnosis account for 0.7% (R2=0.007, p<0.001) of the death for which cases of primary VL were 2.5 times (OR=2.5 (95% C.I. 1.4, 4.6); p<0.002) more likely to die as compared to other categories. The type of treatment regimen accounted for 3.9% (R2=0.039, p<0.001) of the risk of death as reflected in the significant odds shown from the use of ambisome and meltifosine (OR=0.1, 95% (0.0, 0.2) p<0.001).
| Variable | Odds | 95% C.I | p- value | R2 |
|---|---|---|---|---|
| Age | - | - | <0.001 | 0.02 |
| Duration of illnesses | - | - | <0.001 | 0.01 |
| Diagnosis | - | - | <0.001 | 0.007 |
| - Primary Kala- azar | 2.5 | 1.4, 4.6 | 0.002 | - |
| - Primary Kala- azar + HIV | 0.5 | 0.06, 5.1 | 0.63 | |
| - Primary Kala- azar + TB | 0.4 | 0.1, 2.2 | 0.31 | |
| - Relapse | 1.7 | 0.2, 15.3 | 0.6 | |
| - Post Kala- azar dormal | - | - | - | |
| Treatment Regimen | <0.001 | 0.039 | ||
| - Ambisome* | 0.1 | 0.0, 0.2 | <0.001 | |
| - Meltifosine | 0.1 | 0.0, 0.2 | <0.001 | |
| - Sodium Stibogluconate (SSG) | 0.74 | 0.4, 1.3 | 0.32 | |
| - SSG + Paramomycin | 2 | 0.1, 4.2 | 0.56 | |
| - Ambisome + Meltifosine | - | - | - | |
| *Liposomal amphotericin B **PM = Paromomycine |
||||
Table 3: Regression of age, duration of sickness, diagnosis, and treatment regimen to death.
Discussion
VL is known to persist in endemic zones worldwide even though in most endemic areas prevalence reduces over time [3,4]. We found that despite the changing trends of the prevalence of VL in South Sudan, the relative risk remains the same in the endemic areas of Jonglei and Upper Nile states and Kapoeta areas. Even though other studies [3,8] showed the same endemic zones in the country, our study has narrowed endemic areas of the disease to mainly Ayod, Fangak, Baliet and Canal Khorfulus counties (second administrative levels), which together account for 80% of the disease burden over the study period. The ecological risk factors in the counties are consistent with established risks. These areas are highly swampy, with persistent populations of sand flies, dogs, and cattle as well as thatch houses. This study was limited in assessing the extent of ecological risk to the disease and its influences on control measures; however, scanty literature demonstrated insignificant relationship between these factors and the increasing risk of the disease in South Sudan [8]. Further studies are therefore required to understand the socioecological risk factors accounting for the high burden of the diseases in endemic areas over this long period. This notwithstanding, current interventions should be focused in these four endemic zones.
The Eastern Africa block of Kenya, Ethiopia, Sudan and South Sudan have the greater burden of the Kala-azar disease in Africa [1,9]. The risk of spread and re-emergence of non-endemic areas in countries like Ethiopia and cross-border risks have been well documented [1,9,10]. Indeed, the global control measures have incorporated the cross-border activity to ensure sustained gains to reach to a wider population across borders [11]. Evidence from our study showed that since 2010, cases of Kala-azar in South Sudan are emerging and spreading widely to non-endemic counties that share borders with Ethiopia and Sudan. The increasing number of cases around border areas demonstrates the urgent need to re-evaluate interventions and strategies to strengthen cross-border collaboration with partners within and outside the country for effective control.
Annual cycles provide pointers for the critical impact of control measures. In the control of Leishmaniasis, fluctuations of inter-annual peaks have been documented. A climatic condition that correlates with the changing population of the vector and increasing infection has been known yet, insufficient to predict outbreaks [1]. The long incubation period of the disease in humans further worsen predictions for control interventions. Thus, the build-up of healthy carriers could delay screening and treatment of affected populations. This study showed irregular peaks with no seasonal patterns over the five years period. There is, therefore, uncertainty; hence, further studies on the entomological perspective could provide further evidence.
VL affects all ages, but mostly children less than 5 years and more in males than in females [1,7,12]. We found consistency with regards to the gender of the cases reviewed over the period. However, our study shows that a significantly higher percentage of the cases were 5-10 years as compared to all other age groups. This is also different from the earlier observations in South Sudan where the majority of the cases were below 5 years [8,13]. Explanations regarding why older children (5-10 years) were highly infected are limited, however, behavioral tendencies that expose them to the risk to the vector may not be ruled out. Further studies should examine the habits accounting for the high risk among children.
VL of all forms including PKDL is known to be common in East Africa, an observation consistent with our findings. The emergence of co infections of VL with HIV and the guidance on its management are of major concern globally [3,11]. This is informed by the fact that VL is an AIDS-defining condition and prerequisite for initiating antiretroviral treatment with relatively better outcomes, yet VL also negatively affects antiretroviral response. Our study revealed co infections with HIV as well as TB among the diagnosed VL cases. In low resourced settings, for diagnosis of VL, especially in the areas established as treatment sites, it may be necessary to reassess the potential of integrating other services including HIV screening for Kala-azar cases and ensure improved integration of antiretroviral and further monitoring to understand a basic comprehensive clinical management practices that would be responsive to the needs of the clients.
Combination therapies for VL are highly recommended as they provide higher cure rate, low duration of treatment and lower mortality. The Kala-azar control in South Sudan has conformed to the East Africa treatment regimen [3]. We found the use of pentavalent SSG alone and SSG with paramomycin routine irrespective of the form of VL with over 90% cure rate is consistent with findings in Kenya, Ethiopia and Sudan [9]. We were however unable to ascertain the appropriateness of the doses administered and the side effects that could account for the high numbers of relapses, transfers or PKDL among the cases. Evidence of effective management of cases with nutritional supplements was also limited despite the fact that the endemic zones are known to have high malnutrition rates among all ages. Improving treatment notes as part of a comprehensive dataset about VL would enhance greater insights as it takes advantage of the current direct observed treatment model.
Mortality due to VL is associated with many factors including socioeconomic status, treatment regimen and supplementary nutritional interventions [3,9]. Even though we could not find sufficient literature on demographics effects on reducing the risk of death due to VL, our study revealed that a unit change in the age of the patient could account for 2% of the death and 1% due to the duration of sickness. We also found the type of treatment regimen could explain for about 3.9% of the risk of death due to VL. It is imperative that in resource constraint settings, a more effective mechanism for monitoring the quality of care and treatment of VL cases are ensured. Even though death due to co infections of HIV and TB as well as PKDLs could explain these findings; improper choices of the treatment regimen and related administrative procedures on clients cannot be ruled out. Capacity building and general systems strengthening are of the essence in averting deaths.
Conclusion
In appraising prevention and control measures of VL in endemic areas, the impact of intervention should be addressed by redefining the epidemiological characteristics at the second administrative levels over time to account for localization of endemic areas and determine changing trends of diseases to affected populations.
Targeted interventions for prevention and control of VL must account of possible changes in susceptible age groups and their needs and should be well informed by further socio-ecological evidence that could account for behavioral tendencies that could mitigate the risk of exposure to VL.
In the face of limitation of improved treatment regimen with low toxicity and compliance, there is greater need for further research and development of alternative treatment regimen. This is more relevant considering that types of treatment regimen could also contribute significantly to rates of death of VL cases.
References
- Tim KM, Bryan AL, Raphael C, Ryan H, Kimberly CB, et al. (2014) Emerging and Re-emerging Neglected Tropical Diseases: a Review of Key Characteristics, Risk Factors, and the Policy and Innovation Environment. Clin Microbial Rev 27: 949-979.
- Christopher F, Dirk E (2016) Leaving no one behind a neglected tropical disease indicator and tracers for the Sustainable Development Goals. In Health 8: 15-18.
- World Health Organisation (2010) Control of Leishmaniasis. Report on meeting on WHO Expert Committee on Control of Leishmaniasis: Geneva.
- Mary L, Christy H, Angela W, Margaret B, Achille K, et al. (2011) Integrated Implementation of Programs Targeting Neglected Tropical Diseases through Preventive Chemotherapy: Proving the Feasibility at National Scale. Am J Trop Med Hyg 84: 5-14.
- Caryn B, Orin C, Jorge A (2010) Of Cattle, Sand Flies and Men: A Systematic Review of Risk Factor Analyses for South Asian Visceral Leishmaniasis and Implications for Elimination. PLoS Negl Trop Dis 4: e599.
- Ernest T, Lin A, Xia Z, Jun-Hu C, Wei H, et al. (2014) Surveillance-response systems: the key to elimination of tropical diseases. Infect Dis Poverty 3: 17.
- Seaman J, Mercer AJ, Sondorp E (1996) The epidemic of visceral leishmaniasis in western Upper Nile, southern Sudan: Course and Impact from 1984 to 1994: Int J Epidemiol 25: 862-871.
- John LN, Venny CSN, Mugo M, Eric M (2007) Risk factors for the transmission of kala-azar in Fangak, South Sudan. SSMJ 4: 26-29.
- WHO (2015) Visceral Leishmaniasis: control strategies and epidemiological update in East Africa. Report of a WHO bi-regional consultation Addis Abbaba, Ethiopia.
- Endalamaw G, Teshome T, Adugna A, Dia-eldin E, Margriet den B, et al. (2015) Eco-epidemiology of visceral leishmaniasis in Ethiopia. Parasit Vectors 8: 381.
- WHO (2015) Leishmaniasis: strengthening cross-border collaboration for control in Central Asia and Middle Eastern countries of WHO European and Eastern Mediterranean Regions. Report of bi-regional meeting. Awaza, Turkmanbeshi, Turkmenistan.
- Argaw D, Mulugeta A, Herrero M, Nombela N, Teklu T, et al. (2013) Risk factors for visceral Leishmaniasis among residents and migrants in Kafta-Humera, Ethiopia. PLoS Negl Trop Dis 7: 7.
- Gorski S, Collin SM, Ritmeijer K, Keus K, Gatluak F, et.al. (2010) Visceral leishmaniasis relapse in Southern Sudan (1999-2007): a retrospective study of risk factors and trends. PLoS Negl Trop Dis 8: 4.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 3894
- [From(publication date):
March-2017 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 2994
- PDF downloads : 900