Research Article Open Access
The Efficacy Evaluation of Danggui Buxue Tang (DBT) on Menopausal Symptoms: Clinical Trial Protocol Design and Considerations
King-Fai Cheng1,2, Eliza LY Wong3 and Ping-Chung Leung1,2*1Institute of Chinese Medicine, The Chinese University of Hong Kong, Hong Kong, P.R. China
2State Key Laboratory of Phytochemistry and Plant Resources in West China, Hong Kong, P.R. China
3The Chinese University of Hong Kong, Hong Kong, P.R. China
- *Corresponding Author:
- Ping-Chung Leung
Centre for Clinical Trials on Chinese Medicine
5/F, The CUHK Hong Kong Jockey Club School of Public Health Building
Prince of Wales Hospital
Shatin, NT, Hong Kong, P.R. China
Tel: (852)22528868
Fax: (852)26325441
E-mail: pingcleung@cuhk.edu.hk
Received date: November 17, 2014; Accepted date: January 12, 2015; Published date: January 16, 2015
Citation: Cheng K, Wong ELY, Leung P (2015) The Efficacy Evaluation of Danggui Buxue Tang (DBT) on Menopausal Symptoms: Clinical Trial Protocol Design and Considerations. J Community Med Health Educ 5:330. doi: 10.4172/2161-0711.1000330
Copyright: © 2015 Cheng K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Community Medicine & Health Education
Abstract
Background: Almost two third women experience hot flushes after menopause. Conventional treatment is the use of oestrogen replacement therapy or hormone replacement therapy (HRT). Although HRT is considered the most effective treatment for hot flushes, it may increase the risk of breast cancer and thromboembolic disease. Danggui Buxue Tang (DBT) is a combination of Danggui 當æ¸ (Radix Angelicae Sinensis) and Huangqi 黃芪 (Radix Astragali), which has been reported in ancient Chinese medicinal literature to relieve blood deficiency in female. The aims of the clinical studies were to proof the efficacy and to find out the optimal dose of DBT for menopausal symptoms. Methods/Design: The clinical studies were consisted of two stages. Stage I was designed as a single-center, randomized, double blind, placebo-controlled parallel study. Eligible subjects were randomized to one of two groups: DBT or placebo. Stage II was designed as a multiple-dose escalation clinical trial to look for the optimum dose for menopausal women. Eligible subjects were randomized to one of three dosage groups: low, middle and high dose. Results: Through the well-designed and well-conducted clinical trials, we found that in stage I trial, in analysis by severity of flushes, there was a significant reduction in the number of mild hot flushes in DBT group but not in the placebo group. In stage II trial, there were significant reduction in hot flushes and night sweats in high and middle dose groups after 3 months treatment, however, no significant changes were seen in low dose group. Conclusion: Our experiences suggested that it was feasible to perform a well-designed TCM clinical trial although there were challenges had to deal with, such as placebo preparation, outcome/endpoints determination and batch-to-batch consistency of the study medication.
Keywords
Traditional Chinese Medicine (TCM); Danggui Buxue Tang (DBT); Clinical trial protocol; Menopausal symptoms
Background
After menopause, almost two third women experience hot flushes. One third have symptoms lasting up to five years after natural menopause, and 10-20% find symptoms very distressing [1]. The most common menopausal symptoms are hot flushes and sweating, which are due to a decline in serum oestrogen concentrations. In Caucasian women, hot flushes and sweating have been reported in 70% and 84% respectively after a surgical menopause and in 60% and 74% following a physiological menopause [2].
Conventional treatment of the menopause is the use of oestrogen replacement therapy or hormone replacement therapy (HRT), which has been shown to be effective in controlling vasomotor symptoms [3]. HRT is considered the most effective treatment for hot flushes [4]. However, HRT has other biological effects: it prevents osteoporosis and colon cancer [5], but increases the risk of breast cancer and thromboembolic disease [6-8]. Another long-term consequence of the menopause is deterioration in quality of life. There are relatively few publications in this area, but studies on both healthy postmenopausal women as well as those with an underlying medical disorder have suggested that the menopause adversely affects quality of life and that treatment with hormone replacement therapy provides some improvement [9,10]. Oestrogen is therefore a valuable treatment for the menopause, but it is not without side effects. It remains to be seen whether Chinese Medicine as described in this proposal can prove to be an effective, safe and well-tolerated treatment for the menopause.
Danggui Buxue Tang
DBT is a combination of Danggui ç?¶æ¸ (Radix Angelicae Sinensis) and Huangqi é»?è?ª (Radix Astragali) in a weight-to-weight ratio of 1:5 (Figure 1). It is one of the simplest classical Traditional Chinese herbal formulas. The formula has been reported in ancient Chinese medicinal literature to relieve blood deficiency in female especially after menstruation or after giving birth.
From a Chinese medicine perspective, menopausal symptoms are associated with a decline in
Although Traditional Chinese Medicine (TCM) has a long history but its efficacy is not as well documented as one would hope. Evidence of efficacy should come from well-designed clinical trials. The aim of present studies was to proof the efficacy and define the optimal dose of DBT for menopausal symptoms through rigorous clinical trial protocols.
Design of the trials
The clinical studies consisted of two stages.
The objective of the Stage I was to examine the efficacy of DBT on menopausal symptoms. The objective of the Stage II was to find out the optimal dose through the dose response relationship.
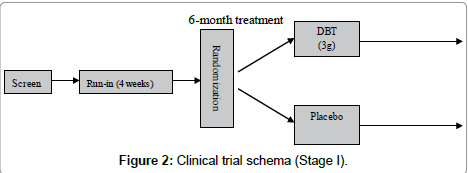
Stage I trial was designed as a single-center, randomized, double blind, placebo-controlled parallel study. Subjects were randomized to one of two treatment groups: Danggui Buxue Tang (ç?¶æ¸è£?è¡?湯) or placebo (Figure 2)
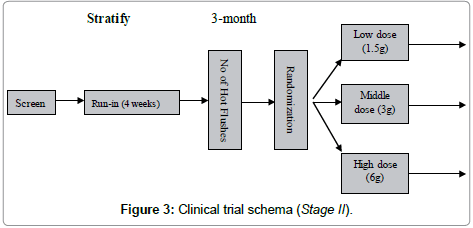
The Stage II was designed as a multiple-dose escalation clinical trial to look for the optimal dose. One group was treated with the normal dose which was same as stage 1 trial. From the normal dose we upgraded 2 times to 6 g daily as high dose and downgraded 2 times to 1.5 g daily as low dose (Figure 3).
Study participants were stratified based on number of hot flushes (strata 1=14-21/week; strata 2≥21/week).
Dose Determination for stage II trial
The results of Stage I clinical trial indicated that the improvement of vasomotor symptoms usually occurred after 4 or 5 months of DBT treatment. The daily dosage of 3 grams extracted from the mixture of Dang Gui and Huang Qi in the ratios of 1 to 5 (w/w) was equivalent to 8.8 g of raw herbs, based on the extraction rate of 34% provided by Hong Kong Institute of Biotechnology (HKIB). This dosage was only one-fourth of traditional dosage according to the record of ã??å??å¤?å?· 辨æ??è«?ã??, in which the formula was consisted of Dang Gui 6 grams and Huang Qi 30 grams, total 36 grams for daily dose.
Previous safety studies have demonstrated that both Dang Gui and Huang Qi have very low toxicity. The LD50 of Dang Gui in mice is 100 g /kg via injection, which is 1000 times of human dose. Long-term ingestion at the dosage of 6 g/kg showed no abnormality in physical activity, food intake, body weight, urine examination, or hematological examination. This dose is 60 times of human dose. China Pharmacopeia (2005) recommended dosage of Dang Gui for human was 6 to 12 grams. Huang Qi is also safe, doses as high as 100 g/kg of raw herb had been given by gavage to rats with no adverse effects. Oral ingestion of Huang Qi decoction (7.5 g/kg) cannot be determined in rats. The LD50 in mice for intraperitoneal injection is approximately 40 g/kg. China Pharmacopeia (2005) recommended dose was 9 to 30 grams. Dose determination was based on the followings:
- Old dose was only one quarter of the traditional dosage and was taken for a long time (4 or 5 months)
- The recommended high dose (6 g/day) was about one-half of the traditional dosage
- The dose was much lower than human safety dosages
- The dose of individual herbs was within the recommended human dosage
- The duration of DBT treatment had been reduced from 6 months to 3 months
Rational of the study design
Placebos and Double-Blind Designs
Blinding of treatment allocation minimizes the risk of bias during the trial and makes sure that the assessment and management of both groups are equivalent. However, real blinding is very difficult, especially for herbal medications [11].
Randomization and stratification
Randomization took place at the time of the first DBT treatment. The best way to achieve the balance was by the use of randomization in which a chance mechanism determines the treatment assignment. The stratified randomization method addresses the need to control and balance the influence of covariates. This method can be used to achieve balance among groups in terms of participants’ baseline characteristics (covariates).
For Stage I trial, the patients were assigned to receive their allocated treatment according to a computerized generated randomization table prepared by biostatistician:
Group 1: taking DBT 3g daily for 6 months
Group 2: taking placebo 3g daily for 6 months
For Stage II trial, each participant was randomized and allocated to one of three dose groups according to a computer-generated randomization code list in a 1:1:1 ratio using a block size of six. After the patient was registered into the study, the values of the stratification factors were recorded, and the patient was randomly assigned to one of the following treatment groups:
Group 1: Taking DBT 1.5 g daily for 3 months
Group 2: Taking DBT 3 g daily for 3 months
Group 3: Taking DBT 6 g daily for 3 months
Screening and eligibility
After a verbal introduction to the trial, prospective participants were asked several screening questions in interview format, including some eligibility criteria and minimal demographic information. This level of screening was typically done over the phone. All subsequent screening procedures took place in the Center for Clinical Trial on Chinese Medicine, and Department of Obstetrics & Gynaecology, CUHK after providing written consent. Procedures differed depending on whether or not subjects were with menopausal symptoms at the start of screening.
Following an initial vasomotor symptom check, patients with hot flushes 14 times per week were evaluated for the potential level of hormones and amenorrhoea at least 12 months in order to participate in the trial. For the Stage II dose finding trial, the potential subjects should be never received treatment for menopausal symptoms and never received menopausal hormone therapy. The evaluation also included a physical exam, clinical history, medication history, Pelvic Ultrasound Endometrial Sampling/ Hysteroscopy, Vaginal Maturation, Arterial Reactivity and assessment of liver function, renal function, lipid profile, urinalysis and a complete blood count.
Additional assessments during screening including self-reported questionnaires to collect information on frequency and severity of hot flushes and night sweats. These questionnaires were completed after consent was obtained.
Eligibility criteria for the randomized trial were listed in Table 1.
| DBT Stage I trial | DBTStage II trial |
|---|---|
| Inclusion criteria 1.Follicle stimulating hormone (FSH), luteinizing hormone (LH), oestradiol in the menopausal range (FSH>18 IU/L, LH>12.6 IU/L, and E2<361 pmol/l). 2. Patients with amenorrhoea for more than 12 months 3. Patient with at least 14 hot flushes or night sweats per week 4. Patient able to give written or witnessed oral informed consent prior to study star and able to comply with study requirements Exclusion criteria 1. Patients with a history of using any form of hormonal replacement therapy within 8 weeks 2. Patients with a history of using Chinese medicine or other therapies which may affect the outcome within 8 weeks 3. Patients who in the judgment of the investigator will be unable to comply with protocol requirements 4. Patients with significant gastrointestinal, renal, hepatic, bronchopulmonary, neurological, cardiovascular, breast or endometrial carcinoma, or allergic diseases 5. Patients with uncontrolled hypertension 6. Patients with undiagnosed vaginal bleeding 7. Patients with a history of significant drug hypersensitivity |
Inclusion criteria 1. Follicle stimulating hormone (FSH), luteinizing hormone (LH), oestradiol in the menopausal range (FSH>18 IU/L, LH>12.6 IU/L, and E2<361 pmol/l) 2. Patients with amenorrhoea for more than 12 months 3. Never received treatment for menopausal symptoms 4. Never received menopausal hormone therapy 5. Reporting a minimum of 14 hot flushes per week at the time of entry into the study 6. Patient able to give written or witnessed oral informed consent prior to study star and able to comply with study requirements Exclusion criteria 1. Patients with a history of using Chinese medicine or other therapies which may affect the outcome within 8 weeks 2. Patients who in the judgment of the investigator will be unable to comply with protocol requirements. 3. Patients with significant gastrointestinal, renal, hepatic, bronchopulmonary, neurological, cardiovascular, breast or endometrial carcinoma, or allergic diseases 4. Patients with uncontrolled hypertension 5. Patients with undiagnosed vaginal bleeding |
Table 1: Eligibility criteria for DBT trials.
Treatment Intervention and Medication
Experimental intervention
Among thousands of traditional Chinese medicine herbal formulae, almost all of which consist of multiple herbs, DBT is one of the simplest containing only two herbs, Radix Astragali (RA) and Radix Angelicae Sinensis (RAS). DBT is traditionally used to treat ailments in women. According to TCM theories, DBT replenishes qi and nourishes xue (the blood) it is therefore believed beneficial for menopausal symptoms [12].
Control intervention
The protocol design for the clinical trial is based on principles of Randomized Controlled Trial (RCT); placebo control is usually involved, which is designed to be inactive. The purpose of using placebo is to control the bias caused by psychological or non-specific effects of treatment and reduce the chance of being recognized by subjects and investigators during the period of clinical trial. To maintain blinding, the placebo is designed to appear, smell, and taste indistinguishably from the trial preparation while pharmaceutical activity and toxicity are both absent [11,13]. If a placebo is easily identified, whether a double blinded design could be implemented is doubtful, because subjects dislike being assigned the placebo group and the quality of the clinical trial will be affected [14,15].
The duration of efficacy study was 6 months and dose-finding study 3 months. The “blinded” doctors saw the patients on weeks 0, 13 and 26 in Stage I trial (Table 2); and saw patients on months 0, 1 and 3 in Stage II trial (Table 3). For the first four encounters, patient’s signs and symptoms had to be evaluated at each visit. The last month (Stage II) was a follow-up period without any medication to see whether a rebound effect would occur.
| Period | Screening | Treatment | ||
|---|---|---|---|---|
| Visit | 0 | Baseline | 2 | 3 |
| Day | -30 to -1 | 0 | 91 | 182 |
| Medical History | X | X | X | X |
| Menopausal Symptoms | X | X | X | X |
| Vital Signs | X | X | X | X |
| Physical Examination | X | |||
| Review of Incl./Excl. Criteria Study | X | |||
| Informed Consent | X | |||
| Urinalysis | X | X | X | |
| Concomitant Medications | X | X | X | X |
| Randomization | X | |||
| Hormone assay | X | X | X | |
| Hematology | X | X | X | |
| Biochemistry | X | X | X | |
| Pelvic Ultrasound Endometrial Sampling/ Hysteroscopy | X | |||
| Vaginal Maturation | X | X | ||
| Body Mass Index (kg/m2) | X | X | X | |
| Cardiovascular markers | X | X | X | |
| MENQOL | X | X | X | |
| Arterial Reactivity | X | X | X | |
| Dispense Study Medication | X | X | ||
| Medication Accountability | X | X | ||
| Record Adverse Event | X | X | ||
Table 2: Schedule of treatment and study assessments (Stage I trial).
| Period | Screening/Run-in | Treatment | Follow-up | ||
|---|---|---|---|---|---|
| Visit | 0 | Baseline | 2 | 3 | 4 |
| Day | -30 to -1 | 0 | 31 | 92 | 120 |
| Medical History | X | ||||
| Menopausal Symptoms | X | X | X | X | X |
| Vital Signs | X | X | X | X | X |
| Physical Examination | X | ||||
| Review of Incl./Excl. Criteria Study | X | ||||
| Informed Consent | X | ||||
| Urinalysis | X | X | X | X | |
| Concomitant Medications | X | X | X | X | X |
| Randomization | X | ||||
| Hormone assay | X | X | X | X | |
| Hematology | X | X | X | X | |
| Biochemistry | X | X | X | X | |
| Pelvic Ultrasound Endometrial Sampling/Hysteroscopy | X | ||||
| Vaginal Maturation | X | X | X | ||
| Body Mass Index (kg/m2) | X | X | X | X | |
| Greene Scale | X | X | X | X | X |
| MENQOL | X | X | X | X | X |
| Dispense Study Medication | X | X | |||
| Medication Accountability | X | X | |||
| Record Adverse Event | X | X | |||
Table 3: Schedule of treatment and study assessments (Stage II trial).
The dosage forms of medication were capsule (Stage I) and sachet (Stage II). All herbs were screened for pesticide residual, microbial exam and heavy metal contamination. Patients in Stage I trial were instructed to take 3 capsules and two times a day. Patients in Stage II were instructed to take one sachet a day.
Outcome measures
Primary end points: Selection of the primary end point is a key design element of an RCT, especially for TCM clinical trial. The primary treatment comparison of Stage I was to test the efficacy of TCM whether DBT has an advantage over placebo control (DBT versus placebo) on menopausal symptoms of hot flushes and sweating.
Other treatment comparison was to evaluate the safety of DBT in patients with menopausal symptoms. Treatment comparison was made for each of the several endpoints:
- Changes in severity and frequency of hot flushes and sweats from baseline to 6 months (primary endpoint)
- Changes in score for the domains measured in the Menopause Specific Quality of Life Questionnaire
- Changes in self-reported menopausal symptoms
The objective of the Stage II study was to investigate the dose response relationship to assess an optimal dose. Treatment comparisons were made for each of several endpoints:
- Change in severity and frequency of hot flushes and sweats
- Changes in score for the domains measured in the Menopause Specific Quality of Life Questionnaire
- Changes in score of Greene Climacteric Scale
- Changes in self-reported menopausal symptoms
The Stage I randomized double blind placebo controlled clinical trial had demonstrated that DBT had effects in improving vasomotor symptoms (hot flushes, sweating and night sweats) over the placebo group. In the Stage II clinical trial, we investigated the optimal dose.
Primary endpoint of both trials was the mean number of hot flushes per 24 hours measured over a 4-week period. The numbers of hot flushes were recorded in a daily diary, and flushes were scored by severity on a scale from of zero to ten. The diary was administered for four weeks during the qualifying period, and for one week after 4, 8 and 12 weeks of the intervention period.
Secondary endpoint was the Women’s Health Questionnaire (MENQOL), a validated, self-administered instrument containing 36 questions assessing a wide range of physical and emotional symptoms of women in the postmenopausal period
Statistical considerations
Primary and secondary hypotheses for Stage I trial were to determine if DBT could reduce the number and severity of hot flushes. If the hypotheses were established, the purpose of Stage II trial was to identify an optimal dose for the treatment of menopausal symptoms.
All analyses were conducted blinded to group allocation. All analyses were done on an “intention-to-treat” basis; i.e. all participants who reported receiving study drugs and taking them at least once would be included. The primary analysis was comparing mean changes from baseline to end of treatment in the two groups, using a two-sample t-test and paired t-test. Comparisons to placebo were conducted using an ANCOVA with baseline as a covariate.
The mean daily number and severity of hot flashes were compared among treatment groups for each month using an ANCOVA, with treatment as a factor in the model and baseline as the covariate.
For the Stage II trial data analysis, the experimental design of the present study involved two factors: dose and time (a within-individual factor with either five levels representing the average frequency or severity scores for hot flashes and night sweats or questionnaire assessments performed at baseline, 0 week, 4 weeks, 12 weeks, and post-treatment). Repeated-measure ANOVA was performed to test the significant dose × time effects of DBT on menopausal symptoms, quality-of-life scores, serum lipids, and female hormones during the treatment period.
Differences in categorical data between groups were explored by Mann-Whitney Test, Wilcoxon Signed Ranks Test or χ2 test. A linear regression analysis was used to analyze the relationship between duration of menopause and the decrease in hot flashes during treatment.
Statistical power and sample size
The sample size determination for the clinical trial is dependent on the primary endpoint that is frequency and severity of hot flushes. The sample size was calculated using data for hot flush frequency from many previous trials of HRT, herbs and acupuncture. In Stage I trial, assuming similar efficacy to that of oestradiol in eliminating hot flushes (expect reduction in prevalence from 0.67 to 0.32), with alpha=0.05 and power 0.90 need 41 patients / group. Therefore, to allow for approximately 20% dropouts, 100 women will be recruited.
Data Management
Clinical data were reported on case report forms (CRFs). The Case Report Form (CRF) is a set of forms for each subject, provides a record of data generated according to protocol. These forms were to be completed on an ongoing basis during the study. During the study, the CRF was monitored for completeness, accuracy, legibility and attention to detail. The CRF was retained for review.
Following is a list of CRFs which were used in Stage I trial: Participant screening form, inclusion/exclusion criteria, medical history, physical examination, laboratory data- hematology, laboratory data – chemistry screen, laboratory data – hormone levels, drug compliance, concomitant medications, adverse event, serious adverse events, drop-out form, menopausal specific quality of life (MENQOL), patient diary of menopausal syndromes, and medication log.
Compliance was monitored via a drug compliance recorded on each visit of the study. Missing data was obtained via the data audit procedures and by the data entry system to the database.
Interim Analysis
Clinical trials tend to have a long duration, because patients are usually recruited one by one, and especially subjects’ responses to herbal medicine treatment are need relatively long time compared with chemical medicines. The utilization of interim analyses to compare treatment arms, terminate the development of ineffective or unsafe drugs, or accelerate the regulatory approval process of breakthrough drugs has gained popularity in the pharmaceutical industry [16]. As the sample-size of a clinical trial is generally based on preliminary and/or uncertain information, an interim check on the unblinded data may be useful to reveal whether or not overall response variances, event rates are as anticipated.
As there are many outcome variables in the DBT clinical trials, but in the interim analyses we limited the number to only the major variables and conducted when half subjects completed the study.
Subject Recruitment Flowchart
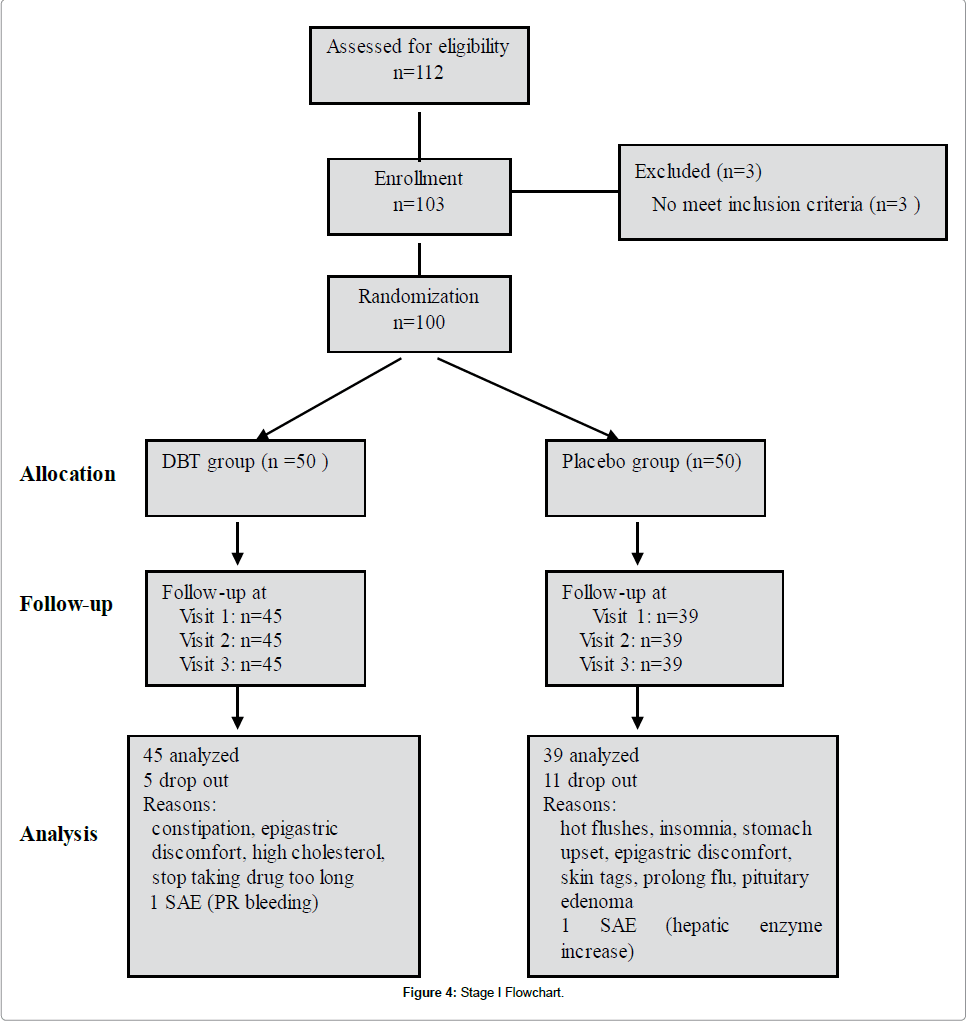
The flowchart of Stage I trial showed that 100 women who were randomized, 84 completed the study (45 in the treatment group and 39 in the placebo group). Eleven withdrawals were in the placebo group and five were in the treatment group (Figure 4).
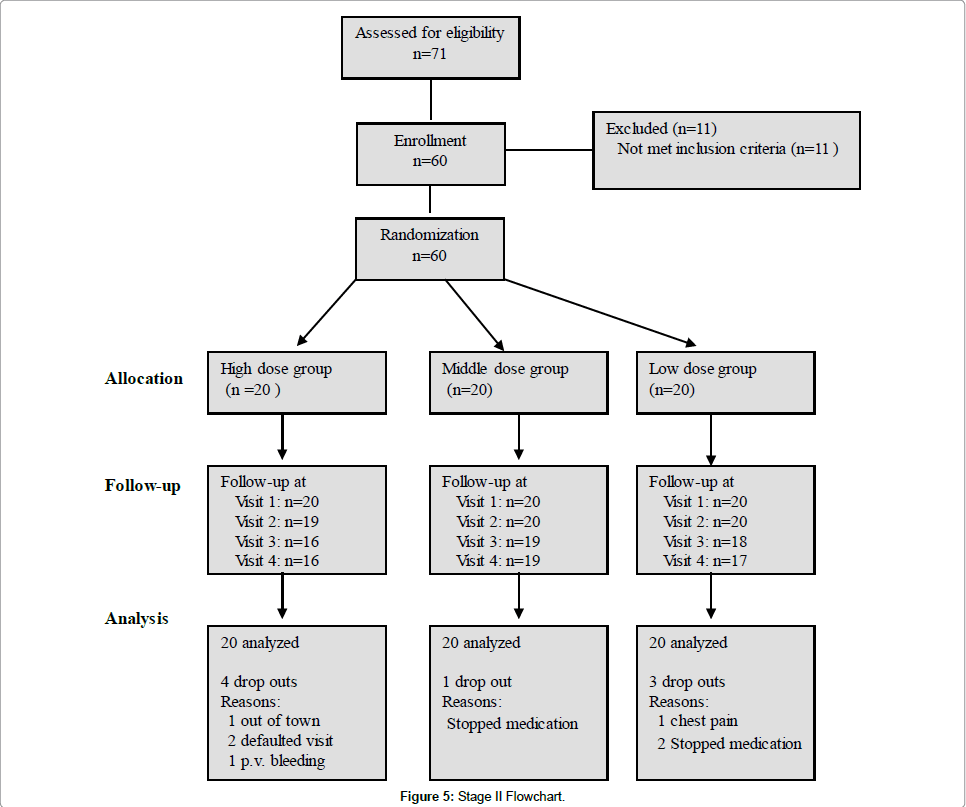
The flowchart of Stage II trial showed that 60 women who were randomized, 52 completed the study (16 in high dose group, 19 in middle dose group and 17 in low dose group). Four withdrawals were in high dose group, one withdrawal was in middle dose group and three were in low dose group (Figure 5).
Discussion
A Chinese herbal formula, whatever how effective in the preclinical studies, if no clinical trial evidences to prove its efficacy and safety, the Chinese herbal formula ultimately cannot be considered valid and cannot get marketing approval. Thus, clinical trial plays a decisive role in the development of Traditional Chinese Medicine.
Reliable evidence of efficacy and safety are from well-designed clinical trial. However, due to lack of proper method to evaluate the efficacy according to its own characteristics of Chinese medicine, it is still in the embarrassing position that is “it’s good but can not tell”. The highest level of efficacy evidence is obtained from randomized controlled clinical trial (RCT) [17,18].
The randomized controlled clinical trial is the cornerstone of clinical evaluation. For Traditional Chinese Medicine there are constraints in carrying out RCT. Our experiences demonstrate that it is feasible to perform a well-designed TCM clinical trial although there are challenges, for example placebo preparation, proper outcome measurement selection and batch-to-batch quality consistency etc.
Instead of following the requirements of RCT, if we chose to adopt the traditional Chinese Medicine experts’ practice, we need to subdivide our patient groups according to their clinical symptom patterns, and then select the appropriate symptom sufferers from each pattern group separately for variable treatment using flexible herbal formulations. DBT might remain as major therapeutic agent, which can be freely modified by the attending physician according to the changes in clinical presentation. We avoid all these difficulties by designing a protocol strictly according to the recommendations of RCT with strict requirements of patient inclusion, standard treatment program and assessment methodology. In order to be recognized globally, the efficacy and safety of Chinese medicines must seek a breakthrough on the Methodology of Evaluation, which include the protocol design, sample size estimation, statistical considerations, case record form design, subject recruitment, placebo preparation, clinical trial implementation, clinical trial data analysis and clinical trial report writing.
As a matter of fact, two clinical trials on post-menopausal women utilizing the protocol had been completed and full manuscripts have been published [19,20].
Acknowledgements
The work reported in this paper was supported by an Area of Excellence Grant from the University Grants Committee of the Hong Kong Special Administration Region, China (Project no. AoE/B-10/01), and it is related to the joint partnership between the Institute of Chinese Medicine, The Chinese University of Hong Kong, and the Kunming Institute of Botanical Research, a state key Laboratory joint venture of China.
References
- Stearns V, Ullmer L, Lopez JF, Smith Y, Isaacs C, et al. (2002) Hot Flushes. Lancet 360: 1851-1861.
- Hagstad J, Janson PO (1986)The epidemiology of climacteric symptoms.ActaObstetGynecol Sand Suppl 134: 59-65.
- Schneider HPG, Gallagher JC (1999)Moderation of the daily dose of HRT: benefits for patients.Maturitas 33:25-29.
- Maclennan AH, Broadbend JL, Lester S, Moore V (2004) Oral oestrogen and combined oestrogen.progestogen therapy versus placebo fot hot flushes. Cochrane Database.
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg,et al.(2002) Risks and benefits of estrogen plus progestin in healthy post-menopausal women: principal results from the Women’s initiative randomized controlled trial. 288: 321-333.
- (1997) Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer 350: 1047-1059.
- Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M,et al. (2003) WHI Investigators.Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial289:3243-3253.
- Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M,et al.(2002)Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) 288: 49-57.
- Blumel JE, Castelo-Branco C, Binfa L (2000) Quality of life after menopause: a population study 34: 17-23.
- Hall G, Pripp U, Schenck-Gustafsson K (1997) Longterm effects of hormone replacement therapy on symptoms of angina pectoris, quality of life and compliance in women with coronary artery disease.28: 235-242.
- Cheng K, Fai, Guan De Qi, Ding A, Wei,et al.(2011)Placebo Preparation for the Proper Clinical Trial of Herbal Medicine -Requirements, Verification and Quality Control. Recent Patents on Inflammation & Allergy Drug Discovery 5: 169-174.
- Tsim KWK (2005)DangguiBuxue Tang (DBT, Chinese Angelica Decoction): a sample trial in TCM standardization. Asia-Pacific Biotech News (APBN)8:1316-1321.
- Guan DQ, Ding AW, Leung P, Chung, Cheng KF. (2008)Placebos Used in Clinical Trials for Chinese Herbal Medicine. Recent Patents on Inflammation & Allergy Drug Discovery 2: 123-127.
- (2002) International Ethical Guidelines for Biomedical Research Involving Human Subjects, The Council for International Organizations of Medical Sciences (CIOMS).
- Chinese Medicine Council of Hong Kong. Good Clinical Practice for proprietary Chinese Medicine.
- (1998) International Conference on Harmonisation. Guidance on statistical principles for clinical trials; availability. Department of Health and Human Services FDA. Federal Register63:49583-49598.
- Altman DG (1996)Better reporting of randomised controlled trials: the CONSORT statement. BMJ 313: 570-571.
- Sackett DL, Straus SE, Richardson WS(2000) Evidence-based Medicine: How to Practice and Teach EBM,(2ndedn.) Edinburgh, UK: Churchill Livingstone.
- Haines CJ,Lam PM, Chung TKH, Cheng KF, Leung PC (2008) A randomized, double-blind, placebo-controlled study of the effect of a Chinese herbal medicine preparation (Dang GuiBuxue Tang) on menopausal symptoms in Hong Kong Chinese women 11: 244-251.
- Wang CC, Cheng F, Lo W(2013)A randomized, double-blind, multiple-dose escalation study of aChinese herbal medicine preparation (Dang GuiBuxue Tang)for moderate to severe menopausal symptoms and quality oflife in postmenopausal women20: 223-231.
Relevant Topics
- Addiction
- Adolescence
- Children Care
- Communicable Diseases
- Community Occupational Medicine
- Disorders and Treatments
- Education
- Infections
- Mental Health Education
- Mortality Rate
- Nutrition Education
- Occupational Therapy Education
- Population Health
- Prevalence
- Sexual Violence
- Social & Preventive Medicine
- Women's Healthcare
Recommended Journals
Article Tools
Article Usage
- Total views: 19397
- [From(publication date):
February-2015 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 14760
- PDF downloads : 4637