The Effects of Tobacco Smoking on Healing of Foot Ulcers and Lower Limb Amputation: A Systematic Review
Received: 01-Jun-2022 / Manuscript No. crfa-22-66543 / Editor assigned: 03-Jun-2022 / PreQC No. crfa-22-66543 (PQ) / Reviewed: 17-Jun-2022 / QC No. crfa-22-66543 / Revised: 24-Jun-2022 / Manuscript No. crfa-22-66543 (R) / Published Date: 30-Jun-2022 DOI: 10.4172/2329-910X.1000351
Abstract
Cigarette smoking is well known to increase the risk of peripheral arterial disease (PAD). Whether current smoking has additional effects upon healing or amputation risk after a foot ulcer is present is unclear.
Aim: We reviewed the literature to evaluate associations between tobacco smoking and the effects on healing of foot ulceration and upon the rates of lower limb amputation (LLA).
Methods: The Cochrane Library, Embase, Medline and PubMed were searched for studies published prior to July 2021. Manuscripts that reported only smoking and the effect on PAD were excluded. Articles that assessed the effect of smoking and reported outcomes of foot ulceration or LLA were included.
Results: Fourteen studies met the inclusion criteria and were included in this review. Overall, there is a surprising lack of outcome data for the association between active tobacco use and healing of foot ulceration or LLA.
Conclusion: Further research should specifically look healing and LLA in those who are current smokers compared to non-current smokers. If current smoking increases risk of new ulcers, impairs healing or increases LLA this may enhance efforts by clinicians to assist patients to cease, and may encourage patients to stop.
Keywords
Foot ulcer; Diabetic foot ulcer (DFU); Smoking; Amputation; Lower limb amputation (LLA); Wound healing
Abbreviations:
ABI, Ankle brachial index; CLI, Critical limb ischaemia; DFD, Diabetic foot disease; DFU, Diabetic foot ulcer; LLA, Lower limb amputation; NDSHS, National Drug Strategy Household Survey; PAD, Peripheral arterial disease; TASC, TransAtlantic Inter- Society Consensus
Introduction
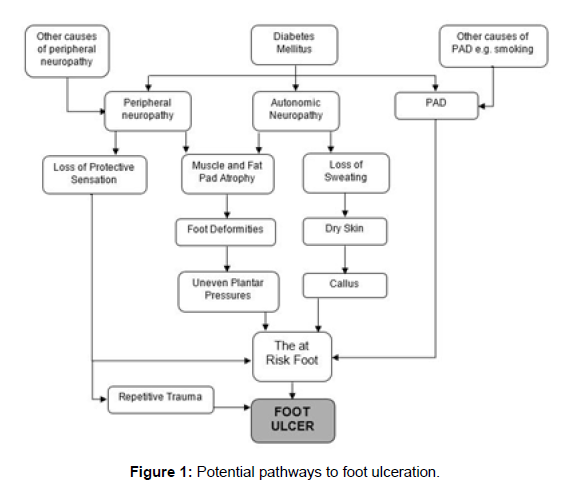
Foot ulceration is an important cause of hospitalisation and lower limb amputation (LLA) worldwide [1]. Most foot ulcers have a multifactorial pathophysiology (Figure 1). Peripheral neuropathy,peripheral arterial disease (PAD), foot deformity, poor glycaemic control, tobacco smoking, duration of diabetes and the presence of other micro and macro vascular complications all contribute to the pathogenesis [2-4]. The majority of non-traumatic lower limb amputations (LLA) are preventable; hence it is important to identify factors contributing to ulceration, delayed healing and LLA.
Tobacco smoking is decreasing worldwide but it still remains a serious problem [5]. According to the National Drug Strategy Household Survey (NDSHS) in 2019, it was estimated that 11.6% of Australian adults smoked daily [6]. Unfortunately, these rates are not significantly lower in people who have diabetes, with the most recent Australian National Diabetes Audit report finding 495 of 4334 cases currently smoked (11.4%) [7]. Smoking is associated with many chronic illnesses including cardiovascular disease, cancer, diabetes and lung disease [8]. Interestingly, smoking increases the risk of developing diabetes [9]. Current cigarette smoking is associated with deterioration in glucose control, as measured by HbA1c [10, 11].
Here, we provide a review of the role of tobacco smoking in foot ulceration or lower limb amputation.
Smoking and Peripheral Neuropathy
Peripheral neuropathy is the most common risk factor for diabetic foot ulceration (DFU); it is found in approximately 78% of patients with foot ulcers 8. The human nervous system is vulnerable to oxidative damage which is believed to be the ultimate mechanism of cell damage leading to diabetic peripheral neuropathy [12]. Tobacco smoking may exacerbate peripheral neuropathy due to the additional oxidative stress, with previous research confirming that tobacco smoking is an independent risk factor for peripheral neuropathy [8,13]. Cigarette smoke also contains glycotoxins which promotes formation of advanced glycated end-products (AGE). Elevated AGE increases risk of peripheral neuropathy [8]. Tobacco smoking may also promote peripheral neuropathy by inducing insulin resistance, hyperinsulinemia [14], and as mentioned above, increased HbA1c
Peripheral Arterial Disease (PAD)
PAD is narrowing of the arteries in the limbs due to atherosclerosis and most commonly affects the lower limbs [15]. It is much more common in the lower limb vessels than in the upper limbs. PAD impairs blood flow, which impairs wound healing, and increases the risk of infection. Patients with PAD are more likely to develop critical limb ischaemia (CLI), which may be diagnosed in the presence of ischaemic foot ulcers, gangrene or ischaemic rest-pain [16]. In 2007, the TransAtlantic Inter-Society Consensus (TASC II) defined ankle pressure <50mmHg, or toe pressure <30mmHg, or transcutaneous oxygen tension (TcPO2) <30mmHg, as diagnostic measures for CLI [17]. When combined with peripheral neuropathy, many people with PAD develop neuro-ischaemic foot ulcers, especially those with CLI. For many patients with neuro-ischaemic foot ulcers, the eventual outcome is a minor or major amputation [15].
Smoking and Peripheral Arterial Disease
Smoking is closely linked to the formation of vascular plaques and previous studies have shown strong associations between smoking and PAD and cardiovascular disease [18,19].
Chronic smokers have weakened vasodilation in the microvasculature which can also further decrease the already reduced blood flow due to PAD [8]. It is reported that amputation rates related to PAD in smokers is twice the rate of that in non-smokers [20].
Smoking and Wound Healing
Wound healing is impaired in individuals with diabetes and their LLA risk is much higher [8]. Smoking is associated with higher risks of postoperative complications, including delayed healing of surgical wounds and increased risk of wound infection. Studies have reported that smoking cessation for at least 3 weeks prior to surgery reduces postoperative morbidity from 52% to 18% [8]. This highlights the role that smoking plays in tissue healing.
The impaired vasodilation caused by smoking leads to tissue hypoxia, which is the primary mechanism for how smoking impairs the wound healing process and increases risk of delayed healing and LLA [8].
However, whether current smoking affects healing of foot ulceration is unclear. Here we review published data relating to tobacco smoking and altered foot ulcer healing or LLA.
Materials and Methods
The following databases were searched to identify relevant studies for inclusion:
(i) Cochrane Library (searched July 25th 2021)
(ii) Ovid Embase (searched July 25th 2021)
(iii) Ovid MEDLINE (searched July 25th 2021)
(iv) PubMed (searched July 25th 2021)
The combination of search terms was as follows:
• (foot ulcer* OR diabetic foot ulcer* OR foot wound* OR DFU) AND (smok*)
• (lower limb amputation* OR lower extremity amputation* OR amputation*) AND (smok*)
The search was limited to Humans and English only. No limitations were placed on date of publication.
One author (N.B.) screened the search results. Titles and abstracts were screened on all databases. The full manuscripts for those considered potentially relevant were read. The reference lists for these papers were also examined for potential studies to be included in this review.
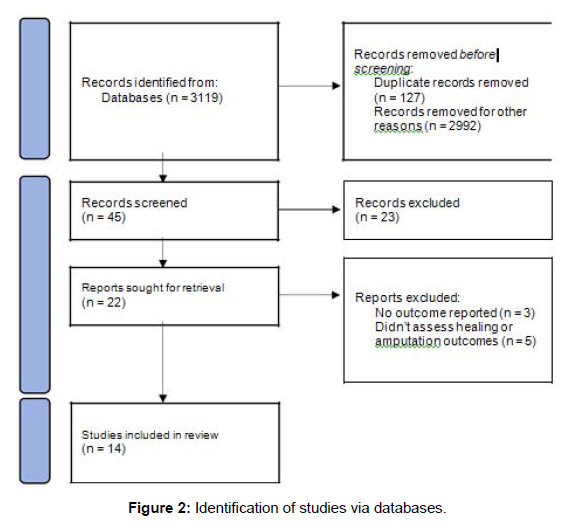
The search process is outlined in Figure 2.
Inclusion and Exclusion Criteria
Studies had to meet the following criteria for inclusion: (a) randomised controlled trials, cohort studies or case-control studies and (b) analyse the relationship between smoking and incidence or healing of foot ulcers or smoking and LLA rates. As mentioned above, papers not written in English were excluded.
Systematic review articles and case reports were not included, however reference lists of systematic reviews were screened and no studies for inclusion were found. For the study to be included there had to be at least one outcome reported for interaction between smoking and foot ulceration or LLA.
Results
Study Selection
Following the database searches, a total of 3119 articles were found. Of these, 3074 papers were excluded after screening, leaving 45 articles for abstract review. Twenty-three of these were excluded as not relevant. Twenty-two articles were obtained for full text review. Of these, fourteen studies were suitable for inclusion in our systematic review. The eight studies which were excluded were for lack of reported outcomes (n = 3) or because they did not assess for association between tobacco smoking and foot ulceration or LLA (n = 5).
Included Studies
Both reviewers (N.B. and J.G.) independently reviewed the data from the included studies. One study was cross sectional in design 1. The remainder were cohort studies; six retrospective [21-26], and seven prospective [27-33].
Four studies were undertaken in the United States of America [22,25, 28, 32], two in the United Kingdom [24, 31], and one in each of the following; Australia [26], China [33], Denmark [23], India [27], Nigeria [29], Pakistan [1], Saudi Arabia [21] and Sweden [30].
Characteristics of the studies are described in Table 1.
Demographic Data of Included Studies
The studies had a combined total of 103,316 participants. Of these, 92,731 were in the control groups and 10,585 were in the case groups.
Of those in the control groups, 92,261 had diabetes with no diabetic foot disease (DFD), 25 had DFU but were ulcer free for 2 years prior, 223 were non-smokers who underwent a lower limb endovascular treatment and the remaining 222 had not undergone a lower limb amputation (LLA) in comparison to the case group.
All studies included both male and female participants. In most studies, consistent with the usual foot ulcer population, there were more males than females.
The characteristics of participants are summarised below in Table 1.
| Reference, Country | Study Design, Sample Sizes | Age, (years) | M:F % | Current Smokers N (%) | Past Smokers N (%) | Diab Hx % | HbA1c, % |
|---|---|---|---|---|---|---|---|
| Al-Rubeaan et al., Saudi Arabia | Retro, 60610 controls, 2071 DFD | 56.7±13.5 | 52:48 | 4076 (6.7) | NR | 100 | 8.8±2.4 |
| 63.8±12.6 | 69:31 | 210 (10.1) | NR | 100 | 9.9±2.3 | ||
| Anderson et al., USA | Retro, N =112 with LLA | 61.5 | 61:39 | 66 (59) | NR | 100 | NR |
| Bajaj et al., India | Prosp, N = 100 all DFU | 59.5 | 64:36 | 32 (32) | NR | 100 | 9.9 |
| Boyko et al., USA | Prosp, 1069 controls, | 62.4±10.8 | 98:2 | 24 (2.2) | NR | 100 | 9.5±3.0 |
| 62.3±9.2 | 98:2 | 19 (8.8) | NR | 100 | 11.8±3.4 | ||
| Engberg et al., Denmark | Retro, N = 780 all DFU | 64.3±12.5 | 69:31 | 187 (24) | NR | 100 | 8.3±3.7 |
| Heald et al., UK | Retro, 15727 controls, 1125 DFU | 73.8±16.9 | 54:46 | 1598 (6.3) | 3174 32) | 100 | 7.5±1.7 |
| 77.9±14.1 | 54:46 | 115 (16) | 232 (32) | 100 | 7.9±1.9 | ||
| Ikem et al., Nigeria | Prosp, 28 controls, 46 DFU | 64.4±8.2 | 50:50 | 2 | NR | 100 | NR |
| 60.4±11.8 | 60:40 | 12 | NR | 100 | NR | ||
| Kokkinidis et al., USA | Retro, 223 non-smokers, 41 smokers | 69.9±11.5 | 66:34 | 0 | NR | 70 | 7.7±2.2 |
| 65.8±10.8 | 66:34 | 41 | NR | 67 | 8.6±2.8 | ||
| Liedberg et al., Sweden | Prosp, 222 controls 188 LLA | NR | 39:61 | 28 | 40 (18) | 6.8 | NR |
| 77.3 | 53:47 | 53 | 32 (17) | 32.4 | NR | ||
| Mantey et al., UK | Prosp, 25 DFU-free, 26 with 3 DFU recurrence | 64.6±13.9 | 52:48 | 2 (8) | 13 (52) | 100 | 7.6±1.2 |
| 66.2±8.0 | 54:46 | 2 (7.7) | 14 (54) | 100 | 8.5±1.7 | ||
| Moss et al., USA | Prosp, N = 1890 watched for LLA | 46.2±11.7 | 47:53 | 343 (18.2) | 409 (21.6) | 100 | NR |
| Riaz et al., Pakistan | Cross-S 14388 controls, 3731 DFU | 51.3±11.9 | 51:49 | 2127 (14.8) | NR | 100 | 9.7±2.3 |
| 53.5±10.6 | 72:28 | 759 (20) | NR | 100 | 9.8±2.3 | ||
| Zaine et al., Australia | Retro, N = 202 all non DFU | 78 | 54:46 | 35 | 72 (36) | 0 | NR |
| Zhong et al., China | Prosp, 439 controls, 57 DFU | Mean NR | 48:52 | 95 | 66 (15) | 100 | NR |
| 6 (10.5) | 16 (28) | 100 | NR |
M = male, F = female. Diab Hx = history of diabetes. Data are presented as mean ± standard deviation, with decimals rounded to 1 decimal place. NR = not reported. Cross-S = Cross-sectional. Retro = retrospective. Prosp = prospective. DFD = Diabetic foot disease. DFU = Diabetic foot ulcer. LLA = Lower limb amputation.
Table 1: Characteristics of the study groups.
Populations evaluated were as follows:
(i) Al-Rubeaan et al. included those with DFU, gangrene, or LLA, and a control group with diabetes but no foot disease [21]
(ii) Five studies included those with DFU, and control groups with diabetes but no DFU [1, 24, 28, 29, 33]
(iii) Two studies only included participants with DFU [23, 27]
(iv) Kokkinidis et al. included people who underwent lower limb endovascular treatment with a case group of current smokers, and a control group of non-smokers [25]
(v) Liedberg et al. included those who had undergone a LLA, and a control group who had not [30]
(vi) Mantey et al. examined those with diabetes who had at least two recurrences of DFU within 2 years, and a control group with previous ulceration who remained DFU free for at least 2 years [31]
(vii) Anderson et al. included participants with diabetes who had undergone a LLA, with a smoking compared to a non-smoking group [22]
(viii) Moss et al. looked at those with diabetes who had undergone LLA; one group who were diagnosed with diabetes before the age of 30 and the other group after the age of 30 [32]
(ix) Zaine et al. included participants with non-diabetes related foot ulcers [26]
Overall results
Results are summarised in Table 2, presented alphabetically by first author.
| Author | Exclusion criteria | Smoking results | Other results | Limitations |
|---|---|---|---|---|
| Al- Rubeaan et al. | <25 years old | Smoking commoner in DFD (10.1% smokers vs 6.7%), DFU and gangrene (p = <0.0001) | DFD assoc DM duration (p = <0.0001). ↑HbA1c and micro-vasc Cx ↑in DFD. ↑ DFD in males, 68.6% (p = <0.0001) | Primary DM clinics not foot ulcer clinics |
| Anderson et al. | Death <3 months of FU | Smokers ↑ LLA and higher amputation level (p = 0.031). Of 11 BKA, 10 smokers | NR | PAD, neuropathy, diabetes duration, HbA1c and age NR. No tests to assess smoking cessation |
| Bajaj et al. | Previous LLA or non-DM related foot ulceration | 67% with NIU and 17% with neuropathic ulcers active smokers. Smoking assoc with NIU (p <0.0001) | DFU assoc with poor glycaemia. NIU assoc with DM duration (p = 0.015) and HT (p =0.0008) vs neuropathic ulcers | Only compared neuropathic to NIU. Small sample size. Other DFU risk factors NR. Duration of ulcer NR |
| Boyko et al. | Current DFU, not able to walk, too ill, unable to consent | 19% of those who developed DFU were smokers vs 24% who did not develop an ulcer (p = 0.46) | ↑ DM duration, insulin-treatment, poor vision, ↑ HbA1c, claudication, past DFU, LLA and foot oedema assoc with DFU (all p = <0.01) | Mostly older, male and T2D. Change in Cx or HbA1c NR. High loss-to-FU |
| Engberg et al. | No prior or current ulceration | Smoking sig risk factor for recurrence or new DFU (univariate analysis) | Critical ischaemia older, ↑DM duration, ↓DiaBP, ↓HbA1c and ↑ creatinine. Ischaemic DFU ↑past LLAs. Smokers and men ↑risk of DFU. Past NIU + HbA1c ≥7.6% ↑ risk of DFU | Information on orthopaedic surgery, re-vascularisation, compliance with footwear and offloading NR |
| Heald et al. | Previous history of DFU. No diabetes | No differences between cases vs controls for smoking (p = 0.42) | DFU older, higher HbA1c and creatinine (p < 0.0001). Peripheral neuropathy and absent foot pulses more common in DFU (p < 0.0001) | Other macro- or microvasc Cx NR. Footwear or offloading NR |
| Ikem et al. | NR | ↑ smoking DFU (p = 0.044). ABI <0.9 correlated with tobacco use (p= 0.044) | DFU assoc DM duration, tobacco use and SysBP. Claudication assoc age, DM duration and neuropathy. PVD in 41.9% with DFU vs 13.4% | Reported outcomes in relation to PVD not overall foot ulceration or LLA |
| Kokkinidis et al. | Acute ischaemia or IC | Incomplete healing at 6 months 91.1% smokers vs 66%. At 9 months 74.8% in smokers vs 54% | Not studied | Loss to FU was high |
| Liedberg et al. | NR | Smokers with LLA but no diabetes. Increased smoking assoc with lower age at LLA (p < 0.001) | Those with diabetes more likely to undergo amputation (p = 0.001) | Results reported unclearly. Length of diabetes and other risk factors assoc with diabetes NR |
| Mantey et al. | Medial wall sclerosis | No sig differences between groups | Glycaemia poorer, and macrovasc Cx and alcohol consumption sig more common with recurrent ulceration | Detailed results for risk factors NR. Small sample size |
| Moss et al. | NR | In younger onset patients pack- years also assoc with LLA | LLA ↑males, with past DFU, ↑retinopathy and ↑HbA1c. LLA in young group assoc with ↑ DiaBP, and ↓daily aspirin | Did not define ex-smokers. Loss to follow-up rate NR |
| Riaz et al. | NR | Smoking sig assoc with foot ulcer (p < 0.0001) | DFU were older, male, had ↑ DM duration and more HT (p < 0.0001). ↑ HbA1c and creatinine in DFU | Not dedicated foot ulcer clinics |
| Zaine et al. | DM, or ulcer healed at initial visit | 53% were smokers or ex-smokers | Hypertension present in 54.5% and hyperlipidaemia in 39.1% of participants | P value of risk factors NR. Single clinic. New diabetes not tested for |
| Zhong et al. | NR | Ex-smokers 19.5% occurrence of DFU, versus 11% non-smokers and 5.9% current smokers | DFU ↑ risk other DM Cx (p < 0.0001). DFU assoc with cracked skin, calluses, and onychomycosis (all p < 0.05) | Did not define/report when ex-smokers ceased |
Assoc = associated. BKA = Below knee amputation. Cx = complications. DFD = Diabetic foot disease. DFU = Diabetic foot ulcer. DiaBP = diastolic blood pressure. DM = diabetes mellitus, FU = follow-up. IC = Intermittent claudication. LLA = Lower limb amputation. Macrovasc = macrovascular. NDFU = non-diabetic foot ulcer. NIU = neuroischaemic ulcers. NR = not reported. SysBP = systolic blood pressure. Vasc = vascular. Yoa = Years of age. ↑ = increased. ↓ = decreased.
Table 2: Overview of study design and results.
Al-Rubeaan et al. reported that rates of smoking were significantly higher in those with DFD at 10.1%, compared to 6.7% in those unaffected by DFD. Participants who smoked were more likely to have DFU or gangrene when compared to the non-smoking cohort (p = <0.0001) [21]. Another study reported that the smoking rate was higher in those with DFU compared to the control group with diabetes (p = 0.044), and an ABI <0.9 correlated with tobacco use (p = 0.044) [29].
Smoking was found to be a significant risk factor for a recurrent or new DFU in 2 studies (p < 0.0001) [1, 23]. Not all studies found that result; in the study by Boyko et al., 24% of those who remained ulcer free were smokers compared to 19% of smokers who developed a DFU, although this result was not significant (p = 0.46) [28].
In the study by Zaine et al. 53% were current or ex-smokers, whereas in the study by Zhong et al. DFU was higher among those who were ex-smokers when compared to current and non-smokers
Bajaj et al. specifically studied neuro-ischaemic and neuropathic ulceration and those with neuro-ischaemic ulceration had a high rate of smoking at 67%. This result was significant when compared to those with neuropathic ulceration (p = <0.0001) [27].
Healing rates: Surprisingly, only one study which assessed rates of healing in DFU and current smoking was identified. The authors studied 264 patients who all had endovascular interventions in the lower leg for chronic limb ischaemia. Of the 264 patients, 41(15.5%) were current smokers. In this study incomplete wound healing was high in both groups. However, it was further increased in current smokers at 6 months (91.1% versus 66% in non-smokers, p = 0.012) [25]. Incomplete wound healing at 9 months was also higher in smokers (74.8% compared to non-smokers 54%, p = 0.026).
Lower limb amputation: In the studies that assessed LLA, there was a significant association between smoking and the rates of amputation. Anderson et al. studied 112 participants who had LLA and they were very likely to be smokers (59% smokers) [22]. Of the 11 participants who underwent a below knee amputation, 10 were current smokers (p = 0.031).
Moss et al. reported an association between pack-years smoked in their younger participants and LLA [32], however they did not examine whether current smoking affected LLA. Another study found that increased smoking was associated with reduced age at LLA (p < 0.001) [30]. In contrast, Al-Rubeaan et al. reported that there was no significant difference between smokers and non-smokers and the rates of LLA [21]. However, in that study DFD was more common in smokers, and there was a 25% rate of smoking in those who underwent LLA versus 6.84% in the background population. It is possible that lack of statistical difference in LLA is due to small numbers for this outcome.
Two of the fourteen studies found no significant differences between the case and control groups for amputations [24, 31]. One is a large retrospective registry study of 49 GP-practices. The second is a small study (51 patients) comparing people who had attended a diabetes service and had relapsing DFU versus those whose ulcer did not relapse within the 2 year study period.
Absence of foot pulses was more common in those with DFU (p <0.0001), and it is well known that smoking leads to the development of PAD, the clinical findings of which are the absence of pedal pulses [8,24]
Discussion
Smoking is a major risk factor for the development and progression of cardiovascular disease and also peripheral arterial disease [34]. There is a significant effect of smoking on large artery function. Research has reported that tobacco smoking increased the pulse wave velocity suggesting an increase in arterial stiffness [35]. These investigators also suggested that smoking decreases elasticity in both large and medium sized arteries in acute and chronic smokers.
Acute smoking causes significant increases in systolic and diastolic blood pressure and heart rate which usually return to baseline within 15 minutes [35]. These effects are also seen in the blood pressure of the aorta following only 1 cigarette in chronic smokers and non-smokers. The greatest changes were seen in the first 5 minutes after a cigarette. These results suggest that acute smoking has important potential for effects on blood flow to the lower limbs.
In addition, acute smoking causes vasoconstriction which can further decrease blood flow and oxygen delivery [36, 37]. In healthy people, there was a 50% reduction in blood flow in finger circulation, and an over 10% reduction in people with diabetes [38].
Importantly, this impairment recovers when smoking is ceased [37]. This suggests that similar effects may be present in toes although we did not identify any studies in this area. If there was a 10-50% reduction in toe blood flow with smoking, that would be very likely to impair wound healing and may increase eventual amputation risk.
Among studies that review this area, Zhang et al. reported that there was a higher percentage of smokers with DFU (29.1%) vs a control group with diabetes (17.4%) [39]. Another meta-analysis reports that smoking history is an influencing factor in amputation rates in DFU patients [40]. Sayiner et al. also indicated that smoking had a negative effect in those with DFU with amputees having significantly longer histories of smoking (p < 0.001) [41].
Two other meta-analyses reported smoking history is a significant risk factor for LLA in those with DFU, one of these studies reporting that smokers had a 2 fold higher risk of amputation than non-smokers [42, 43].
A study by Markuson et al. reported a significant correlation between smoking history and increased HbA1c levels [44]. Other authors have also reported that smoking is associated with worsened glycaemia in diabetes [10, 11]. This increase in HbA1c with smoking may contribute to any effects of smoking on DFU healing as increased HbA1c is associated with delayed healing of ulcers.
Camilleri et al. studied the relationship between toe brachial indices and smoking history. The authors reported that the mean toe brachial index of non-smokers (0.781) was superior to the mean scores of past smokers (0.649) and current smokers (0.544). Low toe brachial indices are a reliable method to detect PAD, and predict delayed healing in foot ulceration [45]. Their study did not assess ulcer healing or amputation.
It is widely acknowledged that tobacco smoking is a major risk factor for development of PAD. The data in this study describe significant associations between smoking and risk of developing DFD, and DFU. In relation to the known association between smoking and PAD, a history of smoking is associated with increased risk of LLA.
However, probably because of the limited, heterogeneous studies, we conclude that an association between smoking and poor healing or DFU is likely but not proven. The data shows that a history of smoking is associated with risk of LLA, as expected.
However, whether current smoking provides a further increase in risk is not conclusively proven. This, and data on wound healing, if proven to be worsened by current smoking, would be important for the counselling of patients with current DFD / DFU.
In summary, tobacco smoking further increases the risks of DFD and DFU in people with diabetes. Data on examining the effects of current smoking on wound healing and LLA are scanty, and further research in this area is desirable. However, given the strong metaanalysis data showing that smoking impairs healing of surgical wounds [46], we recommend that all DFD / DFU patients should be counselled to stop smoking. Data to answer the question of whether current smoking impairs healing of DFU or increases LLA may facilitate smoking-cessation interventions in people with foot ulcers.
Conclusion
The link between tobacco smoking and PAD is widely known. However, it is not yet clear whether current smokers are at greater risk of incidence of delayed healing or LLA when they develop DFU.
For future research, studies comparing patients with foot ulcers according to current, past (including when smoking ceased) and neversmoking should be undertaken to determine whether current smoking affects healing or LLA. This has potentially important implications for people with foot ulcers.
Funding
This work was supported by the Westmead Charitable Trust.
Conflicts of Interest
There are no conflicts of interest to declare.
References
- Riaz M, Miyan Z, Zaidi S, Alvi S, Fawwad A, et al. (2016) Characteristics of a large cohort of patients with diabetes having at-risk feet and outcomes in patients with foot ulceration referred to a tertiary care diabetes unit. Int Wound J 13: 594-599.
- Nigel U (2008) The diabetic foot in the developing world. Diabetes Metab Res Rev 24: S31-S3.
- Ghanassia E, Villon L, Thuan dit Dieudonné JF, Boegner C, Avignon A, et al. (2008) Long-term outcome and disability of diabetic patients hospitalized for diabetic foot ulcers: a 6.5-year follow-up study. Diabetes Care 31: 1288-92.
- Boyko E, Ahroni J, Stensel V, Forsberg R, Davignon D, et al. (1999) A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care 22: 1036-1042.
- Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L et al. (2014) Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA 311: 183-192.
- AIHW (2021) Tobacco smoking. Canberra.
- Clair C, Meigs J, Rigotti N (2013) Smoking behaviour among US adults with diabetes or impaired fasting glucose. Am J Med 126: 541.e15-541.e18.
- Xia N, Morteza A, Yang F, Cao H, Wang A (2019) Review of the role of cigarette smoking in diabetic foot. J Diabetes Investig 10: 202-215.
- Akter S, Goto A, Mizoue T (2017) Smoking and the risk of type 2 diabetes in Japan: A systematic review and meta-analysis. J Epidemiol 27: 553-561.
- Gunton J, Davies L, Wilmshurst E, Fulcher G, McElduff A (2002) Cigarette smoking affects glycemic control in diabetes. Diabetes Care 25: 796-797.
- Nilsson P, Gudbjörnsdottir S, Eliasson B, Cederholm J (2004) Smoking is associated with increased HbA1c values and microalbuminuria in patients with diabetes--data from the National Diabetes Register in Sweden. Diabetes Metab 30: 261-268.
- Smith K, Kapoor R, Felts P (1999) Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 9: 69-92.
- Tesfaye S, Chaturvedi N, Eaton S, Ward J, Manes C et al. (2005) Vascular risk factors and diabetic neuropathy. N Engl J Med 352: 341-350.
- Facchini FS, Hollenbeck CB, Jeppesen J, Ida Chen YD, Reaven G (1992) Insulin resistance and cigarette smoking. Lancet 339: 1128-1130.
- Agarwal S (2009) The association of active and passive smoking with peripheral arterial disease: results from NHANES 1999-2004. Angiology 60: 335-345.
- Ruangsetakit C, Chinsakchai K, Mahawongkajit P, Wongwanit C, Mutirangura P (2010) Transcutaneous oxygen tension: a useful predictor of ulcer healing in critical limb ischaemia. J Wound Care 19: 202-206.
- Norgren L, Hiatt W, Dormandy J, Nehler M, Harris K, et al. (2007) Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg 33: S1-75.
- Willigendeal EM, Teijink JA, Bartelink ML, Kuiken B, Boiten W, et al. (2004) Influence of smoking on incidence and prevalence of peripheral arterial disease. J Vasc Surg 40: 1158-1165.
- Allison M, Criqui M, McClelland R, Scott J, McDermott M et al. (2006) The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol 48: 1190-1197.
- Agarwal S., 'The association of active and passive smoking with peripheral arterial disease: results from NHANES 1999-2004', Angiology, 60 (2009).
- Al-Rubeaan K, Al Derwish M, Ouizi S, Youssef A, Subhani S et al. (2015) Diabetic foot complications and their risk factors from a large retrospective cohort stydney. PLoS One 10: e0124446.
- Anderson J, Boone J, Hansen M, Spencer L, Fowler Z (2012) A comparison of diabetic smokers and non-smokers who undergo lower extremity amputation: a retrospective review of 112 patients. Diabet Foot Ankle 3.
- Engberg S, Kirketerp-Moller K, Ullits Anderson H, Rasmussen A (2019) Incidence and predictors of recurrent and other new diabetic foot ulcers: a retrospective cohort study. Diabet Med 36: 1417-1423.
- Heald A, Lunt M, Rutter M, Anderson S, Cortes G et al. (2019) Developing a foot ulcer risk model: what is needed to do this in a real-world primary care setting? Diabet Med 36: 1412-1416.
- Kokkinidis D, Giannopoulos S, Haider M, Jordan T, Sarkar A et al. (2020) Active smoking is associated with higher rates of incomplete wound healing after endovascular treatment of critical limb ischemia. Vasc Med 25: 427-435.
- Zaine N, Hitos K, Vicaretti M, Fletcher J, Begg L et al. (2016) Characteristics of non-diabetic foot ulcers in Western Sydney, Australia. J Foot Ankle Res 9.
- Bajaj S, Mahajan A, Grover S, Mahajan V, Goyal P, et al. (2017) Peripheral vascular disease in patients with diabetic foot ulcers - an emerging trend: a prospective study from North India. J Assoc Physicians India 65: 14-17.
- Boyko E, Ahroni J, Cohen V, Nelson K, Heagerty P (2006) Prediction of diabetic foot ulcer occurrence using commonly available clinical information: the Seattle Diabetic Foot Study. Diabetes Care 29: 1202-1207.
- Ikem R, Ikem I, Adebayo O, Soyoye D (2010) An assessment of peripheral vascular disease in patients with diabetic foot ulcer. Foot (Edinb) 20: 114-117.
- Liedberg E, Persson B (1983) Age, diabetes and smoking in lower limb amputation for arterial occlusive disease. Acta Orthop Scand 54: 383-388.
- Mantey I, Foster A, Spencer S, Edmonds M (1999) Why do foot ulcers recur in diabetic patients? Diabet Med 16: 245-249.
- Moss S, Klein R, Klein B (1999) The 14-year incidence of lower-extremity amputations in a diabetic population. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care 22: 951-959.
- Zhong A, Li G, Wang D, Sun Y, Zou X, et al. (2017) The risks and external effects of diabetic foot ulcer on diabetic patients: A hospital-based survey in Wuhan area, China. Wound Repair Regen pp: 858-863.
- Tapp R, Shaw J, de Courten M, Dunstan D, Welborn T, et al. (2003) Foot complications in type 2 diabetes: an Australian population-based study. Diabet Med 20: 115-113.
- Mahmud A, Feely J (2003) Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension 41: 183-187.
- Jensen JA, Goodson WH, Hopf HW, Hunt TK (1991) Cigarette smoking decreases tissue oxygen. Arch Surg 126: 1131-1134.
- van Adrichem L, Hovius S, van Strik R, van der Meulen J (1992) Acute effects of cigarette smoking on microcirculation of the thumb. Br J Plast Surg 45: 9-11.
- Acevedo A, Schnell A (1975) Effect of cigarette smoking upon the finger circulation in normal and diabetic subjects. Basic Res Cardiol 70: 350-353.
- Zhang P, Lu J, Jing Y, Tang S, Zhu D, et al. (2017) Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med 49: 106-116.
- Lin C, Liu J, Sun H (2020) Risk factors for lower extremity amputation in patients with diabetic foot ulcers: a meta-analysis. PLoS One 15.
- Sayiner Z, Can F, Akarsu E (2019) 'Patients' clinical charecteristics and predictors for diabetic foot amputation. Prim Care Diab 13: 247-251.
- Sen P, Demirdal T, Emir B (2019) Meta-analysis of risk factors for amputation in diabetic foot infections. Diabetes Metab Res Rev 35.
- Kaminski M, Raspovic A, McMahon L, Strippoli G, Palmer S, et al. (2015) Risk factors for foot ulceration and lower extremity amputation in adults with end-stage renal disease on dialysis: a systematic review and meta-analysis. Nephrol Dial Transplant 30: 1747-1766.
- Markuson M, Hanson D, Anderson J, Langemo D, Hunter S, et al. (2009) The relationship between hemoglobin A(1c) values and healing time for lower extremity ulcers in individuals with diabetes. Adv Skin Wound Care 22: 365-372.
- Camilleri T, Camilleri L, Midolo Y, Papanas N, Gatt A, et al. (2020) Empowering patients living with diabetes mellitus to cease smoking will improve lower limb perfusion. J Addict Dis 39: 1-7.
- Sørensen LT (2012) Wound Healing and Infection in Surgery: The Clinical Impact of Smoking and Smoking Cessation: A Systematic Review and Meta-analysis. Arch Surg 147: 373-383.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Bechara N, Gunton JE (2022) The Effects of Tobacco Smoking on Healing of Foot Ulcers and Lower Limb Amputation: A Systematic Review. Clin Res Foot Ankle, 10: 351. DOI: 10.4172/2329-910X.1000351
Copyright: © 2022 Bechara N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4885
- [From(publication date): 0-2022 - Dec 05, 2025]
- Breakdown by view type
- HTML page views: 4289
- PDF downloads: 596