The Effects of Different Culture Medium on the Growth of a Dinoflagellate (Cochlodinium polykrikoide) in Laboratory Condition
Received: 24-Jul-2018 / Accepted Date: 29-Oct-2018 / Published Date: 03-Nov-2018 DOI: 10.4172/2155-9910.1000263
Abstract
Cultivation of C. polykrikoides was carried out using different culture media including f/2 medium (without silicate), modified f/2 medium, Conway, TMRL and ESM in the Iranian Shrimp Research Center in 2013. C. polykrikoides was cultured in test tubes, 100 ml, 250 ml, 500 ml Erlenmeyer flasks and in a 16 l aquarium, using 32 ppt seawater (27 ± 2°C) and with light intensity of 5000 lux, under cool-white fluorescent illuminations, and in a 12:12 h light-dark cycle. The best result was obtained using modified f/2 medium followed by TMRL medium. With ESM, cultivation was continued, in 100 ml Erlenmeyer flasks but it was not possible in more than 100 ml container. C. polykrikoides cultivation was unsuccessful with the f/2 medium and Conway medium even in the test tube. The results of the study showed that mass cultivation of C. polykrikoides was possible with modified f/2 medium even in an aquarium, but did not have favorable results with TMRL medium in the aquarium.
Keywords: C. polykrikoides ; Culture medium; Cell density; Laboratory condition
Introduction
Of more than 5000 species of marine planktons, only 80 species are able to produce strong poison [1]. Cochlodinium polykrikoides is a dinoflagellate species and its bloom in coastal areas is associated with fish kills in many parts of the world [2-4]. This dinoflagellate is a mixotrophic algae [5,6] with 30-40 μm long and 20-30 μm width [7]. They multiply through binary fission and contain pigments creating what is known as the red tide [7]. It has also been reported that, in the natural condition and on sunny days, C. polykrikoides migrate to deeper area to adjust the intensity of the light received [8] and this algae can produce H2O2 and O2 - which could be one of the reason for fish mortality [4]. In areas where C. polykrikoides occur, fish are suffocated as the result of oxygen depletion [9]. The ichthyotoxic properties of C. polykrikoides blooms have also been described by authors [4,10-12]. Pacific oyster larvae, Crassostrea gigas undergo metamorphosis during C. polykrikoides bloom [13]. Mortality increase of American oyster larvae, Crassostrea virginica during exposures to Cochlodinium has been reported [14]. The first report of C. polykrikoides induced red tide in Iran (Persian gulf) was made in 2007 [15]. Richlen et al. [16] reported long term C. polykrikoides bloom in the Persian Gulf and Oman Sea that caused high fish mortality, affecting fishing activity and damaging coral reefs. In 2008, C. polykrikoides bloom lasted 8 months which resulted in the loss of 34 tons of aquatic organisms [1]. Nevertheless, there has been little research concerning the causes and impacts of C. polykrikoides blooms in the Persian Gulf. Dinoflagellate blooming has been linked to the washing of cobalamin (vitamin B1) from the soil and its entry into the sea [17]. It is also reported that, the increase in sunlight intensity and nutrients contributed to excessive C. polykrikoides bloom [18]. C. polykrikoides has been found in cold and hot water and in salinity of 30-34 ppt [19].
Since ecological factors are changing continuously, it is necessary to find out the underlying causes of blooms and the impacts on ecosystems. Thus, close examinations and laboratory studies may be needed to unravel some of the reasons underlying such blooms. Therefore, it is important to have intensive culture of C. polykrikoides under laboratory condition. Cultivation of C. polykrikoides under laboratory conditions was reported using modified f/2 medium (f/2-Si) [20] and f/2 medium (without silicate) [21,22]. The effects of temperature, salinity and irradiation on C. polykrikoides growth under laboratory condition and using modified f/2 medium (without silicate) have been investigated [23]. Also, the ESM medium has been used for the cultivation of this alga under laboratory condition [24]. The purpose of this study was to determine the proper conditions for the mass cultivation of C. polykrikoides in laboratory condition.
Material and Methods
Water samples were prepared from coastal waters of the Persian Gulf (salinity 39 ± 2.82 ppt, pH=7.95 ± 0.35) along Hormozgan province where C. polykrikoides bloom occurred. The samples were then, passed through 70 μ plankton net and the materials left, were immediately transported to the laboratory where samples were transferred to 1 litter beakers, 3/4 of which were covered with black paper and placed under two fluorescent lamps. Light attraction was used [25] in order to separate, and the C. polykrikoides attracted to the light were separated by sterile micropipette and transferred to the test tube containing modified f/2 medium without silicate [26]. This was repeated several times for more purification. Five culture media were used for the experiments, including f/2 medium without silicate (includes CoCl2.6H2O, CuSO4.5H2O, FeCl3.6H2O, Na2MoO4.2H2O, MnCl2.4H2O, ZnSO4.7H2O, Na2EDTA, NaNO3, NaH2PO4. H2O, Biotin and Thiamine.HCl) [27], Conway (includes KNO3, NaH2PO4, FeCl3, MnCl2.4H2O, H3BO3, viramines B1 and B12 ) TMRL (includes KNO3, NaH2PO4, FeCl3 and SiO2Na), ESM (includes NaNO3, KH2PO4, vitamins B1, B12 and Biotin, EDTA-Fe2+, EDTA-Mn2+ and Tris Aminomethane) [6] and modified f/2 medium (includes NaNO3, NaH2PO4.2H2O, Na3 EDTA, Fe EDTA, Tris, EDTA, MnCl2.4H2O, CoCl2.6H2O, ZnCl2, H2SeO3, Ampicilin, Kanamycin and Neomycin) [26] without silicate [15]. Culture was done in sterile test tubes (100 × 16 mm), each containing 10 ml of modified f/2 medium, 100, 250, 500 ml Erlenmeyer flasks and in a 16 liter aquarium (each containing 10 l of culture medium). The research was carried out, with 10 treatments and 3 replications in each treatment. The cultures were inoculated with 50 cells/ml [23]. To prepare the culture medium, sea water (salinity 40 ± 2 ppt) was first entered to the sedimentary basin and then passed through a sandy filter. In the next step, water was passed through a 0.45 micron filter. To obtain the required salinity (32 ppt), water was diluted with distilled fresh water. Then they were placed in an autoclave (Iran Tab Zaeem-87419) for 15 minutes at a temperature of 121°C. All the tubes and flasks were covered with aluminum foil. Cultures were performed in controlled laboratory conditions, with 27 ± 2°C and light intensity of 5000 lux, under cool-white fluorescent illuminations, on a 12:12 h light–dark cycle. C. polykrikoides cells were counted using a Sedgwick-Rafter counting chamber, under optical microscope.
This experiment was conducted based on a completely randomized design. All statistical analyses were done using SPSS statistical package (version 16.0). One-way analysis of variance (ANOVA) and Duncan’s multiple comparison tests were used to identify significant variations at 0.95 confidence limits (P<0.05).
Results
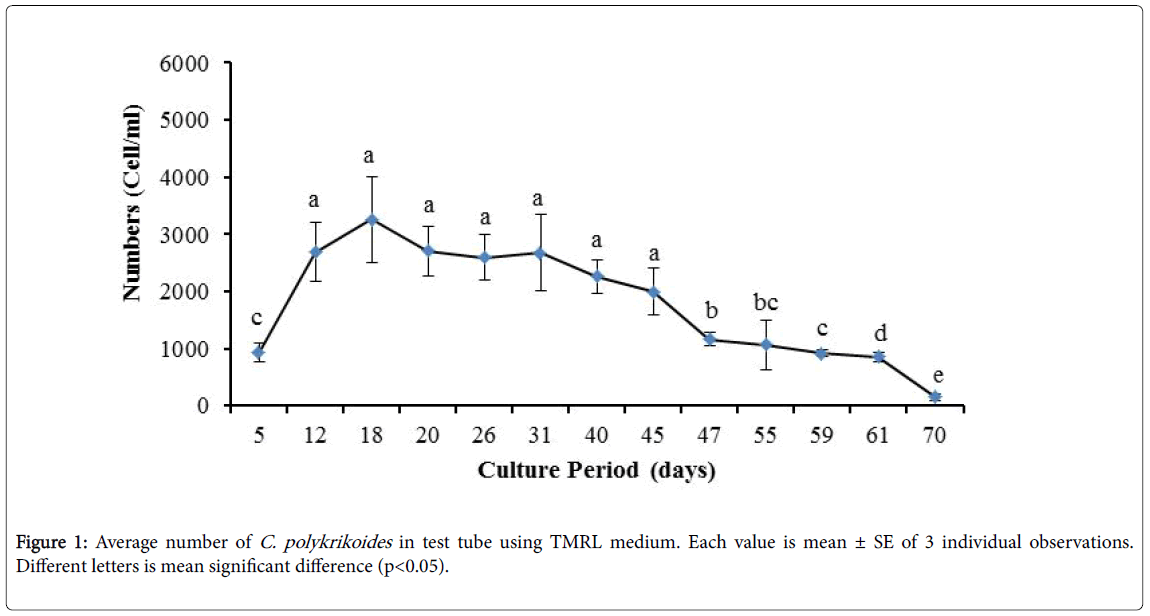
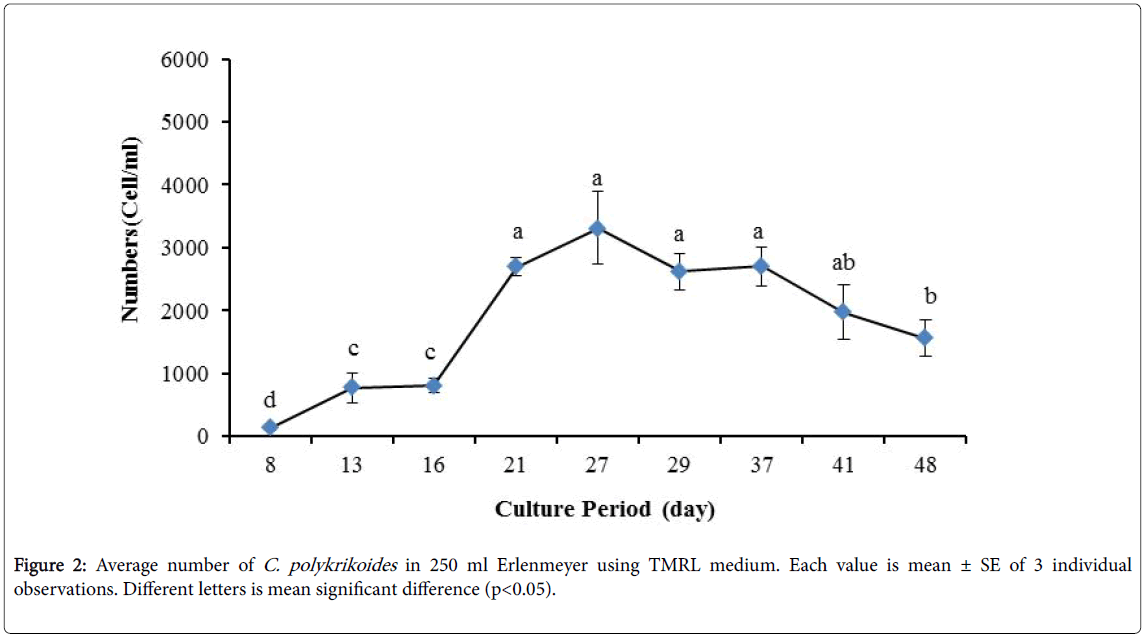
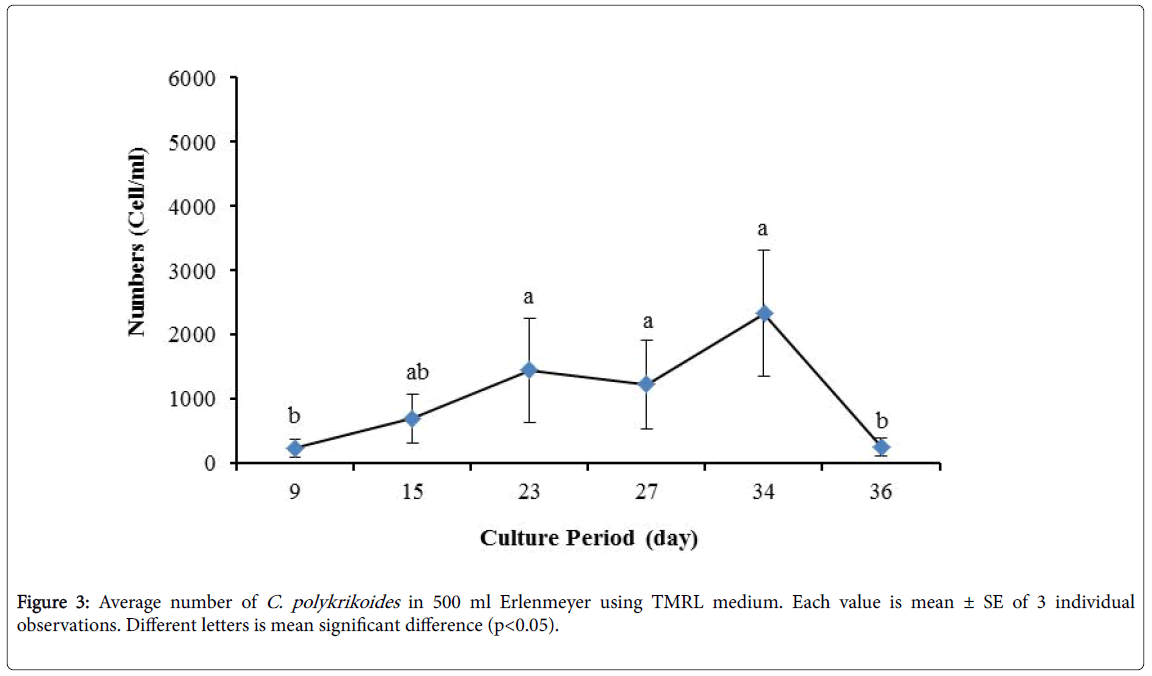
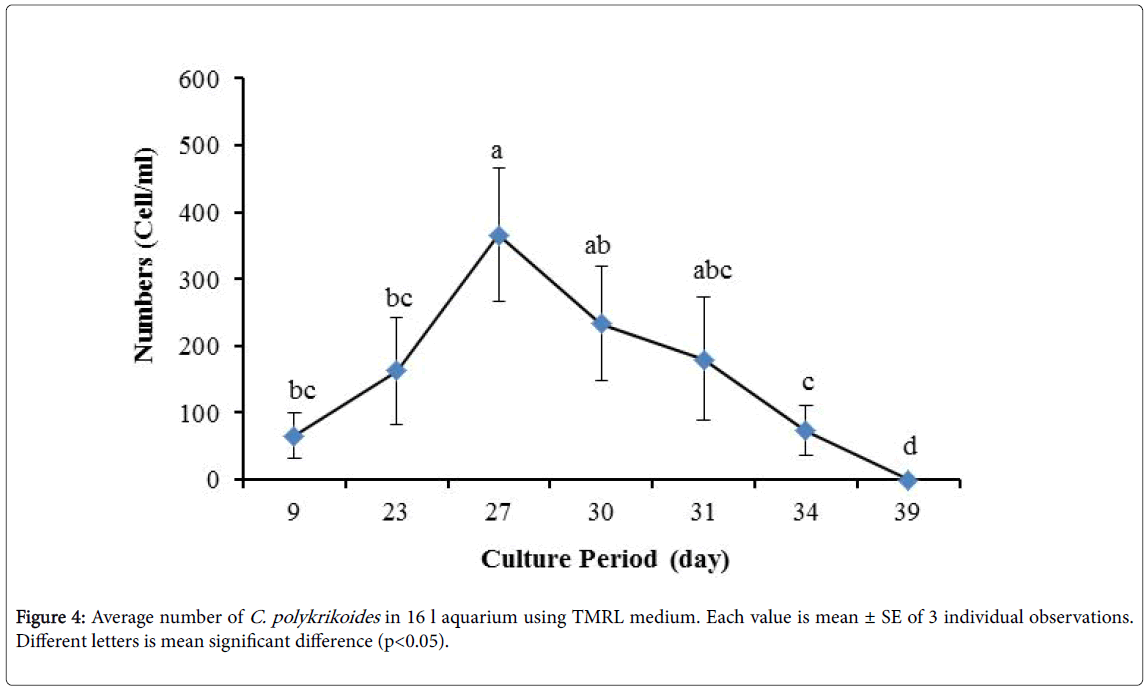
No results were obtained for cultivation of C. polykrikoides , using the Conway and f/2 medium. With ESM medium, C polykrikoides culture was, to some extent successful in the test tubes and 100 ml Erlenmeyer. The result showed that, these mediums were not suitable for C. polykrikoides culture. By the use of TMRL, the highest cell density was obtained in test tubes and 250 ml Erlenmeyer flask, and cell density reduced in 500 ml Erlenmeyer flask and in aquarium. The average cell densities in test tubes, 100 ml, 250 ml and 500 ml Erlenmeyer flasks and in aquariums are presented in Figures 1 to 4 respectively. C. polykrikoides possessed the highest density in test tube and 250 ml Erlenmeyer. But the maximum density in the test tube was occurred earlier (Figures 1 and 2). In the 500 ml Erlenmeyer, the number of cells was lower than that of test tube and 250 ml Erlenmeyer, and the maximum density was occurred later (Figure 3). Cells density in the aquarium was lower than the test tube and Erlenmeyers, but the maximum density of C. polykrikoides was occurred earlier than 500 ml Erlenmeyer (Figure 4). Also, number of C. polykrikoides in the test tube, 250 and 500 ml Erlenmeyer and aquarium reached to 3260, 3310, 2326 and 366 cells/ml after 18, 27, 34 and 27 days respectively (Figures 1-4).
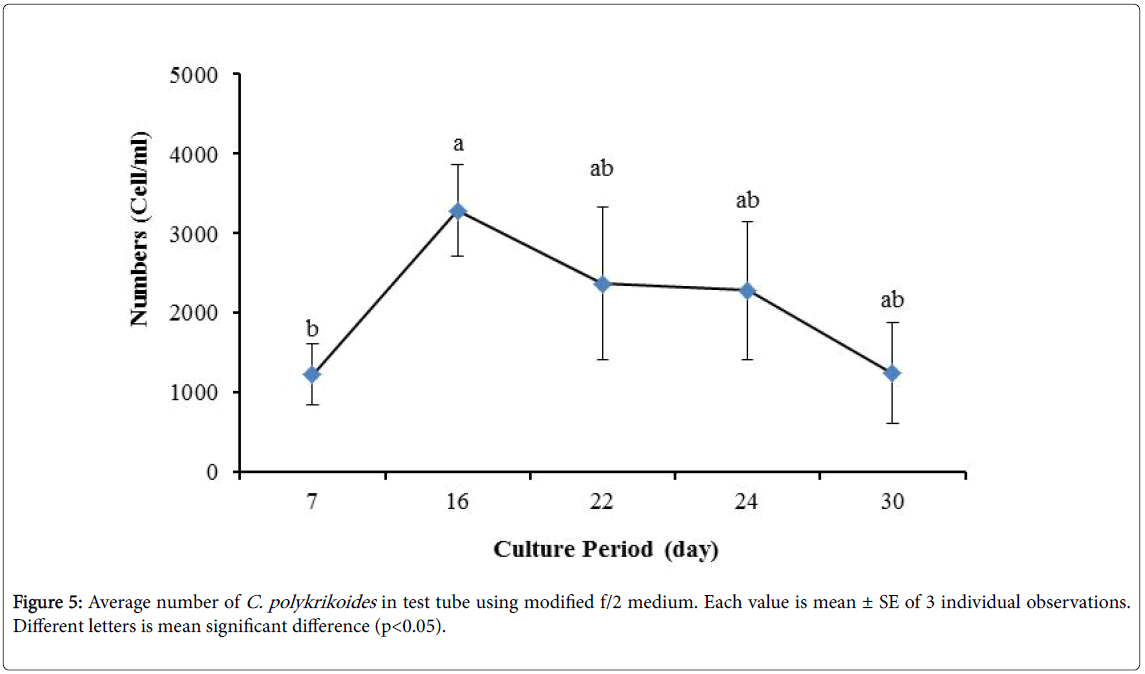
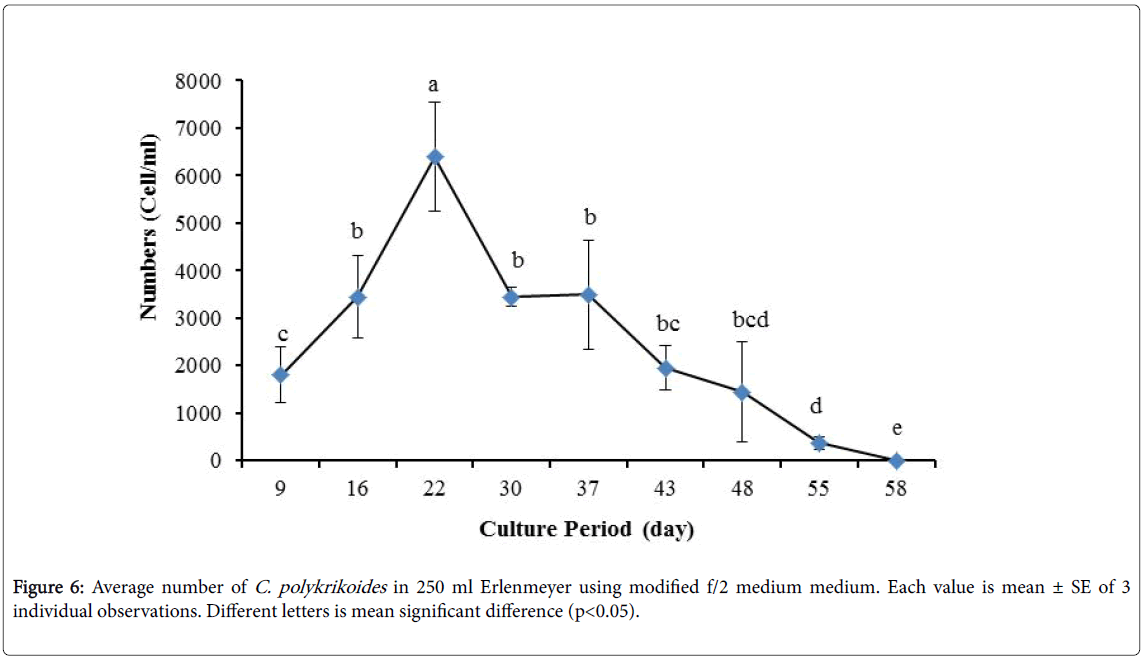
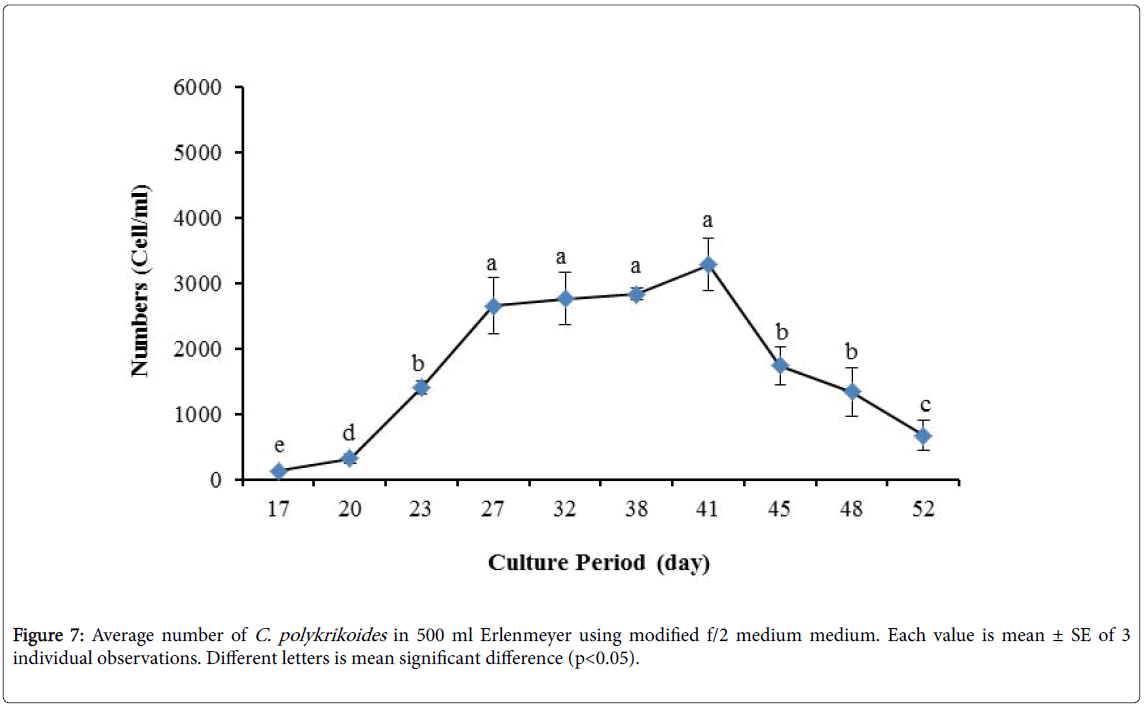
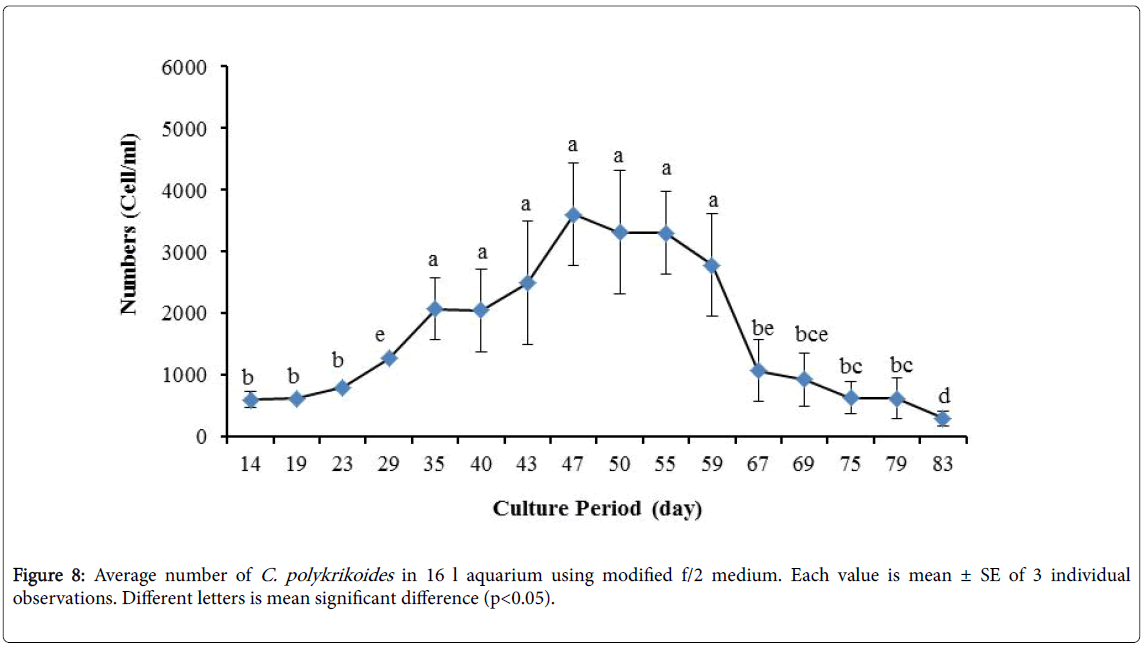
By the use of modified f/2 medium, Cells density in 250 ml Erlenmeyer was significantly more than that test tube, 500 ml Erlenmeyer and aquarium (Figure 5-8). But the maximum density of C. polykrikoides in the test tube was occurred earlier. C. polykrikoides density in the aquarium was higher than test tube and 500 ml Erlenmeyer, but maximum density of C. polykrikoides was occurred later. The maximum C. polykrikoides density obtained through the use of modified f/2 medium in the test tube, 250 ml and 500 ml Erlenmeyer and aquarium were 3283, 6393, 3283 and 3600 cells/ml after 16, 22, 41 and 47 days respectively (Figures 5-8).
Discussion
In the present study, no bloom occurred using f/2 medium (f/2-Si) and this was in agreement with that components of the f/2 medium (N, P, Fe, Mn, Co, Cu, Zn, Mo, B12, biotin, thiamine) did not seem to trigger the occurrence of C. polykrikoides blooms [28]. In the modified f/2 medium, different antibiotics (Ampicilin, Kanamycinand and Neomycin) were used to reduce the activity of a wide range of bacteria. This factor, along with the addition of other nutrients to the modified f/2 medium, created suitable condition for C. polykrikoides blooms. As mentioned earlier, the cultivation of these algae requires special condition, which only supply with a limited number of culture medium. In this regard, in an investigation, C. polykrikoides was cultivated, using the f/2 medium (without silicate) and surface seawater taken between the South Sea of Korea and Jejudo [20]. Since C. polykrikoides could not be cultured using coastal seawater of South Sea of Korea, except during the bloom period [20]. Therefore, as noted in the present research, the role of water compounds in the bloom of C. polykrikoides is of great importance. The area where water is used, may affect the growth of algae [20]. Also, reported that, the cultures of C. polykrikoides , were inoculated with modified f/2 medium [20]. The use of this medium for the massive cultivation of C. polykrikoides is consistent with the present study. Therefore, it can be expected that, in sea waters, the bloom of these algae occurs during the special conditions. In the present study, the maximum algae density reached by using modified f/2 medium was 6393 cells/ml after 22 days in 250 ml Erlenmeyer. But, it was in 250 ml Erlenmeyer after 27 days by using TMRL (3310 cells ml-1).
Due to the suitability of the first culture medium, the maximum number of algae was obtained in less time. In this phase, algae use the nutrients for multiplication. Therefore, as shown in Figures 1 to 8, the total duration of growth phases (included Lag phase, exponential phase, Stationary phase and Decline phase) was longer than TMRL by using modified f/2 medium (except test tube). In a study concerning C. polykrikoides culture in f/2 medium, the total Lag phase, exponential and stationary phases, lasted 22 days [28]. In the mentioned study, the Maximum C. polykrikoides density in the exponential phase was 1500 cell/ml and the culture vessels were 50 ml sterilized polystyrene flasks, containing 20 ml of f/2 medium. In the present study, the minimum and maximum duration were between 31 to 45 days for TMRL medium (Figures 1-4) and 24 to 59 days (Figures 5-8) for modified f/2 medium.
It has been reported that, under laboratory conditions and in the sea, C. polykrikoides forms chain at progressive growth [29]. In the present study, cells were formed in chain consisting of 2 to 8 cells. Maximum C. polykrikoides density, created in laboratory condition was 850 cells/ml [30]. Also, high densities of this algae, 8 × 106 cells/ml [31], 48 × 103 [23] and 6 × 106 cells/ml have been reported [1]. In the present study, the highest density of this algae was 5 × 104 cells/ml at it’s accumulation site in the aquarium (with modified f/2 medium). In relation to the effect of C. polykrikoides cell density on fish mortality, it is reported that, fish survived in water containing less than 1 × 103 cells/ml of C. polykrikoides [10].
The results of the present study showed that modified f/2 medium is the best medium for mass culture of C. polykrikoides followed by TMRL medium in laboratory condition.
Acknowledgement
This project was funded by Iranian Shrimp Research Center and the authors wish to thank the cooperation of Iranian Shrimp Research Center and Persian Gulf and Oman Sea Ecology Research Center and assistance of and other colleagues who helped us with our research.
References
- Attaran-Fariman G, Sharifian S (2014) Abundance and distribution of phytoplankton species with potential of harmful blooming in Southest of Iran. Oceanograp Scient Res J 18: 1-10.
- Azanza RV, David LT, Borja RT, Baula IU, Fukuyo Y (2008) An extensive Cochlodinium bloom along the western coast of Palawan, Philippines. Harmful Algae. 7: 324-330.
- Mulholland MR, Morse RE, Boneillo GE, Bernhardt W, Filippino KC, et al. (2009) Understanding causes and impacts of the dinoflagellate, Cochlodinium polykrikoides, blooms in the Chesapeake Bay. Estuaries and Coasts 32: 734-747.
- Whyte JNCI, Haigh N, Ginther NG, Keddy LJ (2001) First record of blooms of Cochlodinium sp (Gymnodiniales, Dinophyceae) causing mortality to aquacultured salmon on the west coast of Canada. Phycologia 40: 298-304.
- Larsen J, Sournia A (1991) Diversity of heterotrophic dinoflagellates. In The biology of free-living heterotrophic dinoflagellates. Patterson DJ, Larsen J (editors). Oxford: Clarendon, pp 313-332.
- Mitra A, Banerjee K, Gangopadhyay A (2006) Introduction to marine plankton. Daya Publishing House. Delhi. 102.
- Fukuyo Y, Takano H, Chihara M, Matsuoka K (1990) Red Tide Organisms in Japan. An illustrated taxonomic guide. Uchida Rokakuho, Co, Ltd, Tokyo. 407.
- Kim YS, Jeong CS, Seong GT, Han IS, Lee S (2010) Diurnal vertical migration of C. polykrikoides during the red tide in Korean coastal sea waters. 31: 687-693.
- Vincente HJ, Ruth D, Gaid RD, Henry E, Dejarme HE, Roa EC, Azanza RV (2002) Harmful algal bloom in Iligan bay, southern Philippines. Sci Diliman J 14: 59-65.
- Gobler CJ, Berry DL, Anderson OR, Burson A, Koch F, et al. (2008) Characterization, dynamics, and ecological impacts of harmful Cochlodinium polykrikoides blooms on eastern Long Island, NY, USA. J Harmful Algae 7: 293-307.
- Kim HG (1998) Cochlodinium polykrikoides blooms in Korean coastal waters and their mitigation. In: Reguera B, Blanco J, Fernandez ML, Wyatt T (editors). Galicia Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO 227-228.
- Kim CS, Lee SG, Kim HG, Jung J (1999) Reactive oxygen species as causative agents in the ichthyotoxicity of the red tide dinoflagellate Cochlodinium polykrikoides. J Plankton Res 21: 2105-2115.
- Matsuyama Y, Usuki H, Uchida T, Kotani Y (2001) Effects of harmful algae on the early planktonic larvae of the oyster Crassostreagigas. In Proceedings of the Ninth International Conference on Harmful Algal Blooms. Hallegraeff GM, Blackburn SI, Bolch CJ, Lewis RJ (editors). Paris: IOC of UNESCO 411-414.
- Ho MS, Zubkoff PL (1979) The effects of Cochlodinium heterolobatum bloom on the survival and calcium uptake by larvae of the American oyster Crassostrea virginica. In: Toxic Dinoflagellate Blooms. Proc. 2nd Int Conf pp.409-412.
- Abdolalian E, Rohani-Ghadikolaie K, Moezi M, Foroghifard H, Akbarzadeh G (2013) Determination of effective parameters on growth and bloom forming of Cochlodinium polykrikoides. Iranian J Fisheries Sci 21: 31-40.
- Richlen ML, Morton SL, Jamali EA, Rajan A, Anderson DM (2010) The catastrophic 2008-2009 red tide in the Persian Gulf region, with observation on the identification and phylogeny of the fish-killing dinoflagellate Cochlodinium polykricoides. J Harmful Algae 9: 163-172.
- Bilgrami KS, Saha LC (2004) A textbook of algae. CBS Publishers and distributors. New Delhi. India. 260.
- Lee YS (2006) Factors affecting outbreaks of high density Cochlodinium polykrikoides red tides in the coastal seawaters around Yeosu and Tongyeong, Korea. Mar Pollut Bull 52: 1249-1259.
- Kudela RM, Ryan JP, Blakely MD, Lane JQ, Peterson TD (2008) Linking the physiology and ecology of Cochlodinium to better understand harmful algal bloom events: A comparative approach. J Harmful Algae 7: 278-292.
- Lee YS (2008) Utilization of various nitrogen, phosphorus, and selenium compounds by Cochlodinium polykricoides. J Environ Biol 29: 799-804.
- Sun XX, Lee YJ, Choi JK, Kim EK (2004) Synergistic effect of sophorolipid and loess combination in harmful algal blooms mitigation. Marine Pollut Bullet 48: 863-872.
- Honjo T, Imada N, AnrakuY, Kim DI, Muramatsu M, et al. (2002) Removal of harmful red tide plankton by ozone treatment. The Xth International Conference on Harmful Algae. Florida.
- Kim DI, Matsuyama Y, Nagasoe S, Yamaguchi M, Yoon YH, Oshima Y, et al. (2004) Effects of temperature, salinity and irradiance on the growth of the harmful red tide dinoflagellate Cochlodinium polykricoides Margalef (Dinophyceae). J Plancton Res 26: 61-66.
- Kim D, Yamasaki Y, Yamatogi T, Yamaguchi K, Matsuyama Y, et al. (2009) The possibility of reactive oxygen species (ROS)-independent toxic effects of Cochlodinium polykrikoides on Damselfish (Chromis caerulea). Bioscie Biotechnol Biochem 73: 613-618.
- Diwan AD, Joseph S, Ayyappan S (2009) Physiology of reproduction, breeding and culture of tiger shrimp (Penaeus monodon). Naredera Publishing House. Delhi (India). 292.
- Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (editors). Cultures of marine invertebrate animals. Plenum Press: New York, USA. pp 29-60.
- Ojha JS (2006) Aquaculture nutrition and biochemistry. Agrotech Publishing Academy. Udaipur. 186.
- Lee YS, Kim JD, Lim WA, Lee SG (2009) Survival and growth of Cochlodinium polykrikoides red tide after addition of yellow loess. J Environmen Biol 30: 929-932.
- Cho ES, Costa E (2004) Rapid monitoring for the potentially ichthyotoxic dinoflagellate Cochlodinium polykrikoides in Korean coastal waters using fluorescent probe tools. J Plankton Res 26: 175-180.
- Bok TH, Paeng DG, Kim E, Na J, Kang D (2010) Ultrasound backscattered power from Cochlodinium polykrikoides, the main red tide species in the southern sea of Korea. J Plankton Res 32: 503-514.
- Ga´rate-Liza´rraga L, Bustillos-Guzma´n JJ, Morquecho L, Lechuga-De´veze C (2000) First outbreak of Cochlodinium polykrikoides in the Gulf of California. Harmful Algae News, Intergovernmental Oceanographic Commission of UNESCO, 21: 7.
Citation: Vaghei RG, Niamaimandi N, Abdolalian E, Moezzi M (2018) The Effects of Different Culture Medium on the Growth of a Dinoflagellate (Cochlodinium polykrikoide) in Laboratory Condition. J Marine Sci Res Dev 8: 263. DOI: 10.4172/2155-9910.1000263
Copyright: © 2018 Vaghei RG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4082
- [From(publication date): 0-2018 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 3216
- PDF downloads: 866