The Effects of COVID-19 on Some Liver Enzymes Patients at Al Furat General Hospital in Baghdad
Received: 08-Mar-2021 / Accepted Date: 20-Mar-2021 / Published Date: 27-Mar-2021 DOI: 10.4172/2168-9652.1000291
Abstract
Coronavirus has been recognized in October, 2019, but the first infection with the virus in Iraq was identified in the province of Najaf, about 160 km south of Baghdad, on February 24, 2020 and aim of the research Knowing the extent of coronavirus effect on liver function, especially liver enzymes, which play a vital role in the vital processes in the human body 60 samples were collected at Al-Furat General Hospital from patients with COVID-19, of which 38 samples were for men, and 22 samples were for women; the blood was separated to extract serum by the centrifuge, then the results were obtained by an Automatic Biochemistry Analyzer device with Accent-200 and (GPT, ALP, TSB, GOT enzymes) were identified for comparisons between control and patients Through taking a section, analyses were performed for 60 patients, average age was 46, and ages were between 23-75 years. The values of the statistical analyses were clarified when compared to healthy people: the nature of the relationship where it was shown that there is a negative relationship in the amounts of GOT, GPT, ALP Proven by sig, which is higher than 0.05 and research shows that Coronavirus has actual effects on liver function, and the majority of patients have high GOT, GPT ALP due to a clear impact of COVID-19 on the liver enzymes.
Keywords: COVID-19; Virus; Patients; GOT enzymes; Liver enzymes
Introduction
Quality of Coronavirus was recognized in 2019, and the first case was discovered in China in the Wuhan region. When reading the direct impact of these viruses, a significant impact on the work of the liver was found [1]. The occurrence of functional difference and not only this but there was an effect on the clinical characteristics of patients with COVID -19 [2]. This clinical effect was not recognized to this day, and dealing with it has become something vague and not clear-cut and through multiple studies [3] and then proving that liver disease is present in abundance in people with the disease. If we examine it further, we’ll find that SARS-Cov2 is similar in the genetic chain to SARS-Cov. Studies related to this subject published in 2004 show that 60% of people with the disease face a risk of liver damage [4]. The symptoms of COVID19 were not limited only to the liver, but the effects included all parts of the body COVID-19 patients, including the lungs, heart, and kidneys [5]. So that it can damage the intestine and formation Infections in the heart, and this was evidenced by analyzing the urine [6]. This indicates that there was an early presence of kidney damage. Liger said that initial data also shows that 14 to 30% of ICU patients lose kidney function and need dialysis or continuous treatment [7]. The disease can also harm the heart, as doctors in China and New York reported inflammation Heart muscle and arrhythmias, which can lead to cardiac arrest in patients with COVID-19 .Its effect extends to the digestive system, causing disruption in liver function and an increase in its enzymes. It also poses a high risk to liver patients to join the list of groups most vulnerable to severe complications from the virus [8]. As for cirrhosis, which occurs, or its impact on people who already or previously have liver diseases, including functional disorders caused by the disease [9]. Clear evidence of attacking it directly on the part, or it may consist of these functional disorders or infections in the liver as a result of the use of medications such as antagonists Vitality and increase the risk to people who already have a chronic disease or have liver damage in this way will lead to complications depending on the type of patient the liver and as for people who suffer from a fatty liver disease where most patients suffer from heart disease and blood pressure which makes them more vulnerable to health problems when infection with the virus [10]. The symptoms of Coronavirus contribute to high levels of the liver and that 35% of patients have had a rise in the levels of liver enzymes and found the effect of coronavirus on the liver as form where the patient who suffers from high levels of enzymes and increased activity in a virus B will be attacked by Coronavirus. In this situation, it is violent for the patient due to significant weakening of the immune system [11]. As for the patients who have liver transplantation, they do not suffer from any of the many problems when infected with the virus as a result of using anti-immunosuppressive drugs [12].
Materials and Methods
Clinical in Vitro Diagnostic Reagent Kit
• GPT For in vitro diagnostics use R1: 1 x 30 mL R2: 1 x 8 ML.
• GOT For in vitro diagnostics use R1: 1x30 mL R2: 1x8 ML.
• ALP For in vitro diagnostics use R1: 1x30 mL R2: 1x8 ML.
• TSB For in vitro diagnostics use R1: 1x24 mL R2: 1x5 ML
TSB For in vitro diagnostics use R1: 1x24 mL R2: 1x5 ML
TSB = 10 μl serum.
ALP = 4 μl serum
GPT = 15 μl serum
GOT = 10 μl serum
Separation: The device separates the blood components to obtain serum and shown in Figures 1-3 device centrifuge. In these Tables 1-3 that are performed in the results between patients with high levels in some liver enzymes (GPT, TSB, GOT, ALP) and the control group of COVID-19 we notice that the percentage increases by 33% in patients with COVID-19 and the highest rate achieved by 108 if compare enzymes in elevated liver levels [13]. The results also showed that a 5% rate indicates a severe reduction in enzyme levels for patients with COVID-19 patients [14]. In general, we note that In the case of the COVID-19, enzyme levels increase by 15% to 45%, and liver infections are significantly more common among patients [14,15].
| p | AGE | GANDER | TSB (1.70-21) μmol/L | GPT (5-41) U/L | GOT (8-40) U/L | ALP (56-119)U/L |
|---|---|---|---|---|---|---|
| p1 | 64 | M | 9.23 | 26 | 43 | 147 |
| p2 | 62 | F | 14.29 | 26 | 37 | 87 |
| p3 | 48 | M | 12.7 | 48 | 68 | 45 |
| p4 | 46 | M | 12 | 41 | 48 | 65 |
| p5 | 31 | M | 10.61 | 70 | 33 | 80 |
| p6 | 30 | M | 13.5 | 59 | 38 | 60 |
| p7 | 42 | M | 12 | 40 | 27 | 66 |
| p8 | 47 | M | 8 | 35 | 30 | 123 |
| p9 | 44 | M | 12 | 42 | 30 | 48 |
| p10 | 31 | F | 9.82 | 27 | 28 | 60 |
| p11 | 35 | M | 12 | 29 | 34 | 37 |
| p12 | 34 | M | 10 | 33 | 28 | 86 |
| p13 | 35 | M | 8 | 49 | 32 | 73 |
| p14 | 25 | F | 11.27 | 12 | 17 | 49 |
| p15 | 53 | F | 6.13 | 26 | 35 | 73 |
| p16 | 25 | M | 14.81 | 0 | 21 | 48 |
| p17 | 48 | F | 7.64 | 22 | 38 | 141 |
| p18 | 23 | M | 6.11 | 32 | 20 | 87 |
| p19 | 70 | M | 14.07 | 66 | 34 | 128 |
| p20 | 48 | M | 12 | 96 | 68 | 58 |
| p21 | 60 | M | 14.1 | 65 | 56 | 75 |
| p22 | 75 | M | 6.61 | 19 | 50 | 99 |
| p23 | 42 | F | 14.3 | 55 | 45 | 56 |
| p24 | 36 | F | 6.18 | 9 | 20 | 47 |
| p25 | 52 | M | 6.67 | 17 | 21 | 44 |
| p26 | 60 | M | 9.71 | 35 | 38 | 70 |
| p27 | 75 | F | 23 | 36 | 27 | 59 |
| p28 | 51 | M | 12 | 30 | 27 | 66 |
| p29 | 35 | M | 10 | 108 | 7.9 | 45 |
| p30 | 31 | M | 10 | 33 | 37 | 106 |
| p31 | 38 | M | 8.6 | 33 | 30 | 80 |
| p32 | 18 | F | 6 | 10 | 16 | 44 |
| p33 | 35 | M | 14 | 66 | 37 | 70 |
| p34 | 33 | M | 12 | 47 | 27 | 66 |
| p35 | 19 | F | 7.74 | 15 | 24 | 55 |
| p36 | 30 | F | 8.26 | 16 | 31 | 244 |
| p37 | 40 | M | 11.2 | 10 | 39 | 246 |
| p38 | 29 | M | 13.8 | 18 | 22 | 71 |
| p39 | 30 | M | 6.22 | 53 | 35 | 88 |
| p40 | 40 | M | 12 | 13 | 21 | 88 |
| p41 | 25 | M | 19.5 | 29 | 29 | 52 |
| p42 | 40 | M | 12.3 | 60 | 31 | 75 |
| p43 | 50 | M | 34.8 | 15 | 24 | 86 |
| p44 | 44 | M | 13.9 | 36 | 24 | 64 |
| p45 | 27 | M | 27 | 35 | 25 | 56 |
| p46 | 35 | F | 12.5 | 21 | 22 | 76 |
| p47 | 50 | F | 17.6 | 13 | 22 | 72 |
| p48 | 30 | F | 9.4 | 9 | 22 | 70 |
| p49 | 16 | F | 4.6 | 11 | 20 | 114 |
| p50 | 54 | M | 12 | 67 | 47 | 113 |
| p51 | 29 | M | 15.3 | 41 | 35 | 57 |
| p52 | 25 | M | 10 | 14 | 26 | 67 |
| p53 | 45 | F | 7.6 | 29 | 35 | 124 |
| p54 | 39 | F | 12.9 | 11 | 28 | 58 |
| p55 | 49 | F | 11.3 | 10 | 20 | 111 |
| p56 | 33 | F | 9 | 19 | 23 | 65 |
| p57 | 29 | F | 6.1 | 13 | 21 | 111 |
| p58 | 50 | F | 22 | 18 | 38 | 61 |
| p59 | 70 | F | 19.5 | 48 | 57 | 49 |
| p60 | 34 | M | 10 | 35 | 41 | 74 |
Table 1: Results of patients COVID-19.
| p | AGE | TSB (1.70-21) μmol/L | GPT (5-41) U/L | GOT (8-40) U/L | ALP (56-119) U/L |
|---|---|---|---|---|---|
| p1 | 40 | 7.3 | 28 | 11.1 | 73 |
| p2 | 55 | 10.6 | 25.2 | 9.9 | 70 |
| p3 | 70 | 7.9 | 40.2 | 14.7 | 88 |
| p4 | 66 | 12 | 33 | 11.6 | 90 |
| p5 | 67 | 15 | 37 | 21.5 | 59 |
| p6 | 66 | 20 | 48 | 40 | 110 |
| p7 | 45 | 12 | 33.3 | 31.4 | 118 |
| p8 | 54 | 14 | 41.3 | 20.1 | 60 |
| p9 | 77 | 11.7 | 33 | 16.4 | 67 |
| p10 | 83 | 10 | 32 | 18 | 64 |
| p11 | 44 | 12.1 | 19 | 22 | 77 |
| p12 | 23 | 8.5 | 34 | 27.5 | 87.4 |
| p13 | 13 | 18.9 | 27.9 | 22 | 66.9 |
| p14 | 28 | 17.3 | 29 | 31 | 59.9 |
| p15 | 32 | 11 | 19 | 11 | 60.4 |
| p16 | 68 | 9 | 9.8 | 16 | 67 |
| p17 | 45 | 14 | 34.2 | 22.6 | 80 |
| p18 | 44 | 6.7 | 23 | 9.9 | 88.7 |
| p19 | 43 | 10 | 12 | 22.9 | 73.4 |
| p20 | 40 | 11 | 21.5 | 33 | 70.7 |
Table 2: Results of healthy people.
| Correlations | ||||
| GOT Healthy | GOT Co19 | |||
| Spearman's rho | GOT healthy | Correlation | 1 | -0.216 |
| Sig. (2-tailed) | 0.406 | |||
| 20 | 20 | |||
| GOT CO-19 | Correlation | -.216 | 1 | |
| Sig. (2-tailed) | 0.406 | |||
| N | 20 | 60 | ||
Table 3a: Correlations between control and COVID-19 patients in GOT.
| Correlations | |||
| GPT healthy | GPT CO-19 | ||
| GPT healthy | Spearman's rho Correlation | 1 | 0.184 |
| Sig. (2-tailed) | 0.436 | ||
| N | 20 | 20 | |
| GPT CO-19 | Spearman's rho Correlation | 0.184 | 1 |
| Sig. (2-tailed) | 0.436 | ||
| N | 20 | 60 | |
Table 3b: Correlations between control and COVID-19 patients in GPT enzymes.
| Correlations | |||
| ALP healthy | ALP CO-19 | ||
| ALP healthy | Spearman's rho Correlation | 1 | -0.096 |
| Sig. (2-tailed) | 0.689 | ||
| N | 20 | 20 | |
| ALP CO-19 | Spearman's rho Correlation | -.096 | 1 |
| Sig. (2-tailed) | 0.689 | ||
| N | 20 | 60 | |
Table 3c: Correlations between control and COVID-19 patients in ALP enzymes.
Discussion
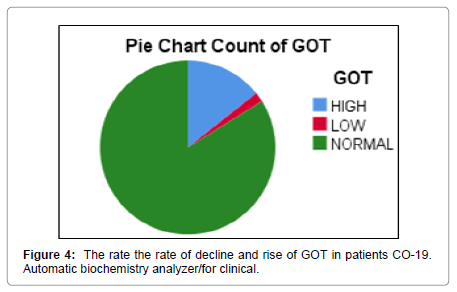
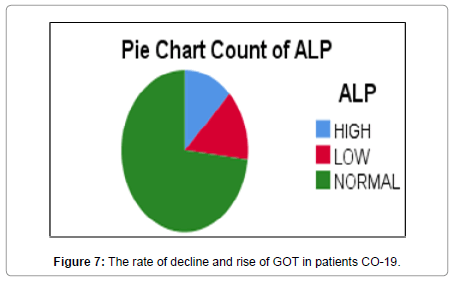
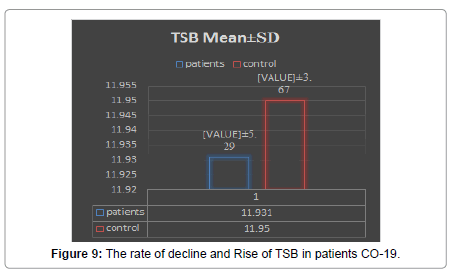
In this research, values were found through TSB, GPT, GOT, ALP analyzes and evaluation of the necessary analysis to the affected person COVID-19 Patients, After that, healthy people were compared, where clinical results were demonstrated by the presence of symptoms appearing on the patient in the beginning [16,17]. As the current results showed that there are significant differences between persons infected with COVID 19 Patients and healthy people and that there is no direct effect to the following analyzes GPT, GOT, ALP, and TSB on the person’s age according to what was shown to us in the outcome. Still, it is difficult to neglect this effect [18]. Relationship it is nonexistent, but it is present, and its existence cannot be denied by TSB analysis (Figures 4-15). Most of the results were normal, and this is what corresponds to it in healthy people, but a percentage was found that represented one value for a person aged 50 years, and the rate was (31.8) [19,20].
Correlation for GOT between COVID - 19
Patients and healthy people: Testing the relationship between GOT between COVID 19 Patients with healthy people noted the Correlation Coefficient is negative and this shows an inverse relationship between GOT between COVID 19 Patients with healthy people and that the significance or (p-value) Sig. (2-tailed) was equal to0.406 and when compared to the level of significance (0.05) we notice that it is smaller than the level of significance and this means that no relationship.
Correlation for GPT between COVID - 19
Patients and healthy people: Through statistical analysis Test the relationship between GPT healthy people with GPT CO-19 Note this Correlation coefficient is negative and this shows an inverse relationship between Test for the importance of (p-value) (Two-way) It was equal to 0.436 and when compared to the importance level (0.05) We note that it is greater than the level of significance and this means that there is not relationship between GPT healthy people with GPT CO-19 patients.
Correlation for ALP between CO- 19 and healthy people
Statistical analysis demonstrated an inverse relationship between ALP between CO- 19and healthy People by taking 60 samples from patients and 20 from healthy, and the statistical indication of the quality of this relationship showed that it was (P-value) 0.689, i.e. it is greater than 0.05 We note that it is greater than the level of significance and this means that there is not relationship between ALP healthy people with ALP CO-19 patients [20-26].
Conclusion
The relationship between GOT, GPT, and ALP analysis was found between infected and healthy people As for TSB, the significant difference between the two groups was not. The effects of GOT on infected people caused major damage to the liver and were among the main factors leading to liver damage about with COVID-19 Patients. According to the results that proved that age was not one of the main factors, but rather a contributing factor to the increase in poor health for the patient if he suffers from other diseases. If a person who suffers from COVID-19 is healthy from other diseases and contains immunity here, age does not become a contributing factor.
References
- Hui DS, Azhar EI, Madani TA, Ntoumi F, Kock R, et al. (2020) The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Inte J Infe Dise 91: 264-266.
- Gorbalenya AE, Baker SC, Baric R, Groot RJD, Drosten C, et al. (2020). Severe acute respiratory syndrome-related coronavirus: The species and its viruses–a statement of the Coronavirus Study Group.
- Chen N, Zhou M, Dong X, Qu, J, Gong F, et al. (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet 395: 507-13.
- Hughes JM, Wilson ME, Luby SP, Gurley ES, Hossain MJ, et al. (2009). Transmission of human infection with Nipah virus. Clin Infe Dise 49: 1743-748.
- Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, et al. (2020). Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann Intern Med 173: 2000-2003.
- Tang N, Li D, Wang X. Sun Z (2020). Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Throm Haemo 18: 844-47.
- Chen G, Wu D, Guo W, Cao Y, Huang D, et al. (2020). Clinical and immunological features of severe and moderate coronavirus disease 2019. The J Clini Inves 130: 2620-2629.
- Chen N, Zhou M, Dong X, Qu J, Gong F, et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lan 395: 507-513.
- Diao B, Wang C, Tan Y, Chen X, Liu Y, et al. (2020). Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front in Immun 11: 827.
- Ianiro G, Mullish BH, Kelly CR, Sokol H, Kassam Z, et al. (2020). Screening of faecal microbiota transplant donors during the COVID-19 outbreak: suggestions for urgent updates from an international expert panel. The Lan Gastro & Hep 5: 430-432.
- Bermingham A, Chand MA, Brown CS, Aarons E, Tong C, et al. (2012). Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euros 17: 20290.
- Geller C, Varbanov M, Duval RE (2012) Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viru 4: 3044-3068.
- Calderwood AH, Day LW, Muthusamy VR, Collins J, Hambrick RD, et al. (2018). ASGE guideline for infection control during GI endoscopy. Gastro Endo 87: 1167-1179.
- Xiao F, Tang M, Zheng X, Liu Y, Li X, et al. (2020) Evidence for gastrointestinal infection of SARS-CoV-2. Gastro 158: 1831-1833.
- Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GV, et al. (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The J of Patho: A J Patho Socie 203: 631-637.
- Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, et al. (2004). SARSâ€associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepa 39: 302-310.
- Su L, Ma X, Yu H, Zhang Z, Bian P (2020). The different clinical characteristics of corona virus disease cases between children and their families in China–the character of children with COVID-19. Emer Micro & Infec 9: 707-713.
- Kramer A, Schwebke I, Kampf G (2006). How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infec Dises 6: 130.
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lan 395: 497-506.
- Riley S, Fraser C, Donnelly CA, Ghani AC, Abu-Raddad LJ, et al. (2003) Transmission dynamics of the etiological agent of SARS in Hong Kong: impact of public health interventions. Scien 300: 1961-1966.
- Yi Y, Lagniton PN, Ye S, Li E, Xu RH, et al. (2020) COVID-19: what has been learned and to be learned about the novel coronavirus disease. Inter J Bio Scien 16: 1753.
- Van Deursen VM, Damman K, van der Meer P, Wijkstra PJ, Luijckx GJ, et al. (2014) Co-morbidities in heart failure. Hear Fail Revie 19: 163-172.
- Zhang C, Shi L, Wang FS (2020) Liver injury in COVID-19: management and challenges. The Lan Gastro & Hepato 5: 428-430.
- Cowling BJ, Leung GM (2020) Epidemiological research priorities for public health control of the ongoing global novel coronavirus (2019-nCoV) outbreak. Eurosu 25: 2000110.
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, et al. (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lanc 395: 1033.
- Monteleone G, Sarzi-Puttini PC, Ardizzone S (2020) Preventing COVID-19- induced pneumonia with anticytokine therapy. The Lan Rheuma 2: 255-256.
Citation: Shanshool MT, Shanshool ET, Al Ghuraibawi DFF (2021) The Effects of COVID-19 on Some Liver Enzymes Patients at Al Furat General Hospital in Baghdad. Biochem Physiol 10: 290. DOI: 10.4172/2168-9652.1000291
Copyright: © 2021 Shanshool MT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2511
- [From(publication date): 0-2021 - Dec 05, 2025]
- Breakdown by view type
- HTML page views: 1681
- PDF downloads: 830