Research Article Open Access
The Effects of Carbon and Gold Ion Implanted Surfaces on Neuronal Stem Cells' Functions
Emel Sokullu1*, Taner Dağcı2, Oğuz Gözen2, Fulya Ersoy3and Ahmet Öztarhan41Department of Engineering Sciences, Division of Bioengineering, Izmir Katip Celebi University, Izmir, Turkey
2Department of Physiology, School of Medicine; Center for Brain Research, Ege University, Izmir, Turkey
3Department of Biomedical Technologies, Izmir Katip Celebi University, Izmir, Turkey
4Department of Bioengineering, Ege University, Izmir, Turkey
- *Corresponding Author:
- Mr. Emel Sokullu
asistant professor, IKCU, Engineering Sciences-Bioengineering Division
Cigli ana yerleskesi, Izmir, Cigli 35620, Turkey
Tel: +905332300582
E-mail: emelsu@gmail.com
Received date: November 28, 2016; Accepted date: January 27, 2017; Published date: February 03, 2017
Citation: Sokullu E, Dağcı T, Gözen O, Ersoy F, Öztarhan A (2017) The Effects of Carbon and Gold Ion Implanted Surfaces on Neuronal Stem Cells’ Functions. J Biotechnol Biomater 7:253. doi:10.4172/2155-952X.1000253
Copyright: © 2017 Sokullu E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Biomaterials have been used in medicine for decades to improve the functions of tissues and organs. They are also used as prosthesis and implants which are designed to substitute functions of a lacking organ or tissue. Carbon (C) and Gold (Au) were particularly chosen due to their biocompatibility and applied as implants for decades. Carbon and gold were great ion sources for medical applications, as well. In this study, polystyrene dishes were modified using gold and carbon ions via ion implantation technique. Using this surface modification method, it was aimed to improve surface characteristics and achieve a bioactive surface for neural stem cells. Even though the integration of stem cells was promising, neural stem cell studies still have many milestones to reach. Neuro-regeneration was the most desired function for people who suffer from neural system diseases. Changing surface characteristics of scaffolds was a way to promote regeneration and ion implantation was one of the methods to modify surface properties which play a huge role in enhancing the proliferation and integration of cells. In this study, it was observed that the ion implantation stimulated the neural proliferation and the implantation of different ions on cell culture surfaces was essential to determine the effects of this technique on adhesion, proliferation, differentiation and apoptosis properties of cells in details.
Keywords
Ion implantation; Carbon; Gold; Stem cells; Nerve regeneration.
Introduction
There are two important phases for cell attachment; 1- Affinity phase which occurs very fast. Ionic forces between cells and material and Van der Walls forces which make short term physicochemical bonding have active roles during this phase. 2- Adhesion phase takes longer and makes a stronger attachment.
The adhesions between cultured cells and the material surface are called focal attachments or adhesion plaques. Focal attachments have 10-15 nm distance between the cell membrane and the material surface and they are tight connections. There are specific receptor proteins like integrin on the external surface of focal attachments. And the internal surface has actin filaments such as talin, paxillin, vinculin, tensin and proteins that provide interactions between membrane receptor proteins [1-3]. Many proteins in adhesion plaque such as integrin, cytoskeleton proteins, proteases, protein kinases, phosphatases take part in signal transferring by co-localizing with vinculin and talin [3]. Focal interactions occur in low motility in the cells and it is supported by extracellular matrix proteins like fibronectin or vitronectin in vitro. On the other hand, adhesion molecules are characterized via the interaction ability with a specific ligand. These ligands can either be present on the membrane of a neighbor cell or they can be an extracellular matrix protein. Selectins, immunoglobulin superfamily, cadherins and integrins are the four main groups of the adhesion proteins.
Almost all in vitro cell studies are performed onto a material which is either a scaffold or a petri dish. It is a fact that unless cells attach to the material surface, no cell proliferation is to occur [3,4]. Hence, adhesion proteins which promote cell attachment play a huge role in cell proliferation and integration. Thus, the surfaces where cells are inoculated are treated [5,6] in order to stimulate the secretion of adhesion proteins.
Since 1960s Carbon (C) and since 1970s Gold (Au) has been used as implants. Many different cells are studied with gold and carbon implants, particularly osteoblastic ones [2,7-9]. On the other hand, neural cell studies are relatively at their early stages and mostly they are studied for peripheral neural therapies as tube and cuff forms [10-14]. In a study which was performed by Bieberich et al., C and Au electrodes are located in PC12 cell culture which is a model cell line for neural differentiation and it is reported that both synapsis formation and neural differentiation were observed on Au side [15]. When a defect occurs in the neural system, the healing process is quite limited and both individuals and the society are affected by these injuries. For years, studies have been focused on reducing the effects of neural injuries or complete recovery from them. One of the recent approaches for neural injury treatments is using embryonic, fetal or adult neural stem cells [16- 19]. The advantage of neural cells as grafts is providing a replacement of the neural loss with a new cell line. Neural cells accelerate the axon regeneration and complementary signalization pathways are provided. Recently, scientists have been studying on stem cells intensively to regain neural functions after injury. Even though stem cell injection to the injured region was promising for the recovery, stem cell studies still have many drawbacks both functionally and ethically.
This study has been performed to observe how the function and the behavior of neural stem cells on the carbon (C) and gold (Au) implanted materials change.
Materials and Methods
Study groups
In this study, two different cell lines at three different culture states and two different materials were used and the study was divided into seven study groups including controls (n=8) (Table 1).
Neuron and astrocyte cell lines
In this study, brain cortex was isolated from rat embryos at E12.5. The preparation of neuron-astrocyte stem cell mixture was described in detail previously [20]. These cells were grown in PNGMTM Primary Neuron Growth Medium (Lonza) and AGMTM Astrocyte Growth Medium (Lonza) at 37°C in 5% CO2 containing incubator.
MTT
2 × 104 cells/ml was suspended with 10% FCS solution and 100 μl of the solution was added into a 96-well plate. 100 μl benzene derived solution was added onto cell solutions with a concentration of 0,0002- 0,001-0,002-0,01-0,02-0,1 mg/ml. The 96-well plate was incubated at 37°C for 24 h or 48 h. After incubation, 20 μl MTT dye (5 mg/ml) was added into every well and incubated at 37°C for 2 hours. After this step, 200 μl DMSO was added to the well and incubated for 10 min to dissolve formazan salts which were synthesized by live cells. Color change in 96 well plates was examined using EL×808-IU Bio-Tek plate reader at a wavelength of 540 nm. Each concentration was repeated three times and viability of control group was assumed 100% [21].
Ion implantation
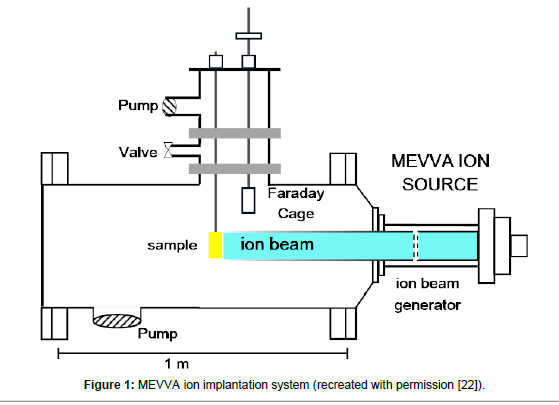
Polystyrene Petri dishes were picked as they were the most used in vitro surfaces for cell attachment. Carbon (C) and Gold (Au) were used as an ion source. C and Au ions were implanted at a dose of 1 × 1015 ions /cm2 and an ion energy of 20 keV using MEVVA ion source (Figure 1). Duty cycle was set to 10-2 and the repetitive rate was set 250 μs per 1-2 pulse. Total extracted ion beam flux typically reached to a couple of mA peak point. Average beam flux was around 1% deviation degree. Vacuum pressure was set to 10-5 torr during the implantation process. Implantation occurred at broad beam mode. The diameter of the ion beam which forms at extractor grids of the accelerator was 10 cm and the specimen was 60 cm away from the ion generator. The ions were accelerated from the grids and crushed the polystyrene surfaces which were located 60 cm far from the grids and penetrated into the polystyrene surface. The average ion energy was determined using average ion charge which measured via time of flight (TOF) [22].
Spectrophotometric measurement of RNA
The quality and quantity of isolated RNA were determined via spectrophotometric measurements at the wavelengths of 260 nm and 280 nm and optic density analysis. RNA quality was evaluated using the ratio of A260/A280. The integrity of RNA samples was evaluated by agarose gel electrophoresis. One OD260 corresponded to RNA concentration of 40 μg/ml and concentration of molecules were calculated with this formula:
Concentration (μg/ml)=A260 × Dilution Factor × Molecular Constant
RNA isolation
The phenol-chloroform extraction method was used for total RNA isolation. Cells were washed with PBS at 4°C and centrifuged at 1800 rpm for 5 min at 4°C. After removing the supernatant, 1 ml TRIZOL reactive was added and cells were slowly homogenized via pipetting and incubated for 5 min at room temperature. 200 μl chloroform was added into cell solution and incubated 3 min more before centrifugation for 15 min at 13000 rpm and 4°C. After centrifugation, the solution became three layers and the top layer which includes transparent RNAs was transferred into a microcentrifuge tube. 500 μl isopropanol was added and incubated 10 min on the ice. After incubation, RNA mixture is centrifuged for 10 min at 13000 rpm and 4°C. RNA pellets were visible in all tubes. The supernatant was removed and the pellet was washed twice with 500 μl 75% ethanol. After washing, ethanol was allowed to evaporate in room temperature and RNA pellet was dissolved in distilled water. All primer and probe sets used in this study were designed to span an exon-exon junction.
cDNA synthesis via reverse transcription RT
cDNA synthesis was performed using Roche Transcriptor First Strand cDNA Synthesis Kit the reaction details are given in Table 2. Negative transcriptase control reactions were also performed using RNA-free water as a sample. cDNA samples were stored at -20°C.
Quantitive real time PCR (RT-PCR)
Primer and probe sequences are shown in Table 3 and reaction ingredients were shown in Table 4. The RT-PCR binding temperature was 60°C for all genes. Specified gene sequences were synthesized according to IDTDNA (USA) company report. TaqMan probe (Roche Diagnostics, Germany) was used for reactions. The details of the RTPCR reaction were shown in Table 5. Gene expressions were analyzed via Roche Light Cycler 1.5 with 40 cycle reaction. Each sample was studied in duplicates. Comparative threshold cycle method (comparative threshold cycle=ΔΔCt) that was reported by Pfaffl [23] was used to assess targeted gene expressions. β-actin (ACTB) expression data was used as reference gene.
Scanning electron microscopy (SEM)
Ion implanted and unimplanted culture plates were seeded with cells. Cells were tamponed with 0.1 M sodium cacodylate and then incubated with 2.5% glutaraldehyde solution for 1 h on the ice. After this step, samples were washed with sodium cacodylate solution for 30 min and tamponed with 0.1 M sodium cacodylate and subjected to 2% osmium tetroxide as a post-fixation step. At the end of this process, samples were washed with DI water for 10 minutes twice and dehydrated in an ethanol series (35%, 70%, 85%, 95% and 100%, 5 min for each concentration). After dehydration, samples were located into hexamethyl disilazane solution for 30 min to dry out the samples. Once samples were dried, they were placed onto brass plates and observed with a scanning electron microscopy (SEM, Philips XL 30S-FEG) which was located in IYTE Materials Research Institute, Izmir, Turkey. SE detector was provided 3-dimensional topographic images while BSE detector was provided 2-dimensional images depending on the atomic contrast.
| Cells | Culture State | Culture Surface | |
|---|---|---|---|
| Control | Neuron-Astrocyte Stem cells | 7th, 14th, 28th Day | Polystyrene culture plate |
| Group I | Neuron-Astrocyte Stem cells | 7th Day | Carbon implanted polystyrene surface |
| Group II | Neuron-Astrocyte Stem cells | 14th Day | Carbon implanted polystyrene surface |
| Group III | Neuron-Astrocyte Stem cells | 28th Day | Carbon implanted polystyrene surface |
| Group IV | Neuron-Astrocyte Stem cells | 7th Day | Gold implanted polystyrene surface |
| Group V | Neuron-Astrocyte Stem cells | 14th Day | Gold implanted polystyrene surface |
| Group VI | Neuron-Astrocyte Stem cells | 28th Day | Gold implanted polystyrene surface |
Table 1: Study groups for tests.
| Component | Amount | Final concentration |
|---|---|---|
| RNA | 1 µg | 50 ng/µl |
| Oligo dT primer (10 µM) | 1 µl | 0.5 µM |
| dNTP mix (10 mM) | 2 µl | 1 mM |
| RNase inhibitor (20 u/µl) | 1 µl | 1 u/µl |
| RT tampon (5X) | 4 µl | 1X |
| dH2O | Add until solution reaches to 20 µl | |
Table 2: Reaction of cDNA synthesis.
| Accession Number | Gene | Oligos (5’à3’) | |
|---|---|---|---|
| NM_00102529 | myelin basic protein (MBP), transcript variant 1, mRNA | Forward Primer | CCTCTTAGAAAGAACTCATTC |
| Reverse Primer | GGACATTAGAAGACTGGAA | ||
| Probe | CTGTCGCTGAATCAAGTCGCTG | ||
| NM_017009 | glial fibrillary acidic protein (GFAP), mRNA | Forward Primer | GAAGCTCCAAGATGAAAC |
| Reverse Primer | CCTCAAGAACTGGATCTC | ||
| Probe | TCCAGCGACTCAACCTTCCTC | ||
| NM_139254 | tubulin, beta 3 class III (Tubb3), mRNA | Forward Primer | GACGCCAAGAACATGATG |
| Reverse Primer | CTCCACGAAGTAACTACTG | ||
| Probe | CATCTGCTCATCCACCTCCTTCA | ||
| NM_012987 | Nestin (Nes), mRNA | Forward Primer | GGTCTCTTGAAGATAGAAATG |
| Reverse Primer | CAGTGATTCATGGTTCTC | ||
| Probe | TCTCCATCACCTGCTCTTCTTCTTC | ||
| NM_031144 | β - actin (ACTB) | Forward Primer | CCCGCGAGTACAACCTTCT |
| Reverse Primer | CGTCATCCATGGCGAACT | ||
| Probe | AGCTCCTCCGTCGCCGGTCCA | ||
Table 3: RT-PCR primer and probe sequences.
| Component | Amount |
|---|---|
| cDNA | 2 µl |
| Primer (F/R) | 0.5 µM |
| Probe | 0.1 µM |
| 2X TaqMan buffer | 10 µl |
| Total Volume | 20 µl |
Table 4: Components of RT-PCR reaction.
Statistical analysis
Experimental data (n=6) was analyzed using SPSS 17.0 software and ANOVA (Newman-Keuls, Posthoc) tests and p<0.05 was accepted as significant results.
Results
MTT
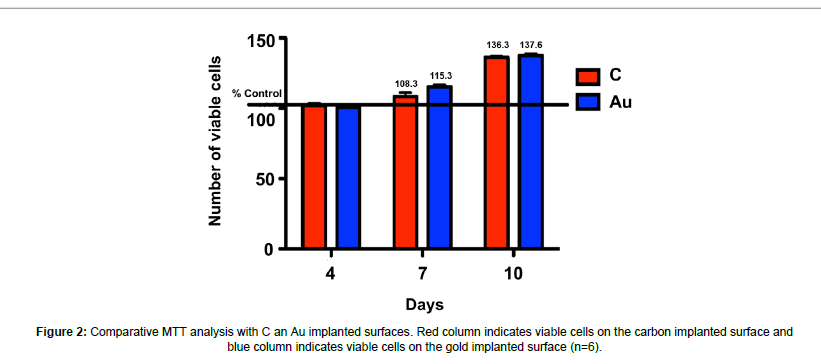
The viability of cells which attach to ion implanted surfaces was evaluated and it was reported that cell viability increased significantly at day 4, 7 and 10 with respect to time (p<0.05). The most significant viability increase was observed at day 10 and there was no significant difference between carbon and gold implanted samples (Figure 2).
SEM imaging
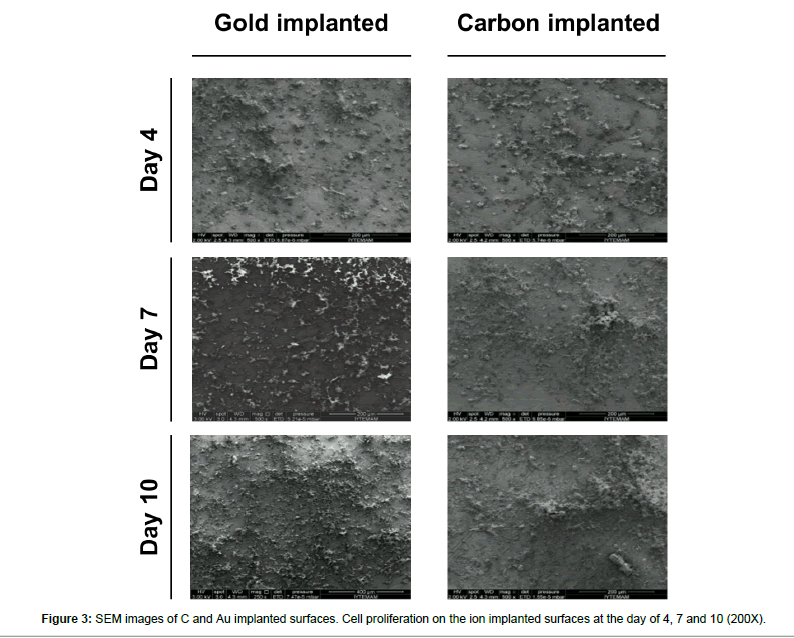
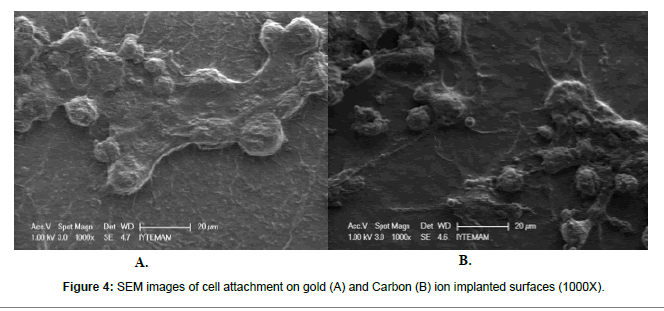
Carbon and gold implanted surfaces were seeded with neural stem cells and their SEM images were taken at the day of 4, 7 and 10. Cell proliferation was tracked via SEM images and a significant cell proliferation was observed at day 4 and 7. On the other hand, the most obvious proliferation was observed at day 10 (p<0.05). Even though a significant cell proliferation was noticed on ion implanted surfaces, there is no overall difference between carbon and gold implanted samples were reported (Figures 3 and 4).
RT-PCR analysis
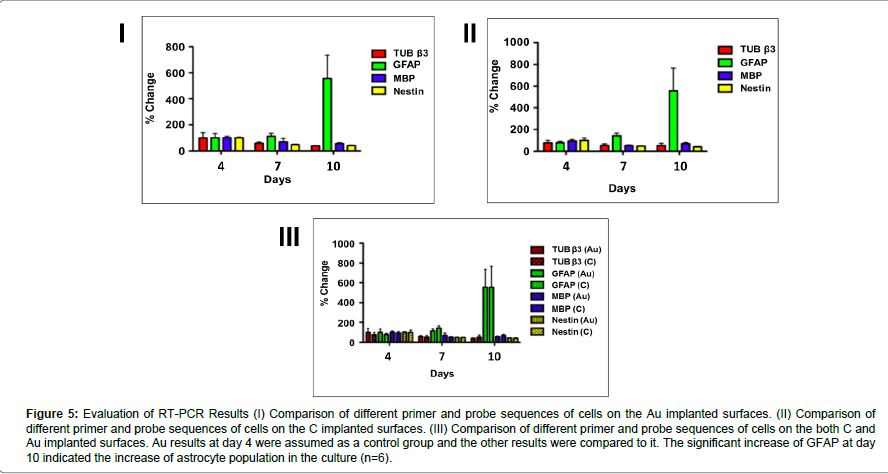
RT-PCR analysis showed that GFAP expression at day 10 increased significantly both on the carbon and gold implanted surfaces (one-way ANOVA, [F (5,39) =4,84, p=0,002]; p<0.05 for both carbon and gold implanted surfaces). Also, a significant decrease of nestin expression was observed [one-way ANOVA, F(5,38)=9,61, p=0,000]. However, there were no significant differences between groups (Figure 4).
Discussion
The regeneration ability of neural cells is considerably limited and slow after neural injuries. For this reason, many different methods and materials were tried to increase regeneration ability of neurons. It was shown that Au and C are beneficial for recovering from neural injuries, specifically acute and chronic spinal cord injuries [15,24].
In this study, the attachment tests of neural cells which seeded onto Au and C implanted surfaces were compared to each other. The data of Au at the day for was assumed as control while evaluating the results. A significant increase of cell attachment was observed with respect to time (p<0.05) and the maximum cell attachment was determined at the day of 10. There was no difference between Au and C implanted samples. Both Au and C ions affected the neural cell regeneration positively during culture period. These findings were supported by other studies, as well [24-26].
Ion implantation at different energy and doses was started to be applied in the 1960s. This method was applied most abundantly on metals and other industrial materials on its early use [27]. In the 1980s, the behavior of cells on the ion implanted surfaces was studied [21]. In 2004, Tsuji et al. published a considerable study about neural cell attachment and regeneration on negative carbon ion implanted silicon surfaces [28].
| Reaction | Temperature (°C) | Duration (s) |
|---|---|---|
| First denaturation | 95 | 30 |
| Denaturation | 95 | 30 |
| Annealing | 60 | 40 |
| Extension | 72 | 80 |
| Cooling | 40 | 30 |
Table 5: Details of RT-PCR reaction.
In this study, an enhanced neural cell attachment and proliferation were observed on the ion implanted surfaces in SEM images with respect to time (n=6, p<0.05) (Figure 3).
First studies of Au implantation began in the early 1970s. However, its effect on neural cells only started to be studied in the last couple of decades. In 2000, Fan et al. reported that neurons attached to the Au implanted silicon surfaces significantly better. However, the effect of any other ion was not studied in this work [29].
Tsuji denoted on two of his works that carbon ion implantation stimulates the nerve regeneration positively [25,30]. Sommani [24] and Oztarhan [22] also reported that carbon ions increased the nerve development and regeneration.
In our study, it was shown that neurons both proliferate and enhance their regeneration capability on the carbon and gold implanted surfaces. On the other hand, any difference between carbon and gold implanted surfaces cannot be detected. The nerve regeneration and proliferation were affected similarly by the presence of C and Au ions.
Figure 5:Evaluation of RT-PCR Results (I) Comparison of different primer and probe sequences of cells on the Au implanted surfaces. (II) Comparison of different primer and probe sequences of cells on the C implanted surfaces. (III) Comparison of different primer and probe sequences of cells on the both C and Au implanted surfaces. Au results at day 4 were assumed as a control group and the other results were compared to it. The significant increase of GFAP at day 10 indicated the increase of astrocyte population in the culture (n=6).
In 2011, it was also reported by Sokullu et al. [26] that gold and carbon ions did not have any advantage against to one another in terms of nerve development and regeneration.
The studies on ion implantations were focused on neural cells bodily and did not go into specific cell types individually thus far. In our study, cell types of neuron cells were analyzed using specific primers and probes via RT-PCR. On both Au and C implanted surfaces, GFAP was increased significantly at day 10 and this indicated that the astrocytes which are a specific type of glial cells, proliferated and matured in comparison with other cell types. On the other hand, the concentration of nestin decreased significantly during the culture. Since nestin demonstrates the immature neural stem cells, this significant decrease denoted the maturation of embryonic stem cells. Any other significant difference between other cell types was not found.
When damage occurs in neural systems neurons, and glial cells actively take part in the recovery of the damaged area. Xu at al. [31] reported in 2013 that damage stimulated the secretion of glial cell line-derive neurotrophic factor (GDNF) and suggested that glial cells supported the injured area. Since astrocytes are the most abundant glial cells in central neural system, our findings also confirmed this study with the RT-PCR results which showed a significant increase of GFAP that proves the increase of the astrocyte population.
Conclusion
With this work, it was shown that carbon and gold implantation stimulate the increase of neural cell proliferation, specifically glial cells and induce the nerve regeneration. There was no difference between gold and carbon implanted samples in terms of cell regeneration and proliferation.
The importance of this research was examining the cell functions such as attachment, proliferation, migration, differentiation and apoptosis on the cell culture surfaces which were implanted with different ion types via ion implantation method. The results will contribute to biomedical and implant technologies and initiate the new researches on this topic. It will also be a new approach to the treatment process of neurodegenerative diseases.
Acknowledgement
This study is funded by 2011-TIP-37 research fund. We acknowledge to Ege University Rectorate Commission of Scientific Research Projects and Ege University Faculty of Medicine the sub-commission of Research Projects and all the staff of our faculty.
References
- Gould TR, Brunette DM, Westsury L (1981) The attachment mechanism of epithelial cells to titanium in vitro. J Periodontal Res 16: 611-616.
- Howlett CR, Evans MD, Walsh WR, Johnson G, Steele JG (1994) Mechanism of initial attachment of cells derived from human bone to commonly used prosthetic materials during cell culture. Biomaterials 15: 213-222.
- Gumbiner BM (1996) Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 84: 345-357.
- Vogler EA, Bussian R (1987) Short-term cell-attachment rates: A surface-sensitive test of cell–substrate compatibility. J Biomed Mater Res 21: 1197-1211.
- Kaulambayeva MZ, Toleubekova AS, Akhmetsadykov NN (2015) Evaluating the effectiveness of organic coatings based on natural polymers for the treatment of wounds in the experiment. J Biotechnol Biomater 5: 211.
- Cannatelli MD, Ragauskas AJ (2015) Value added biomaterials via Laccase-mediated surface functionalization. J Biotechnol Biomater 5: 175.
- Puleo DA, Holleran LA, Doremus RH, Bizios R (1991) Osteoblast responses to orthopedic implant materials in vitro. J Biomed Mater Res 25: 711-723.
- Gronowicz G, McCarthy M (1996) Response of human osteoblasts to implant materials: Integrin-mediated adhesion. J Orthop Res 14: 878-887.
- Gawalt ES (2003) Bonding organics to Ti alloys: Facilitating human osteoblast attachment and spreading on surgical implant materials. Langmuir 19: 200-204.
- Lundborg G (1982) Nerve regeneration in silicone chambers: Influence of gap length and of distal stump components. Exp Neurol 76: 361-375.
- Faweett J, Keynes RJ (1990) Peripheral nerve regeneration. Annu Rev Neurosci 13: 43-60.
- Frostick SP, Yin Q, Kemp GJ (1998) Schwann cells, neurotrophic factors and peripheral nerve regeneration. Microsurgery 18: 397-405.
- Stoll G, Müller HW (1999) Nerve injury, axonal degeneration and neural regeneration: Basic insights. Brain Pathol 9: 313-325.
- Hadlock T, Sundback C, Hunter D, Cheney M, Vacanti JP (2000) A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng 6: 119-127.
- Bieberich E, Guiseppi-Elie A (2004) Neuronal differentiation and synapse formation of PC12 and embryonic stem cells on interdigitated microelectrode arrays: Contact structures for neuron-to-electrode signal transmission (NEST). Biosensors and Bioelectronics 19: 923-931.
- Tohill M, Mantovani C, Wiberg M, Terenghi G (2004) Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett 362: 200-203.
- Santiago LY (2006) Peptide-surface modification of poly (caprolactone) with laminin-derived sequences for adipose-derived stem cell applications. Biomaterials 27: 2962-2969.
- Caplan AI (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213: 341-347.
- di Summa PG, Kingham PJ, Raffoul W, Wiberg M, Terenghi G, et al. (2010) Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg 63: 1544-1552.
- Konyalioglu S, Armagan G, Yalcin A, Atalayin C, Dagci T (2013) Effects of resveratrol on hydrogen peroxide-induced oxidative stress in embryonic neural stem cells. Neural Regene Res 8: 485-495.
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55-63.
- Öztarhan A, Brown I, Bakkaloglu C, Watt G, Evans P, et al. (2005) Metal vapour vacuum arc ion implantation facility in Turkey. Surface and Coatings Technology 196: 327-332.
- Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45.
- Sommani P, Tsuji H, Sato H, Kitamura T, Hattori M, et al. (2007) Minimum line width of ion beam-modified polystyrene by negative carbon ions for nerve-cell attachment and neurite extension. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 257: 118-121.
- Tsuji H, Sommani P, Hattori M, Yamada T, Sato Y, et al (2008) Adhesion patterning of mesenchymal stem cells on polystyrene surface by carbon negative-ion implantation and neuron differentiation on the position. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 266: 3067-3070.
- Sokullu-Urkac E, Oztarhan A, Gurhan IS, Gulce-Iz S (2011) Neural cell attachment on metal ion implanted glass surfaces. MRS Proceedings.
- Silver FH, Christiansen DL (2012) Biomaterials science and biocompatibility. Springer Science & Business Media.
- Tsuji H, Izukawa M, Ikeguchi R, Kakinoki R, Sato H (2004) Surface treatment of silicone rubber by carbon negative-ion implantation for nerve regeneration. Applied Surface Science 235: 182-187.
- Fan Y, Cui FZ, Chen LN, Zhai Y, Xu QY (2000) Improvement of neural cell adherence to silicon surface by hydroxyl ion implantation. Surface and Coatings Technology 131: 355-359.
- Tsuji H, Sommani P, Kitamura T, Hattori M, Sato Y, et al. (2007) Nerve-cell attachment properties of polystyrene and silicone rubber modified by carbon negative-ion implantation. Surface and Coatings Technology 201: 8123-8126.
- Xu P, Rosen KM, Hedstrom K, Rey O, Guha S, et al (2013) Nerve injury induces glial cell line-derived neurotrophic factor (GDNF) expression in Schwann cells through purinergic signaling and the PKC-PKD pathway. Glia 61: 1029-1040.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 3317
- [From(publication date):
March-2017 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 2459
- PDF downloads : 858