The Effects of Apelin on IGF1/FSH Induced Steroidogenesis, Proliferation, Bax Expression, and total Antioxidant Capacity in Granulosa Cells of Buffalo Ovarian Follicles
Received: 16-Sep-2022 / Manuscript No. cmb-22-74948 / Editor assigned: 19-Sep-2022 / PreQC No. cmb-22-74948(PQ) / Reviewed: 30-Sep-2022 / QC No. cmb22-74948 / Revised: 01-Nov-2022 / Manuscript No. cmb-22-74948(R) / Accepted Date: 08-Nov-2022 / DOI: 10.4172/1165-158X.1000247 QI No. / 10.4172/1165-158X.1000247
Abstract
Apelin was believed to be an adipokine secreted from adipose tissue. However, studies demonstrate that it is a pleiotropic peptide and has several effects on the female reproductive system. In this study, the effect of different doses of IGF1 and FSH in the presence of apelin-13 on the production of estradiol and progesterone was evaluated in the follicular granulosa cells of buffalo ovaries, in addition, the effects of different doses of apelin isoforms (apelin-13 and apelin-17) on proliferation, the expression of Bax protein and total antioxidant capacity activity of the same cells were investigated. Granulosa cells of buffalo ovaries were cultured in the presence of different doses of IGF1 and FSH with or without apelin-13 (10-9M) to evaluate its effect on the secretion of estradiol and progesterone. WST-1 method was used to survey the effect of apelin on granulosa cells proliferation and cytotoxicity. In addition, the antioxidant capacity of the cells in the presence of apelin was assessed using FRAP method. mRNA and protein levels of Bax protein were measured in granulosa cells treated with apelin using real-time PCR and western blot techniques. Apelin-13 stimulated the effect of IGF1 on the production of estradiol and progesterone, and the progesterone production levels were affected by apelin-13 dose-dependently. However, it did not significantly stimulate the effect of FSH on the secretion of estradiol or progesterone. Apelin-13 (all doses) and -17 (10-8 and 10-9 M) improved the proliferation of granulosa cells. Moreover, preincubation of the cells for an hour by apelin receptor antagonist (ML221, 10 μM) did not significantly affect the proliferation of cells. Neither apelin-13 nor apelin-17 were not cytotoxic for the cells compared to the control treatment. Apelin-13 at the doses of 10-6 and 10-8 M substantially up and down-regulated Bax protein expression; however, such effects were not observed when the cells were preincubated with ML221. In addition, apelin-17 did not influence the expression amount of Bax. Furthermore, both apelin-13 and -17 improved the total antioxidant capacity of the ovarian granulosa cells, but such effects were not seen when the cells were preincubated with ML221. These findings indicate that apelin enhanced the IGF1-induced steroidogenesis and improved the cell proliferation and antioxidant capacity of follicular granulosa cells of buffalo ovaries; however, its effect on Bax expression was divergent.
Keywords
Apelin; Steroidogenesis; Granulosa Cells; Proliferation; Antioxidant Capacity; Bax expression
Introduction
As a new adipokine produced by adipose tissue, apelin is derived from preproapelin (77 amino acids). Preproapelin is cleaved into a 55-amino-acid fragment and later into different apelin isoforms such as APLN-36, APLN-17, APLN-13, and the pyroglutamyl APLN-13 (Pyr- APLN-13). (Pyr) APLN-13 and -17 are more active isoforms in more quantity in the bloodstream. The receptor of APLN is APJ (an orphan G protein-coupled receptor) with a similar structure to the Angiotensin II Type 1 Receptor (AT1). APJ can bind to different isoforms of APLN with various affinities and activate several signaling pathways that lead to the appearance of different functions in the body. In addition to adipose tissue, APLN and APJ are extensively distributed in the various tissues of the body, such as the heart, brain, lung, blood vessels, spleen, intestine, breast, and reproductive tract. Moreover, the known roles of APLN and APJ are participation in angiogenesis, cardiovascular function, fluid homeostasis, cell proliferation, food intake, and regulation of energy metabolism. The reproductive roles of APLN have been reported in recent years, in addition to its localization in the gonads and the arcuate, supraoptic, and paraventricular hypothalamic nuclei. By attempts from the last 15 years ago, the presence of APLIN has been verified in the Granulosa (GCs), theca luteal cells, and oocytes of bovine, human, and porcine. The considerable effects of APLN have been reported on follicle selection in the bovine species, steroidogenesis in different species such as rats, humans, cattle, sheep, and pigs. In our previous study, we defined the expression of Apelin and APJ in the different developmental stages of ovarian GCs in buffalo. Likewise, it is stated that apelin, in the presence of different factors such as IGF1 and FSH, has meaningful effects on the secretion of Progesterone (P4) and Estradiol (E2) in cattle and porcine as well as buffaloes through various signaling pathways [1].
The effects of APLN on IGF1/FSH-induced steroidogenesis and cell proliferation, apoptosis, and scavenging activity of ovarian follicular GCs in buffaloes are unmasked. Therefore, in this study, for the first time, we surveyed these functions. Thus, the effect of APLN-13 on IGF1/FSH-induced steroidogenesis was evaluated. In addition, the effects of APLN-13/-17 proliferation, cytotoxicity, Bax expression, and scavenging activity of GCs were studied [2].
Materials and Methods
Reagents
Unless otherwise stated, all chemicals and media used in the current study were obtained from Sigma-Aldrich (MO, USA).
Hormones and Antibodies
Recombinant porcine FSH, recombinant human IGF1 (ab270062), APLN-13 (ab141010), APLN-17 (ab141011) were purchased from Abcam. Also, APLN (ab141011), Bax (ab77566), and beta-Actin (ab8226) antibodies were obtained from Abcam. APJ (20341-1-AP), anti-rabbit IgG (SA00001-2), and anti-mouse IgG (SA00001-1) were purchased from Proteintech. Primary and secondary antibodies were used at 1: 500 to 1:1000 and 1/3000 for western blotting [3].
Follicle Collection and Granulosa Cells Culture
Buffalo ovaries were collected from a local slaughterhouse and transported on ice within 2 h after slaughter to the laboratory in Phosphate-Buffered Saline (PBS) supplemented with 0.05 mg/mL streptomycin and 0.06 mg/mL penicillin. In the laboratory, the ovaries were washed adequately with physiological saline solution.
To assess the effect of the APLN on IGF1/FSH-induced steroidogenesis, cell proliferation, apoptosis, and cell redox status, the GCs culture model was established. Therefore, all the healthy (well vascularized and having transparent follicular wall and fluid) and visible follicles were aspirated by a 17-gauge needle attached with a 10-mL syringe. The aspirates were transferred to a 60-mm dish under sterile conditions with PBS, and all cumulus-oocyte complexes were removed. The remaining cells and liquids were centrifuged in 15-mL conical tubes at 700 g for 5 min. Then, GCs were resuspended in Dulbecco's Modified Eagle Medium (DMEM) medium containing 10% Fetal Bovine Serum (FBS) and antibiotics and antimycotic solution (penicillin 100 U/mL, streptomycin 100 mg/mL, amphotericin B 0.25 mg/mL). Cell viability was evaluated using trypan blue exclusion dye, exceeding 80%. The cells were then seeded in a 48/96-well plate in a humidified CO2 (5%) incubator at 37.5°C and having approximately 1.5 × 105 viable cells per well. The cells were allowed to attach and grow (75%-80% confluence) for 48 h. Then cells were treated with fresh media (FBS free) containing different doses of porcine FSH or human recombinant IGF-I (0, 10-6, 10-7, 10-8, 10-9, and 10-10 M) singly or in the presence of 10-9 M APLN- 13 and were maintained for 48 h. Control cells were grown in similar conditions as other cells except for the addition of the peptides. For each experimental condition, six replicates were tested. After 48 h, the spent media were collected and stored for E2 and P4 assay. APLN-13 or APLN-17 were applied to GCs after culturing for 48 hours with 10% FBS to investigate whether they affected the mRNA and protein expression of Bax, the cells were treated with different doses of APLN- 13 and APLN-17 (0, 10-6, 10-8, 10-9 M) for additional 48 hrs and then mRNA and protein were extracted from cells [4].
Total RNA Extraction, cDNA Synthesis
Total RNA was extracted from GCs of follicles using TRIZOL reagent by the manufacturer's instruction, and a fixed amount of RNA (100 ng) was directly reverse-transcribed into a 20 μL first-strand cDNA using a PrimeScript RT reagent Kit with gDNA Eraser (Perfect Real Time, TAKARA BIO INC, Japan) following the manufacturer's instructions [5].
Quantitative Real-Time PCR analysis
Rt-qPCR was done in a total volume of 20 μL, containing equally distributed cDNA (100 ng), 10 mM each of the forward and reverse primers, and 10 μL of 2 × SYBR Green Master Mix (SYBR® Premix Ex Taq™ II (Tli RNaseH Plus, TAKARA, Japan). All reactions for all genes of interest were performed in triplicate and were run on the light Cycler 480 system (Roche Diagnostics) under the following conditions: 95°C for 30 s, followed by 40 cycles at 95°C for 5 s and 60°C for 30 s. β-actin and RPS15 were used as the internal control (reference genes) to normalize the relative gene expression levels. All reactions were performed in triplicate. The gene expression levels were analyzed with the 2-ΔΔCT method described previously by concerning the housekeeping genes. The details of the selected genes and the primer pairs used in the study are provided in Table 1.
| Gene | Primer Sequence (5’-3’) | Amplicon size (bp) | Accession No. |
|---|---|---|---|
| β-actin | F: TCTCACGGAGCGTGGCTACAG R: CTGCTCGAAGTCCAGGGCCACGTA |
100 | NM_001290932.1 |
| RPL15 | F: TGGGCTACAAGGCCAAACAA R: GCTTCGAGCAAA CTTGAGCTGG |
140 | MG969348 |
| Bax | F: AACATGGAGCTGCAGAGGAT R: CCAGCTCTTGGTCGCTGTAGAG |
84 | 46 |
Western Blot Analysis
Total proteins were obtained from cultured GCs of different experiments by lysing in RIPA buffer containing PMSF (R0010; Solarbio, China) at 4°C for 30 min followed by collection and centrifugation at 12,000 rpm for 5 min at 4°C. The pellet was eliminated, and lysates were diluted with 6X protein loading buffer (DL101-02; TransGen, China) and heated to 100°C for 5 min. After cooling on ice, the samples were stored at -80°C until the western blotting. Western blotting was started by loading the samples on a 12% gradient polyacrylamide gel (P0012AC; Beyotime, China) and then transferred to a PVDF membrane (ISEQ00011; Millipore, China), followed by blocking in 8% (wt/vol) Difco Skim Milk in Tris-buffered saline containing 0.1% (vol/ vol) Tween-20 (TBST) for 2 h. Overnight incubation with the primary antibody was performed. Then, after four washes, 10 min each, with TBST, membranes were incubated for 1 h at 37°C with goat antimouse IgG for beta-Actin and with goat anti-rabbit IgG for Bax. The membranes were washed four more times in TBST for 10 min each, then developed using ECL Plus (P0018, Beyotime, China) followed by detection with a multi-function imager (Syngene, Cambridge, UK). The intensities of individual bands were normalized to the expression of beta-Actin. Western blots were performed three times [6].
The Proliferation and Cytotoxicity Effects of GCs
The effects of APLN-13/-17 on GCs proliferation and cytotoxicity were evaluated by WST-1 ((4-[3-(4-iodophenyl)-2-(4-nitrophenyl)- 2H-5-tetrazolium]-1, 3-benzene disulfonate) method. This method is based on the cleavage of the tetrazolium salt WST-1 to formazan by mitochondrial dehydrogenases. An increase in the number of viable cells causes an increase in the activity of mitochondrial dehydrogenases, which in turn enhances the amount of formazan dye produced. The formazan dye produced from WST-1 by viable cells can be quantified by measuring the absorbance of the dye at OD=440 nm. Briefly, 4 × 104 cells per 200 μL of culture media were seeded in 96-well plates treated with APLN-13/17 (0, 10-9, 10-8, 10-6 M) with or without preincubation of cells with APJ antagonist (ML221 10 μM) for 1 hour and incubated for 48 hours. According to the product manual, 10 μL of WST-1 was added to the cells during the last 4 hours of incubation. Then, the absorbance was detected by a plate reader machine at a wavelength of 44 Cytotoxicity 20 nm [7].
The rate of cytotoxicity of APLN-13/-17 was calculated according to the following formulae:

Where CTA is the control group, and ATC is APLN treated cells.
Total Antioxidant Capacity Assessment by the FRAP Method The FRAP method is a colorimetric assay based on the ability of the antioxidant molecules to reduce Ferric Tri Piridyl Tria Zine (Fe3+TPTZ) to a ferrous form (Fe2+TPTZ). Fe2+ is assessed spectrophotometrically through determination of its colored complex with 2, 4, 6-Tris (2-Pyridyl)-Stria Zine (Fe2+ TPTZ). TPTZ reagent was prepared before use, mixed with 25 mL of acetate buffer, 2.5 mL of 2, 4, 6-Tris (2- Pyridyl)-s-Tria Zine (TPTZ) 10 mM in HCl 40 mM, and FeCl36H2O solution. In total, 4 × 104 cells per 200 μL of culture media were cultivated in a 96-well plate and treated with APLN-13/-17 for 48 h. Then, the plates were centrifuged for 10 min at 400 g, the supernatants were discarded, and cells were lysed by adding cold Triton 0.5% (v/v) + PMSF in PBS (200 mL/well) and incubating on ice for 30 min. The test was carried out on 40 mL of cell lysates added to Fe3+ TPTZ reagent and then incubated at 37°C for 30 min. The absorbance of Fe2+ TPTZ was detected at 595 nm. The ferric reducing ability of cell lysates was estimated by plotting a standard curve of absorbance against FeSO4- 7H2O standard solutions [8].
Steroids ELISA Assay
E2 and P4 concentrations were estimated in serum-free medium from buffalo GCs after 48 h of culture using ELISA kits supplied by Miebiao biology, China. Cells were plated in 48-well plates (105 viable cells/well), and eight replicates were tested for each experimental condition (IGF1 and FSH in the presence or absence of APLN-13 or -17). The results were expressed as the concentration of the steroid (pmol/L). The intra- and inter-assay coefficients of variation for progesterone were less than 10%. Results are given as mean ± SEM. Data were obtained from 3 independent cultures.
Statistical Analysis
All the data have been shown as mean ± SEM. One or two-way ANOVA followed by Duncan's multiple range test was used to test the differences among groups by GLM procedure of SAS software (Version 9.4). A significant difference was considered if P < 0.05. The data were checked for normality and homogeneity of variance between the groups.
Results
The effect of varying IGF-1 and FSH doses, with or without APLN-13, on the secretion of E2 and P4 from GCs of buffalo ovaries
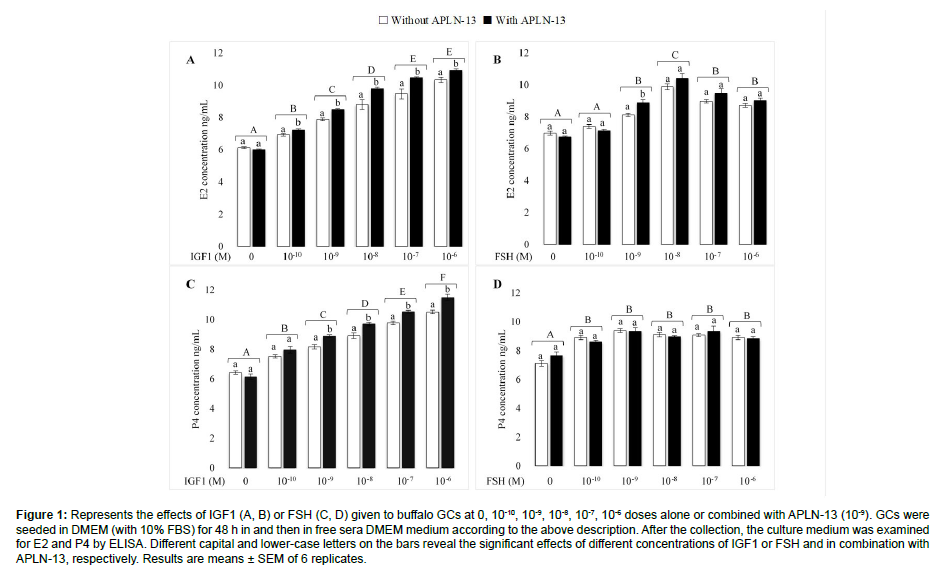
Figure 1 portrays the effect of different doses of IGF1 and FSH, with and without APLN-13, on the secretion of E2 and P4 in buffalo ovarian GCs. To understand the effects of APLN with two main hormones influencing on GCs biological function, we treated the GCs with different doses of IGF1 or FSH (0, 10-10, 10-9, 10-8, 10-7, 10- 6) with or without APLN-13 (0, 10-9) in a sera-free DMEM culture medium for 48 h following the incubation of the cells in the same medium with 10% FBS. The results showed that different doses of IGF1 significantly increased the secretion of E2 and P4 from buffalo GCs (P < 0.01). The effects of IGF1 on E2 and P4 were substantially triggered when combined with APLN-13. There were no significant differences between the effects of IGF1 at 10-7 versus 10-6 M on the secretion of E2 (P<0.05); nevertheless, IGF1 affected P4 production from GCs in a dose-dependent manner, increasing with higher doses. A 10-10 M FSH dose had no significant effect on E2 secretion. Despite this, other doses led to increased production of E2, with the highest effect being 10-8 M. Furthermore, treatment of GCs with FSH increased the secretion of P4, even though there was no substantive difference between various doses of FSH. In addition, we observed no significant differences between FSH alone and combined with APLN-13 (except for 10-9 M of FSH on E2) on E2 and P4 concentration production in the treated buffalo GCs [9].
Figure 1: Represents the effects of IGF1 (A, B) or FSH (C, D) given to buffalo GCs at 0, 10-10, 10-9, 10-8, 10-7, 10-6 doses alone or combined with APLN-13 (10-9). GCs were seeded in DMEM (with 10% FBS) for 48 h in and then in free sera DMEM medium according to the above description. After the collection, the culture medium was examined for E2 and P4 by ELISA. Different capital and lower-case letters on the bars reveal the significant effects of different concentrations of IGF1 or FSH and in combination with APLN-13, respectively. Results are means ± SEM of 6 replicates.
The effects of various doses of APLN-13 and APLN-17 on the buffalo GCs proliferation and cytotoxicity
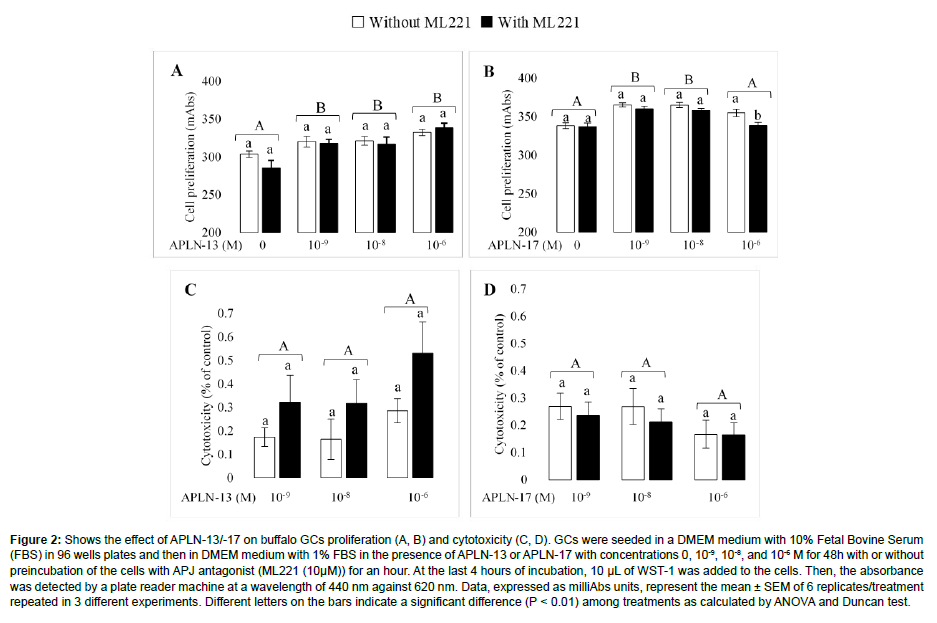
The effects of different doses of APLN-13/-17 on the GCs proliferation and cytotoxicity are presented in Figure 2. APLN-13 caused a significant increase in cell proliferation compared to control cells; however, there were no substantial differences among various doses of APLN-13 in cell proliferation (Figure 2A). APLN-17 also increased the proliferation of GCs at 10-8 and 10-9 M doses, but not at 10-6 (Figure 2B). Neither APLN-13 nor APLN-17 did not have a substantially cytotoxic effect on the GCs (Figure 2C, 2D). Results also showed that preincubation of the cells with APJ antagonist (ML221 10 μM) before APLN-13/-17 treatment did not affect the GC proliferation (Except APLN-17 at 10-6 M).
Figure 2: Shows the effect of APLN-13/-17 on buffalo GCs proliferation (A, B) and cytotoxicity (C, D). GCs were seeded in a DMEM medium with 10% Fetal Bovine Serum (FBS) in 96 wells plates and then in DMEM medium with 1% FBS in the presence of APLN-13 or APLN-17 with concentrations 0, 10-9, 10-8, and 10-6 M for 48h with or without preincubation of the cells with APJ antagonist (ML221 (10μM)) for an hour. At the last 4 hours of incubation, 10 μL of WST-1 was added to the cells. Then, the absorbance was detected by a plate reader machine at a wavelength of 440 nm against 620 nm. Data, expressed as milliAbs units, represent the mean ± SEM of 6 replicates/treatment repeated in 3 different experiments. Different letters on the bars indicate a significant difference (P < 0.01) among treatments as calculated by ANOVA and Duncan test.
The effects of various doses of APLN-13 and APLN-17 on the expression abundance of Bax in the GCs of buffalo ovaries
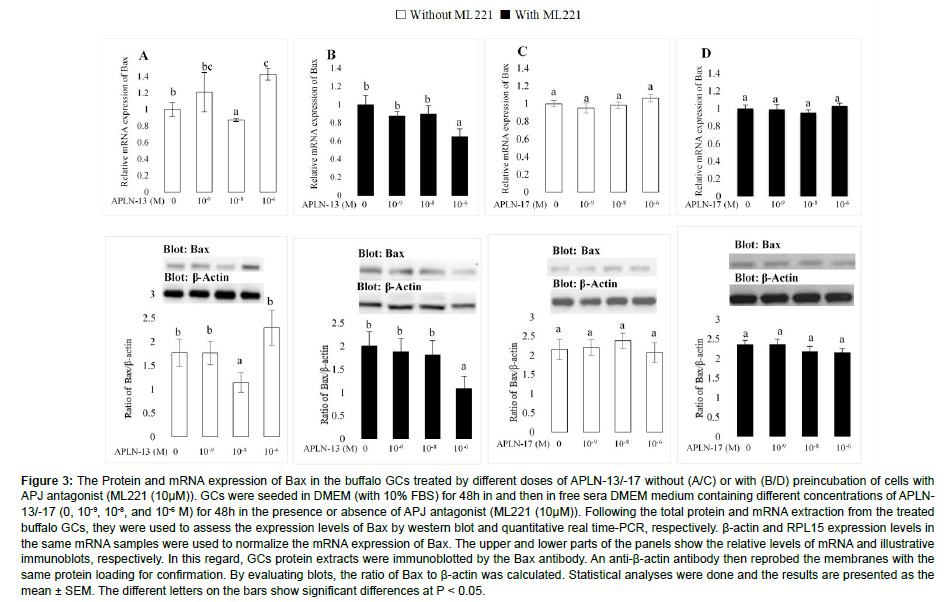
The effects of various doses of APLN-13/-17 on the expression amount of Bax are shown in Figure 3. APLN-13, when administered at 10-8 and 10-6 M, significantly decreased and increased the mRNA expression of Bax without preincubating the cells with ML221 (Figure 3A). Surprisingly, the same effects were not detected when the cells were preincubated with ML221 (10 μM; Figure 3B) and APLN-13 at 10-6M, which significantly decreased the mRNA and protein expression levels of Bax. On the other hand, different doses of APLN-17 did not significantly affect the expression abundances of Bax (Figure 3C, 3D).
Figure 3: The Protein and mRNA expression of Bax in the buffalo GCs treated by different doses of APLN-13/-17 without (A/C) or with (B/D) preincubation of cells with APJ antagonist (ML221 (10μM)). GCs were seeded in DMEM (with 10% FBS) for 48h in and then in free sera DMEM medium containing different concentrations of APLN- 13/-17 (0, 10-9, 10-8, and 10-6 M) for 48h in the presence or absence of APJ antagonist (ML221 (10μM)). Following the total protein and mRNA extraction from the treated buffalo GCs, they were used to assess the expression levels of Bax by western blot and quantitative real time-PCR, respectively. β-actin and RPL15 expression levels in the same mRNA samples were used to normalize the mRNA expression of Bax. The upper and lower parts of the panels show the relative levels of mRNA and illustrative immunoblots, respectively. In this regard, GCs protein extracts were immunoblotted by the Bax antibody. An anti-β-actin antibody then reprobed the membranes with the same protein loading for confirmation. By evaluating blots, the ratio of Bax to β-actin was calculated. Statistical analyses were done and the results are presented as the mean ± SEM. The different letters on the bars show significant differences at P < 0.05.
The effects of various doses of APLN-13 and APLN-17 on total antioxidant capacity of GCs
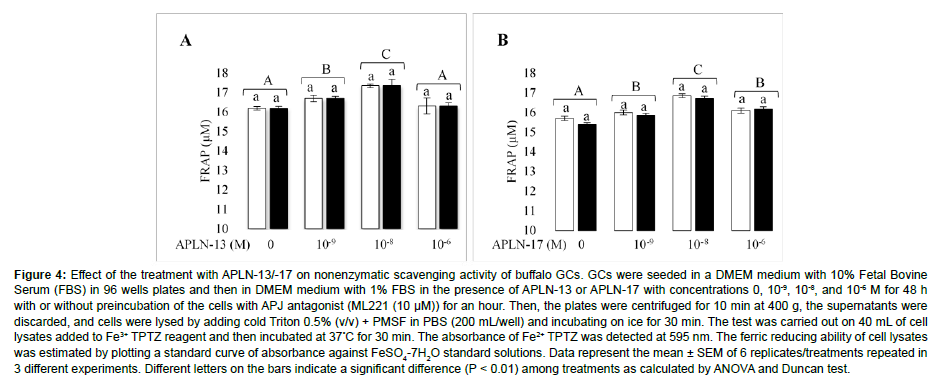
The effects of different doses of APLN-13/-17 on the antioxidant capacity of GCs are shown in Figure 4. APLN-13 at the doses of 10-9, 10-8 M significantly increased the reduction power of GCs compared to the control cells; however, it did not have a significant effect at the dose of 10-6 M (Figure 4A). Contrastingly, all doses of APLN-17 had a statistically significant effect on the antioxidant power of GCs (Figure 4B). The cells treated with APLN-13 and APLN-17 at the doses of 10-8 had the highest impact on the antioxidant power of GCs. When cells were preincubated with ML221 (10 μM) for an hour before being treated with APLN-13/-17, antioxidant capacity of the cells was not significantly affected [10].
Figure 4: Effect of the treatment with APLN-13/-17 on nonenzymatic scavenging activity of buffalo GCs. GCs were seeded in a DMEM medium with 10% Fetal Bovine Serum (FBS) in 96 wells plates and then in DMEM medium with 1% FBS in the presence of APLN-13 or APLN-17 with concentrations 0, 10-9, 10-8, and 10-6 M for 48 h with or without preincubation of the cells with APJ antagonist (ML221 (10 μM)) for an hour. Then, the plates were centrifuged for 10 min at 400 g, the supernatants were discarded, and cells were lysed by adding cold Triton 0.5% (v/v) + PMSF in PBS (200 mL/well) and incubating on ice for 30 min. The test was carried out on 40 mL of cell lysates added to Fe3+ TPTZ reagent and then incubated at 37°C for 30 min. The absorbance of Fe2+ TPTZ was detected at 595 nm. The ferric reducing ability of cell lysates was estimated by plotting a standard curve of absorbance against FeSO4-7H2O standard solutions. Data represent the mean ± SEM of 6 replicates/treatments repeated in 3 different experiments. Different letters on the bars indicate a significant difference (P < 0.01) among treatments as calculated by ANOVA and Duncan test.
Discussion
In recent years, adipokines have been the most focused peptides in the reproduction system. The effect of the APLN/APJ system on various aspects of reproduction has been studied in different species, but the findings are contradictory. There are no reported results regarding the effects of APLN on the reproduction of buffaloes. As part of the current study, we surveyed for the first time the effects of different FSH and IGF1 doses on E2 and P4 production in buffalo ovaries with and without APLN-13. Also, we evaluated the effects of APLN-13 and APLN-17 on cell proliferation, Bax protein expression, cytotoxicity, and antioxidant capacity in the same cells. Our findings showed APLN-13 stimulated the increasing effect of IGF1 on the secretion of E2 from GCs of buffalo ovaries; however, the effect of different doses was not the same. In addition, APLN-13 triggered the effect of IGFI on the production of P4 in a dose-dependent manner. In the presence of APLN-13, neither E2 nor P4 were significantly affected by FSH doses from buffalo GCs. These results agree with the literature. As one of the main adipokines, apelin has a crucial role in energy metabolism. It affects glucose transfer and mimics the insulin effect in skeletal muscles. As insulin and IGF1 have similar structures and receptors, APLN can enhance IGF1 responsiveness in GCs and increase E2 and P4 production. Furthermore, previous studies showed that APLN enhances the phosphorylation of PI3K/Akt induced by IGF1, and it causes the regulation of steroidogenesis in GCs. It was suggested that the effects of APLN on IGF-1-induced steroidogenesis in GCs are exerted through PI3K/Akt signaling pathway [11].
The current results showed that APL-13/-17 was significantly increased the proliferation of buffalo GCs; certainly, APLN-17 at 10-6 M did not have a significant effect on GCs proliferation compared to the control group. The same results were gained when the cells were preincubated with ML221 for an hour. Additionally, we showed that APLN-13/-17 was not cytotoxic to buffalo GCs. Literature demonstrated that APLN restrains cell apoptosis and retina angiogenesis and improves the proliferation of vascular smooth muscle cells, osteoblasts, and microvascular endothelial cells. Furthermore, the proliferative effect of APLN on various carcinoma cell lines has been verified in different studies. In concurrence with our results, APLN promoted the GCs proliferation in the rat, bovine, and porcine. However, the results of one study suggested that APLN does not affect human GCs proliferation, but in line with our findings, they explained that it does not affect the viability of GCs. Another report declared that APLN prevents the proliferation and migration of pulmonary arterial smooth muscle cells. We did not investigate the cellular pathways of APLN's effect on proliferation or apoptosis. However, the reported results showed that APLN promoted in vitro cell proliferation through activation of the ERK1/2 signaling pathway in porcine GCs, lung adenocarcinoma, MCF-7 (breast cancer cells), and ovarian cancer cells. On the other hand, in some other studies, it is illustrated that cell proliferation promotion can occur via the IP3/Akt signaling pathway. ERK1/2 signaling pathway activation is the main pathway for cell proliferation improvement by APLN. ERK1/2, as an essential pathway to cell proliferation, triggers the expression of cyclin proteins (mainly cyclin D1) and improves cell cycle move on. APLN also improves steroidogenesis and proliferation of GCs through increasing IGF1 sensitivity [12].
This study also revealed that APL-13 only at the doses of 10-8 M (without ML221) and 10-6 M (with ML221) decreased Bax protein expression; nonetheless, 10-6 M (in the absence of ML221) elevated the protein expression of Bax compared to control cell; therefore, the results were divergent. Bcl-22 protein family is a necessary governor for apoptosis in various types of cells. Different members of the BCL2 family are expressed in growing follicles, such as antiapoptotic and proapoptotic factors. It is shown that Bax, as a proapoptotic protein, is engaged in apoptosis of follicle cells under different conditions and, along with Bcl-2 as an antiapoptotic factor, plays a critical role in apoptosis of different cells. The antiapoptotic effect of APLN has previously been shown in mouse and human osteoblasts, rat cardiomyocytes, and rat neurons. Furthermore, it showed that APLN decreases apoptosis of rat ovarian GCs. However, in contrast to parts of our study, they found that APLN was upregulated expression of Bax. In an older study, it was found that the mRNA expression of APJ was increased in apoptosis-induced bovine GCs. They concluded that there is a correlation between GCs atresia and expression of APJ. However, they did not find any effect of APLN-13/-17 on the apoptosis of bovine ovarian GCs. In line with our findings, APLN diminished mRNA and protein expression of proapoptotic proteins (including Bax) in BeWo cells. APLN decreases apoptosis through PI3K/Akt and ERK1/2 signaling pathways, and activation of these pathways causes suppression of BAX/BAD proapoptotic proteins. Moreover, it is demonstrated that APLN enhances Bcl-2 expression levels, reduces Bax protein's expression amount, and arrests the release of cytochrome C. We did not assess the expression of Bax in luteinizing GCs. However, they have implied that down-regulation of the apelin-APJ system could be associated with cell apoptosis and leutolysis in bovine [13].
The present study results revealed that APLN-13/-17 had a significant effect on the antioxidant power of buffalo ovarian GCs, and APLN improved the antioxidant potential of GCs. Oxidative stress is a crucial factor causing Reactive Oxygen Species (ROS) aggregation and subsequent cell death. In human adipocytes, found APLN reduced ROS production and release, as well as oxidative stress and oxidativeinduced cellular degeneration. Likewise, in agreement with the results of this study, APLN enhanced the response of rat adipocytes and adrenal medulla cells to oxidative stress and decreased the levels of oxidative stress indicators. They have outlined that APLN reduced oxidative stress by enhancing extracellular oxygen utilization. APLN increases GC antioxidant power and is directly correlated with improving cell viability and decreasing apoptosis. Another possible explanation for APLN's positive effect on cell death could be its antioxidant properties [14].
Conclusion
Our results showed that apelin stimulated the IGF1 induced production of estradiol and progesterone in the buffalo ovarian granulosa cells, improving the proliferation and antioxidant power of the same cells. However, the effect of apelin on the Bax protein as a proapoptotic factor varied. In general, these data demonstrate that apelin may have an essential role in steroidogenesis and proliferation of follicular granulosa cells of buffalos; nonetheless, these roles, in addition to Bax expression and antioxidant power of the cells are required more investigations [15].
Availability of Data
All data supported the conclusions during this study are included within the article.
Conflict of Interests
There are no conflict of interests to declare.
Funding
This work was financially supported by grants from the Key Research and Development Program in Guangxi (GuiKe AB1850013), the Special Fund for Guiding Local Scientific and Technological Development by the Central Government (GuiKe ZY21195007), the Guangxi Natural Science Foundation (2018GXNSFDA050013) and the National Key Research and Development Program (2017YFE0113800) of China.
Acknowledgment
Not applicable
References
- Mederos MA, Reber HA, Girgis MD (2021) Acute Pancreatitis: A Review. JAMA 325: 382-390.
- Konok GP, Thompson AG (1969) Pancreatic ductal mucosa as a protective barrier in the pathogenesis of pancreatitis. Am J Surg 117: 18-23.
- Dalbec KM, Max Schmidt C, Wade TE, Wang S, Swartz-Basile DA, et al. (2010) Adipokines and cytokines in human pancreatic juice: unraveling the local pancreatic inflammatory milieu. Dig Dis Sci 55: 2108-2112.
- Yuan X, Wu J, Guo X, Li W, Luo C, et al. (2021) Autophagy in Acute Pancreatitis: Organelle Interaction and microRNA Regulation. Oxid Med Cell Longev 2021: 8811935.
- Wang H, Li C, Jiang Y, Li H, Zhang D (2020) Effects of Bacterial Translocation and Autophagy on Acute Lung Injury Induced by Severe Acute Pancreatitis. Gastroenterol Res Pract 2020: 8953453.
- Yang H, Ma S, Guo Y, Cui D, Yao J (2019) Bidirectional Effects of Pyrrolidine Dithiocarbamate on Severe Acute Pancreatitis in a Rat Model. Dose Response 17: 1559325819825905.
- Kong L, Deng J, Zhou X, Cai B, Zhang B, et al. (2021) Sitagliptin activates the p62-Keap1-Nrf2 signalling pathway to alleviate oxidative stress and excessive autophagy in severe acute pancreatitis-related acute lung injury. Cell Death Dis 12: 928.
- Yue J, López JM (2020) Understanding MAPK Signaling Pathways in Apoptosis. Int J Mol Sci 21.
- Pietrocola F, Izzo V, Niso-Santano M, Vacchelli E, Galluzzi L, et al. (2013) Regulation of autophagy by stress-responsive transcription factors. Semin Cancer Biol 23: 310-322.
- Cao W, Li J, Yang K, Cao D (2021) An overview of autophagy: Mechanism, regulation and research progress. Bull Cancer 108: 304-322.
- Chen Z, Chen Y, Pan L, Li H, Tu J, et al. (2015) Dachengqi Decoction Attenuates Inflammatory Response via Inhibiting HMGB1 Mediated NF-κB and p38 MAPK Signaling Pathways in Severe Acute Pancreatitis. Cell Physiol Biochem 37: 1379-1389.
- Wei S, Huang Q, Li J, Liu Z, You H, et al. (2012) Taurine attenuates liver injury by downregulating phosphorylated p38 MAPK of Kupffer cells in rats with severe acute pancreatitis. Inflammation 35: 690-701.
- Beaudoin AR, St-Jean P, Grondin G (1989) Pancreatic juice composition: new views about the cellular mechanisms that control the concentration of digestive and nondigestive proteins. Dig Dis 7: 210-220.
- Hegyi P, Petersen OH (2013) The exocrine pancreas: the acinar-ductal tango in physiology and pathophysiology. Rev Physiol Biochem Pharmacol 165: 1-30.
- Tan JH, Cao RC, Zhou L, Zhou ZT, Chen HJ, J, et al. (2020) ATF6 aggravates acinar cell apoptosis and injury by regulating p53/AIFM2 transcription in Severe Acute Pancreatitis. Theranostics 10: 8298-8314.
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Citation: Shokrollahi B, Zheng HY, Li LY, Tang LP, Ma XY, et al. (2022) The Effects of Apelin on IGF1/FSH-Induced Steroidogenesis, Proliferation, Bax Expression, and Total Antioxidant Capacity in Granulosa Cells of Buffalo Ovarian Follicles. Cell Mol Biol, 68: 247. DOI: 10.4172/1165-158X.1000247
Copyright: © 2022 Shokrollahi B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1208
- [From(publication date): 0-2022 - Mar 04, 2025]
- Breakdown by view type
- HTML page views: 980
- PDF downloads: 228