The Effects of Aerobic Training on the IL-17 and IFN-γ Expression in the Hippocampus of Male Wistar Rats with Alzheimer Disease
Received: 12-Oct-2019 / Accepted Date: 21-Nov-2019 / Published Date: 28-Nov-2019 DOI: 10.4172/2161-0460.1000480
Abstract
Background: Inflammation is a prominent risk factor for Alzheimer’s disease. Then, lifestyle interventions such as exercise training, can target the inflammatory pathway. The purpose of this study was to investigate the effect of 4 weeks of aerobic training on the expression of IL-17 and IFN-γ in the hippocampus of rats with Alzheimer’s disease induced by amyloid-beta injection.
Methods: The 21 adult male Wistar rats were randomly divided into 3 groups of control (CG), Alzheimer’s, (AG) and Alzheimer’s+aerobic training (AAG). Beta-amyloid oligomers were used to induce Alzheimer’s disease in the hippocampus. A week after Alzheimer’s induction, rats of the AAG group performed exercise 5 days a week for 4 weeks. In the first and second weeks, the training session consisted of two 15 min sets with speed 10 m/m and a 5 min rest between sets. In the third and fourth weeks, the speed increased to 15 m/min and the number of sets to three and four 15 min, respectively (with 5 min rest between sets). After 4 weeks, hippocampal isolation from all rats and was used to evaluate cytokines gene expression.
Results: The results showed that IFN-γ gene expression was significantly lower in AAG group than AG group and significantly higher in the AAG and AG groups than CG group (p ≤ 0.05). Also, IL-17 gene expression was significantly lower in the AAG than AG group. While the IL-17 expression was significantly higher in the AG group than the CG group, there was no significant difference between the AAG and CG groups (p ≤ 0.05).
Conclusion: Aerobic training may help to alleviate the neuro-inflammation conditions that occur in Alzheimer’s disease by reducing the expression of pro-inflammatory cytokines such as IL-17 and IFN-γ.
Keywords: Aerobic training; Alzheimer; IFN-γ; IL-17; Neuroinflammation
Introduction
Alzheimer’s disease is a progressive neurodegenerative disorder characterized by irreversible cognitive and memory impairment and various physical problems. This disease is one of major and increasing global health challenges and with increased life expectancy, it is expected to become increasingly prevalent [1,2].
The etiology of Alzheimer’s disease is so complex that it is not fully understood. Various pathogenic factors can be involved in the pathology of this disease. One of the major pathological features of Alzheimer’s is the abnormal accumulation of amyloid plaques and tau tangles in outside and inside neurons, respectively [3]. By interfering neuron-to-neuron communication at synapses and blocking the transport of nutrients and other essential molecules inside neurons, beta-amyloid plaques and tau tangles block may contribute to cell death [3]. Some studies show that the distribution of tau tangles and NFTs are more closely correlated with the severity of AD correlates than the amyloid plaques [4-6]. Although, the abnormal accumulation of amyloid plaques is diagnostic of AD, some studies have shown that the Alzheimer population has a high amyloid burden but no cognitive impairment [7]. Some strong evidence has indicated that some factors than amyloid plaques, such as NFTs and neuroinflammation are important in the loss of memory and other cognitive functions typical of AD [7,8].
Immunopathological studies have shown that inflammatory reactions can be involved in the neuronal degeneration of Alzheimer’s patients [9]. Some studies have shown that activation of microglia (brain-specific macrophages) is associated with increased levels of chemokines and inflammatory cytokines [10]. Besides, the protective effects of non-steroidal anti-inflammatory drugs against the risk of Alzheimer’s disease support the involvement of neuroinflammation hypothesis in Alzheimer’s disease [11]. Although inflammation is a normal process designed to protect the body from injury, disease and infection in the short term, inflammation that persists can be harmful. Pro-inflammatory cytokines such as IFN-γ and IL-17 are abundantly expressed adjacent to amyloid-beta plaques and neurofibrillary tangles [12]. IFN-γ is mainly produced by T cells, natural killer cells and NT cells. Also to a lesser degree, astrocytes, macrophages and microglia can secrete this cytokine [13]. The potential and diverse functions of this cytokine lead to various consequences for the central nervous system [14]. In some studies, elevated levels of IFN-γ were found in the brains of Alzheimer’s patients compared to healthy controls [15]. Also, a decrease in glial inflammation in response to Encephalomyelitis has been observed in IFN-γ deficient mice [16].
IL-17 is proinflammatory cytokine that produced by Th17 cells and other immune cells including neutrophils and macrophages [17]. In Alzheimer’s disease, Th17 cells can infiltrate into the brain parenchyma and thereby induce neuronal inflammation by secreting IL-17 [18]. Also, a study of transgenic Alzheimer’s models found that IL-17 is released by the neutrophils around beta-amyloid plaques [18]. This suggests that IL-17 is probably involved in the pathogenesis of Alzheimer’s and cognitive impairment [17].
As yet, there is no an effective treatment for AD. Although a fuller understanding of the pathogenesis and progression of the disease is required for the discovery of effective treatments for AD, it has seen that because involvement inflammation in Alzheimer’s, anti-inflammatory strategies can be effective. Exercise training with its unique characteristics creates an anti-inflammatory environment in the body [19]. Many studies suggest that regular exercise training reduces pro-inflammatory cytokines and increases anti-inflammatory cytokines [20]. Besides, exercise training can improve neuroprotection and modify the structural and functional changes of neurons in some diseases by improving cardiopulmonary fitness [21]. Animal studies have shown that endurance training can accelerate angiogenesis, neurogenesis, learning and memory in rats [22]. Also, there has been an inverse relationship between brain atrophy and cardiopulmonary fitness in Alzheimer’s patients [23]. Although exercise has anti-inflammatory properties and is effective in improving Alzheimer’s disease, little study has focused on this issue. Therefore, the purpose of the present study was to investigate the effect of aerobic exercise training on IL-17 and IFN-γ as two proinflammatory cytokines, in Alzheimer’s rats.
Materials and Methods
Animals
In this experimental study, 21 male wistar rats (8 weeks old, 195 ± 20 g weight) were used. For familiarization with the environment, all rats were acclimatized under a 12 h/12 h light/dark cycle in 22 ± 3ºC with 45 ± 2 humidity for a week. There was no restriction on access to food and water. The experiments were approved by the Animal Ethics Committee of Azad University of Tehran (Approval No. IR.TUZ.FUM. REC.1398.02).
After acclimatization, all rats have exposed the treadmill to familiarization with it (10 min, speed: 10 m/min in a day for 5 days). In the next stage, the rats were divided randomly into 3 groups: Alzheimer’s (AG), Alzheimer’s+aerobic training (AAG) and control (CG) (without exercise and injection) (7 rats). A week after injection Aβ in the hippocampus, rats of AAG groups had undergone aerobic training for four weeks.
Exercise training protocol
The rats of AAG group trained on a treadmill with zero degrees gradient five days a week for 4 weeks. In this protocol, the speed and duration of training were gradually increased. During the training sessions, these rats were monitored and encouraged by a weak electrical shock (0.5 mA intensity). This tool did not cause stress in the animal (Table 1).
| Week | Set *time (min) |
Rest between sets (min) |
Speed (m/min) |
Days |
|---|---|---|---|---|
| Week 1 | 2*15 | 5 | 10 | 5 |
| Week 2 | 2*15 | 5 | 10 | 5 |
| Week 3 | 3*15 | 5 | 15 | 5 |
| Week 4 | 4*15 | 5 | 15 | 5 |
Table 1: Exercise training protocol.
Preparation of Aβ1–42
In this study, intra-hippocampal injection of Aβ1-42 oligomers was used for induced Alzheimer’s disease in rats. Injection of Aβ1- 42 oligomers into the hippocampus can induce neurodegenerative changes and imitates both behavioural and pathological characteristics of AD [24].
The preparation of Aβ1-42 oligomers has been shown in detail in previous studies [23]. In first, Aβ1-42 peptide as a stock solution was prepared at a concentration of 1 μg/μLin sterile saline solution. Then, this solution was incubated at 37ºC for 4 days to form Aβ1-42 oligomers.
In the next stage, the rats of AAG and AG groups were anesthetized with the intraperitoneal injection of ketamine and xylazine (10 mg/ kg) and underwent stereotaxic surgery [25]. Then, rats were bilaterally injected with Aβ1-42 oligomers into the CA1 area of the dorsal hippocampus by the Hamilton syringe. The injections were performed once daily for 4 consecutive days [26]. Some studies have shown that the in vivo rats model of AD based on 4 consecutive intra-dCA1 injections of Aβo(1-42) could be a valuable tool to study the Aβo(1-42) impact in the hippocampus [26].
Tissue biopsies
24 h after the last training session, all the rats were anesthetized by sodium pentobarbital (40 mg/kg BW). Subsequently, the brain of rats was removed from the skull bone and the hippocampal was separated from the rest of the whole brain on ice and weighed and then frozen with liquid nitrogen and stored at -80ºC for future analysis.
RNA extraction, cDNA synthesis and real-time PCR
In first, right hippocampus tissue was homogenized in TRIzol solution (1 ml) and then, the total RNA was extracted by the kit according to the manufacturer’s instructions (Omega Bio-Tek, USA). After determination of RNA concentration by the Nanodrop spectrophotometer, cDNA was synthesized by using 1000 ng RNA and a high-capacity cDNA reverse transcription kit (Applied Biosystems, USA) according to the manufacturer’s instructions. In next stage, Realtime PCR was performed by using the RealQ Plus 2x Master Mix Green (AMPLIQON, Denmark) and add 1 μl cDNA, 10 μl Master Mix, 1 μl of each of the forward and reverse primers and 7 μl RNase-free water. The detailed operating procedure referred to a previous study [27].
Statistical analysis
Data were expressed as means ± standard deviation. One-way analysis of variance (ANOVA) followed by Bonferroni’s test was used for group analysis (P ≤ 0.05). SPSS software V.22 version was used for statistical analysis.
Results
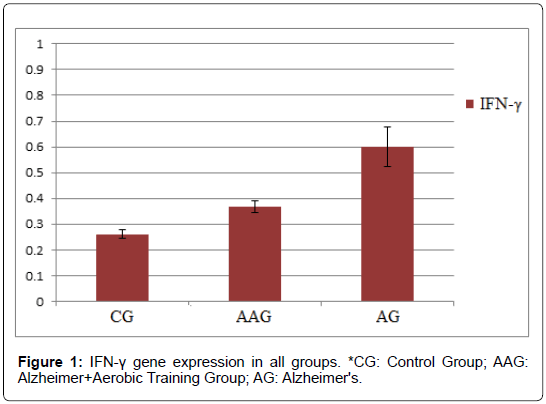
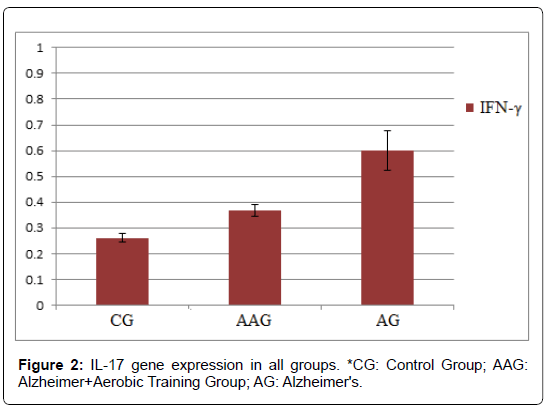
According to the results, the mean ± standard deviation of IFN-γ gene expression in CG, AAG and AG groups were 0.261 ± 0.017, 0.368 ± 0.024 and 0.602 ± 0.077, respectively. Also, mean ± standard deviation of IL-17 gene expression in CG, AAG and AG groups were 0.157 ± 0.029, 0.188 ± 0.045 and 0.389 ± 0.039, respectively.
The results of this study showed that IFN-γ gene expression was significantly lower in AAG group than AG group. Also, IFN-γ gene expression was significantly higher in the AAG and AG groups than CG group (p ≤ 0.05) (Figure 1).
The results showed that amyloid-beta injection significantly increased IFNg mRNA expression in the hippocampus of rats. On the other hand, 4 weeks of aerobic exercise decreased IFNg mRNA expression in these rats. Also, IL-17 gene expression was significantly lower in the AAG than AG group. While the IL-17 expression was significantly higher in the AG group than the CG group, there was no significant difference between the AAG and CG groups (p ≤ 0.05). This showed that amyloid-beta injection significantly increased LI- 17 mRNA expression in the hippocampus of rats. However, 4 weeks of aerobic exercise decreased IL-17 expression in the hippocampus of Alzheimer’s rats induced by amyloid-beta injection (Figure 2).
Discussion
Irregular inflammatory processes are one of the main causes of the progression of certain age-related neurodegenerative diseases, such as Alzheimer’s. Environmental factors and genetic characteristics can trigger inflammatory reactions and lead to release proinflammatory cytokines such as interferon-gamma and interleukin-17 [12]. If these neuro-immune interactions chronically active and not well controlled may lead to changes in neurotransmission, behavior and pathogenesis associated with the disease [12]. In general, inflammation is one of the important factors involved in Alzheimer’s pathology.
The present study showed that IL-17 expression was significantly higher in the hippocampus of Alzheimer’s rats than the control group, which is consistent with other studies [28]. IL-17 is a pro-inflammatory cytokine produced by Th17 cells. The high expression of this cytokine in the hippocampus of Alzheimer’s rats indicates high Th17 inflammatory responses in the central nervous system.
T helper 17 (Th17) cells are highly proinflammatory that produce some proinflammatory cytokines, including interleukin (IL)-17, IL-21 and IL-22 [29-31]. Although, role and mechanism of Th17 cells in AD occurrence and development remains to be provided, some studies showed that cytokines generated by Th17 cells (IL-17, IL-21 and IL- 22) and transcriptional factor involved in differentiation of Th17 cells (RORγ) are significantly higher in AD patients [32]. These cytokines generated by Th17 cells can bind to receptors on neurons and lead to induces neuronal apoptosis or death [33]. This direct contact between Th17 cells and neurons has been seen in Parkinson’s disease (PD) [34]. It is likely that the direct contact mechanism be due to interaction of Fas and Fas ligand (FasL). Fas is a member of the superfamily of tumor necrosis factor receptors that expressed on a wide variety of cell types. However, FasL as membrane and soluble forms, is expressed predominantly in activated T cells. The occupancy of Fas receptor by its ligand induces cell apoptosis [35].
Elevated Th17 inflammatory response can both stimulate nerve inflammation and cause neurodegeneration in Alzheimer’s disease [35]. Kebir et al. reported that endothelial cells of the blood-brain barrier increase the expression of IL-17 receptors in some autoimmune inflammatory diseases, including MS [28]. By binding interleukin-17 to these receptors, tight junctions of the blood-brain barrier are impaired, which facilitates Th17 entry into the brain [28]. It can be hypothesized that a similar mechanism also occurs in Alzheimer’s where increased interleukin-17 in peripheral blood disrupts blood-brain barriers and leading to greater migration of Th17 cells into the brain parenchyma and production of interleukin-17 in the brain. These effects eventually increase the inflammation and neurological degeneration in Alzheimer’s disease [35].
In the present study, it was also found that interferon-gamma expression was higher in the hippocampus of rats with Alzheimer’s disease than the control group, which is consistent with other studies [36]. Interferon-gamma is a cytokine that naturally produced by natural killer cells, T cells and neurons. This cytokine has various immune functions in the central nervous system, including activation of macrophages/microglia and stimulation of macrophages to release toxic oxygen radicals [37]. The expression of interferon-gamma increases with aging and neurological degeneration. Also, interferongamma increases vascular permeability to normal T-cells and natural killer cells as well as expression of TNF-a and IL-1B by microglial [38]. This suggests that interferon-gamma is a key player in stimulating inflammatory reactions in the central nervous system.
Some research has shown that there is a relationship between the expression of interferon-gamma and its effect on neurodegenerative processes with Alzheimer’s disease. Blasko et al. reported a link between gamma interferon and amyloid plaque formation [39]. Meda et al. also reported that interferon-gamma increased microglial TNF-a production, which stimulated the formation of AB peptides in vitro [40]. Overall, these studies show that interferon-gamma increases the accumulation of AB peptide and reduces the clearance of pathogenic peptides in Alzheimer’s rats [13].
The present study also found that exercise training significantly decreased the expression of IL-17 and IFN-γ. To our knowledge, no research has examined the effect of exercise training on IL-17 in Alzheimer’s animal models in the literature. There are also very few studies examining the effects of exercise training on IFN-y in these models. Jensen et al. examined the impact of 16 weeks of moderate to vigorous aerobic exercise (60 min per session and 3 sessions per week) on interferon-gamma plasma and cerebrospinal fluid in patients with Alzheimer’s disease. Results showed that after 16 weeks of training, plasma levels of interferon-gamma were higher in the control group than in the exercise group. There was also a significant correlation between body mass index and cerebrospinal fluid interferon-gamma levels. The results of this study indicate the effect of exercise on markers of inflammation in Alzheimer’s patients [41]. Also, Tuon et al. showed that strength training and treadmill training reduced gamma interferon in Parkinson’s mice. This suggests that strength and endurance training can have a neuroprotective role [42].
Some studies have shown that exercise training can potentially affect inflammation by enhancing the tight junctions of blood-brain barriers, reducing microglial activity, reducing macrophage infiltration into the brain, decreasing the expression of inflammatory cytokine receptors in blood-brain barriers, as well as increasing anti-inflammatory cytokines. However, further research is needed in this subject said the current study supports the anti-inflammatory role of exercise training in Alzheimer’s disease [43]. However, the molecular mechanisms of the effect of exercise on neuroinflammation have not been well understood. Therefore, more research is needed to provide better interpretations.
Conclusion
As seen in the present study, exercise training may help to alleviate the neuro-inflammation conditions that occur in Alzheimer’s disease by reducing the expression of pro-inflammatory cytokines IL-17 and IFN-γ. In the absence of effective pharmaceutical treatment of AD, such information is of even greater importance to the individual and society.
References
- Wimo A, Guerchet M, Ali GC, Wu YT, Prina AM, et al. (2017) The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 13: 1-7.
- Wu YT, Beiser AS, Breteler MM, Fratiglioni L, Helmer C, et al. (2017) The changing prevalence and incidence of dementia over time-current evidence. Nat Rev Neurol 13: 327-339.
- Shoghi-Jadid K, Small GW, Agdeppa ED, Kepe V, Ercoli LM, et al. (2002) Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. Am J Geriatr Psychiatry 10: 24-35.
- Crews L, Masliah E (2010) Molecular mechanisms of neurodegeneration in Alzheimer's disease. Hum Mol Genet 19: R12-20.
- Ingelsson M, Fukumoto H, Newell KL, Growdon JH, Hedley-Whyte ET, et al. (2004) Early Abeta accumulation and progressive synaptic loss, gliosis and tangle formation in AD brain. Neurology 62: 925-931.
- Nelson PT, Braak H, Markesbery WR (2009) Neuropathology and cognitive impairment in Alzheimer disease: A complex but coherent relationship. J Neuropathol Exp Neurol 68: 1-14.
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, et al. (2008) Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 65: 1509-1517.
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, et al. (2012) Clinical and biomarker changes in dom- inantly inherited Alzheimer’s disease. New Engl J Med 367: 795-804.
- Dorey E, Chang N, Liu QY, Yang Z, Zhang W (2014) Apolipoprotein E, amyloid-beta and neuroinflammation in Alzheimer’s disease. Neurosci Bull 30: 317-330.
- Swardfager W, Lanctôt K, Rothenburg L, Wong A, Cappell J, et al. (2010) A meta-analysis of cytokines in Alzheimer's disease. Biol Psychiatry 68: 930-941.
- Vlad SC, Miller DR, Kowall NW, Felson DT (2008) Protective effects of NSAIDs on the development of Alzheimer disease. Neurology 70: 1672-1677.
- Su F, Bai F, Zhang Z (2016) Inflammatory cytokines and Alzheimer’s disease: A review from the perspective of genetic polymorphisms. Neurosci Bull 32: 469-480.
- Yamamoto M, Kiyota T, Horiba M, Buescher JL, Walsh SM, et al. (2007) Interferon-γ and tumor necrosis factor-α regulate amyloid-β plaque deposition and β-secretase expression in Swedish mutant APP transgenic mice. Am J Pathol 170: 680-692.
- Huberman M, Shalit F, Roth-Deri I, Gutman B, Brodie C, et al. (1994) Correlation of cytokine secretion by mononuclear cells of Alzheimer patients and their disease stage. J Neuroimmunol 52: 147-152.
- Tran EH, Prince EN, Owens T (2000) IFN-γ shapes immune invasion of the central nervous system via regulation of chemokines. J Immunol 164: 2759-2768.
- Zenaro E, Pietronigro E, Bianca VD, Piacentino G, Marongiu L, et al. (2015) Neutrophils promote Alzheimer's disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med 21: 880-886.
- McManus R, Higgins S, Mills K, Lynch M (2014) Respiratory infection promotes T cell infiltration and amyloid-β deposition in APP/PS1 mice. Neurobiol Aging 1: 109-121.
- Isanejad A, Amini H (2019) Physical exercise and heat shock proteins. In: Chaperokine Activity of Heat Shock Proteins. Springer pp: 247-277.
- Hess N, Smart N (2017) Isometric exercise training for managing vascular risk factors in mild cognitive impairment and Alzheimer’s disease. Front Aging Neurosci 9: 48.
- Bugg J, Head D (2011) Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol Aging 1: 506-514.
- Mattson M, Chan S, Duan W (2002) Modification of brain aging and neurodegenerative disorders by genes, diet and behavior. Physiol Rev 7: 637-672.
- Erickson K, Voss M, Prakash C (2011) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 15: 3017-3022.
- Liu T, Bai W, Wang J, Tian X (2016) An aberrant link between gamma oscillation and functional connectivity in Aβ1-42-mediated memory deficits in rats. Behav Brain Res 15: 51-58.
- Gandy S, Greengard P (1994) Processing of Alzheimer aβ-amyloid precursor protein: Cell biology, regulation and role in Alzheimer’s disease. In: Amyloid protein precursor in development, aging and Alzheimer ’s disease. Springer, Berlin, Heidelberg. pp: 100-120.
- Adler JR (1993) Apparatus for and method of performing stereotaxic surgery. Accuray Inc, assignee United States patent US-5207223A.
- Faucher P, Mons N, Micheau J, Louis C, Beracochea DJ (2016) Hippocampal injections of oligomeric amyloid β-peptide (1-42) induce selective working memory deficits and long-lasting alterations of ERK signaling pathway. Front Aging Neurosci 11: 245.
- Liao W, Zhang R, Dong C, Yu Z, Ren J (2016) Novel walnut peptide-selenium hybrids with enhanced anticancer synergism: Facile synthesis and mechanistic investigation of anticancer activity. Int J Nanomedicine 11: 1305-1321.
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, et al. (2007) Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 13: 1173-1175.
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, et al. (2005) IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201: 233-240.
- Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, et al. (2007) IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 448: 484-487.
- Miossec P, Korn T, Kuchroo VK (2009) Interleukin-17 and type 17 helper T cells. N Engl J Med 361: 888-898.
- Saresella M, Calabrese E, Marventano I, Piancone F, Gatti A, et al. (2011) Increased activity of Th-17 and Th-9 lymphocytes and a skewing of the post-thymic differentiation pathway are seen in Alzheimer’s disease. Brain Behav Immun 25: 539-547.
- Appel SH, Beers DR, Henkel JS (2010) T cell-microglial dialogue in Parkinson's disease and amyotrophic lateral sclerosis: Are we listening? Trends Immunol 31: 7-17.
- Nagata S, Golstein P (1995) The Fas death factor. Science 267: 1449-1456.
- Zhang J, Ke KF, Liu Z, Qiu YH, Peng YP (2013) Th17 cell-mediated neuroinflammation is involved in neurodegeneration of aβ1-42-induced Alzheimer’s disease model rats. PLoS One 8: e75786.
- Blasko I, Veerhuis R, Stampfer-Kountchev M, Saurwein-Teissl M, Eikelenboom P, et al. (2000) Costimulatory effects of interferon-and interleukin-1 or tumor necrosis factor on the synthesis of A1-40 and A1-42 by human astrocytes. Neurobiol Dis 7: 682-689.
- Mastrangelo MA, Sudol KL, Narrow WC, Bowers WJ (2009) Interferon-γ differentially affects Alzheimer’s disease pathologies and induces neurogenesis in triple transgenic-AD mice. Am J Pathol 175: 2076-2088.
- Mohammadpour JD, Hosseinmardi N, Janahmadi M (2015) Non-selective NSAIDs improve the amyloid-β-mediated suppression of memory and synaptic plasticity. Pharmacol Biochem Behav 132: 33-41.
- Blasko I, Veerhuis R, Stampfer-Kountchev M, Saurwein-Teissl M, Eikelenboom P, et al. (2000) Costimulatory effects of interferon-and interleukin-1 or tumor necrosis factor on the synthesis of A1-40 and A1-42 by human astrocytes. Neurobiol Dis 7: 682-689.
- Meda L, Cassatella MA, Szendrei GI, Otvos L, Baron P, et al. (1995) Activation of microglial cells by β-amyloid protein and interferon-γ. Nature 374: 647.
- Jensen CS, Bahl JM, Ostergaard LB, Hogh P, Wermuth L, et al. (2019) Exercise as a potential modulator of inflammation in patients with Alzheimer's disease measured in cerebrospinal fluid and plasma. Exp Gerontol 121: 91-98.
- Tuon T, Souza PS, Santos MF, Pereira FT, Pedroso GS, et al. (2015) Physical training regulates mitochondrial parameters and neuroinflammatory mechanisms in an experimental model of Parkinson’s disease. Oxid Med Cell Longev.
- Kelly AM (2018) Exercise-induced modulation of neuroinflammation in models of Alzheimer’s disease. Brain Plast 4: 81-94.
Citation: Samadi F, Amini H (2019) The Effects of Aerobic Training on the IL-17 and IFN-γ Expression in the Hippocampus of Male Wistar Rats with Alzheimer Disease. J Alzheimers Dis Parkinsonism 9:480. DOI: 10.4172/2161-0460.1000480
Copyright: © 2019 Samadi F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4456
- [From(publication date): 0-2019 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 3651
- PDF downloads: 805