The Effectiveness of Motor Imagery on Motor Performance in Individuals with Parkinson's Disease: A Systematic Review
Received: 02-Jan-2023 / Manuscript No. JADP-23-82384 / Editor assigned: 04-Jan-2023 / PreQC No. JADP-23-82384 (PQ) / Reviewed: 18-Jan-2023 / QC No. JADP-23-82384 / Revised: 25-Jan-2023 / Manuscript No. JADP-23-82384 (R) / Published Date: 31-Jan-2023 DOI: 10.4172/2161-0460.1000559
Abstract
Background: Research has shown that Motor Imagery (MI) can effectively improve symptoms related to neurological conditions such as Parkinson's Disease (PD). PD is a neurodegenerative, progressive condition with no known cause or cure. As PD progresses, individuals affected may have distinct PD symptoms that negatively impact motor performance, including tremors, stiffness, bradykinesia, gait, and balance issues. MI is a cognitive process in which an individual visualizes they are performing an action or movement without carrying out the physical activity. This systematic review aims to analyze the effectiveness of using MI as an intervention on motor performance in individuals with PD.
Methods: A search string of "parkinson’s disease” OR “parkinson disease” AND “motor imagery” with modifiers of peer review, 2016-2022, and the English language was run across four databases. This returned 277 results that were further screened with inclusion and exclusion criteria and quality of evidence measures, leaving four articles for analysis. To evaluate the risk of bias, the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) tool was used. Articles were analyzed and coded by sample size, intervention, results, and the overall impact of MI on PD. This systematic review was registered through PROSPERO.
Results: MI was found to have positive outcomes in improving participants’ general motor functions.
Conclusion: Evidence supports using MI as an intervention to improve motor performance for individuals with PD and other neurodegenerative conditions. Further research would provide parameters on how to incorporate MI as an intervention.
Keywords: Alzheimer’s disease; Dementia; Anti-hypertensive drugs; ALLHAT trial; Medicare claims data
Introduction
The number of Americans living with Parkinson's Disease (PD) is currently at one million, with projections to increase to 1.2 million by 2030. According to the Centers for Disease Control and Prevention, PD is the 14th most common cause of mortality in the United States. PD is a neurodegenerative, progressive condition with no known cause or cure [1-8]. As PD progresses, individuals affected may have distinct PD symptoms that negatively impact motor performance, including tremors, stiffness, bradykinesia, gait, and balance issues. Non-movement symptoms, such as cognitive changes, sleep disturbances, urinary incontinence, and constipation, may co-occur with PD [9]. In addition, mental health issues may arise, including depression and anxiety. Both motor and non-motor symptoms may negatively influence the quality of life for individuals, specifically, abilities related to self-care, sleep quality, mobility, and community management.
Motor Imagery (MI) is a cognitive process in which an individual visualizes they are performing an action or movement without carrying out the physical movement. MI requires deliberate inhibition of movement and conscious activation of brain areas involved in movement planning and execution. Studies have indicated that identical brain areas are activated during actual movement performance as during MI. MI leads to the brain development of neuroplasticity in the primary motor cortex and movement-specific central activation patterns, similarly to how motor performance activates that area [10]. MI is safe, non-invasive, cost-efficient, easily facilitated by practitioners, and can be integrated into an individual's treatment plan in various settings [11]. No training or specialized certification is required to implement MI as an intervention.
This systematic review aimed to investigate how MI affects motor performance in individuals diagnosed with Caligiore et al. published a systematic review on a similar topic however incorporated the use of action observation as a component of the intervention [12].
Conversely, this systematic review will focus solely on the impact of MI on motor performance. Since PD primarily affects an individual’s motor output, the researchers hypothesize that using MI alone as an intervention will produce positive outcomes on motor performance.
MI has been used with various populations, as found in the research literature. For example, Silva et al. reported that stroke survivors who had difficulty completing basic daily tasks that required the initiation of motor movement improved their walking speed after using MI as an intervention approach [13]. Yin et al. found lower body function improved in stroke survivors after using MI techniques [14].
Materials and Methods
The researchers conducted a scoping search to review the current literature on MI and PD. This systematic review followed the PRISMA guidelines. In collaboration with a research librarian, a search string was developed and used across four databases (Table 1).
| Database | Search string and filters |
|---|---|
| CINAHL | (“parkinson’s disease” OR “parkinson disease”) and “motor imagery” Date: 2016-Present (2022) |
| APA Psych Info and Psych Articles | (“parkinson’s disease” OR “parkinson disease”) and “motor imagery” Date: 2016-Present (2022) |
| PubMed | (“parkinson’s disease” OR “parkinson disease”) and “motor imagery” Date: 2016-Present (2022) |
| ProQuest Healthcare Administration | (“parkinson’s disease” OR “parkinson disease”) and “motor imagery” Date: 2016-Present (2022) |
Table 1: Databases and search terms.
Literature search
Articles were retrieved from the following databases: CINAHL (n=34), APA Psych Info and APA Psych Articles (n=68), PubMed (n=102), and ProQuest Healthcare Administration (n=73). The search was filtered from 2016 to 2022 removing 147 articles. Researchers were assigned to a database and individually ran the search string with the additional modifier of 2016 to 2022. Results were imported to RefWorks, an online reference management software. One researcher (AP) completed a grey literature search across the following databases: OpenGrey, System for Information on Grey Literature in Europe, NY Academy of Medicine Grey Literature Report, WHO Library Database, and MedNar returning zero results that met the inclusion criteria (Figure 1).
Screening and selection
Duplicate articles were removed from RefWorks using an automatic deduplication tool and documented on the PRISMA flowchart (n=6). The remaining 124 articles were divided and screened based on titles and keywords. Ten additional articles were identified as duplicates and removed manually. All researchers (SS, EF, CM, EO, AP) screened the remaining articles based on abstracts relevant to the review and compared against inclusion and exclusion criteria. Full-text review and quality appraisal were completed on ten articles (Table 2).
| Inclusion criteria | Exclusion criteria |
|---|---|
| Parkinson's disease | Mental Imagery |
| Motor Imagery | Action Observation |
| Peer-reviewed research articles | Diagnoses other than Parkinson’s Disease |
| Adults (18 and older) | Articles published prior to 2016 |
| Articles in English | Virtual Reality Intervention |
| Published 2016-2022 | Dance |
| - | Audiology |
| - | Systematic Reviews |
| - | Pediatric Population |
| - | Parkinsonism Diagnosis |
| - | Qualitative Reviews |
| - | Video Guided Motor Imagery |
Table 2: Inclusion and exclusion criteria.
Quality appraisal
Two teams of two researchers (EO, AP and CM, EF) each evaluated five articles using the McMasters Quantitative Critical Review Form. Individually, researchers completed this review before collaborating and coming to a consensus on the quality of each article. Four researchers (EF, CM, EO, AP) discussed the quality review, and the results were reviewed until an agreement was reached. Three articles were immediately deemed appropriate for this review as they included populations other than those with PD, three were removed due to exclusion criteria, and four were undecided. The researchers re-analyzed the four articles until a consensus was reached. One article was included, and one required further discussion after two were deemed inappropriate for this review. In consultation with the fifth researcher (SS), the final article was removed due to exclusionary criteria.
Data extraction
To ensure all necessary data were extracted and analyzed, the research team piloted the evidence table with one article making minor adjustments. Two researchers (EF, AP) then extracted the data (citation, sample size, level of evidence, intervention, results, and overall effect) from the remaining three articles (Table 3).
| Author (date) | Sample size (age, gender) | Level of evidence | Intervention | Results | End effect of MI on PD |
|---|---|---|---|---|---|
| Fischer et al. (2017) | 11 (1 drop-out), 7 male, 3 female Age range | Level III | Deep brain stimulation (DBS) surgery in the Subthalamic nucleus soft tissue neck (STN) 2-7 days prior to recording the DBS improvement motor symptoms | Results show that in the early stages of imaging the gripping strength, gamma activity within the brain increased (p<0.01). | + |
| Bilaterally grip the dynamometer with max | |||||
| effort 3x and hold for 4.5 s. | |||||
| Grip 15%, 50%, and 85% of max force for 4.5s. Then grip max force for 4.5s. | |||||
| Completed all for 3-5 trials | |||||
| Motor imagery- Dynamometers set aside and patients were asked to imagine gripping without activating any muscles for 2.5s. | |||||
| Tinaz et al. (2022) | (Mot or Imagery- Neurofee dback) MI-NF Group Age=66.2 ± 8.1 (45.3-79. 3) Gender= 10 females, 12 males |
Level I | Visit1: Researchers completed several evaluations which included the MDS-Unified Parkinson's Disease Rating Scale (MDS-UPDRS), motor function tests, the Montreal Cognitive |
Although there was not a significant difference between the groups, the subjects showed improvement in their anxiety, depression and fatigue based upon the MOCA scores | + |
| Assessment (MOCA) and several others | Both groups reported improved body awareness | ||||
| Visit 2: Subjects were educated on the main principles of the imagery tasks and the overall benefits related to function |
Both groups reported improved awareness during daily tasks | ||||
| (Visual Imagery) VI Group Age 65.7 =-8.1 (47.8-79. 7) Gender= 10 females, 12 males |
The remainder of the visits were two weeks apart from each other | related to gross movement (arm swing and walking awareness, “hook and punch” boxing) |
|||
| MI-NF group: subjects participated in mindful activities via guided audio recordings | |||||
| followed by guided motor recordings. Afterwards, the subjects participated in a functional task |
|||||
| VI group: subjects participated in guided visual imagery via audio recordings minus the functional task participation after listening to the audio recordings | |||||
| Tinaz et al. (2018) | Group 1: 5 females, 5 males Age=62.6 ± 10.8 Group 2:4 Male 4 Female Age= 66.0 ± 8.5 |
Level III | Participants showed positive outcomes related (Motor Imagery) MI practice for 10-15 minutes daily Participants documented in a diary daily Researchers measured heartbeat counting via Magnetic Resonance Imaging (MRI) | MI demonstrates positive and negative neurofeedback based upon body sensations and emotional state related to MI being practiced in a relaxed state resulting in improved motor outcomes | + |
| Subram anian et al. (2016) | 30 PD patients (26 males; 4 females) |
Level I | Group 1: completed homework using MI strategies in the first 4 weeks and then virtual reality component for the next 6 weeks Group 2: participated in Multiple Object Tracking (MOT) via gaming device for 10 weeks |
No statistical difference between group 1 and 2 however; there was an increased activity in the supplementary motor area (SMA) of the brain when using MI in group 2 | + |
Table 3: Evidance table for data extracted.
Risk of bias
To assess the risk of bias, a team of two researchers (EO, CM) adapted and piloted a Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) Risk of Bias table from the Grade Handbook on all four articles [15]. The GRADE tool is a systematic approach to rating the certainty of evidence. A third researcher (SS) was involved in resolving questions and disagreements between the two reviewers' judgments.
Effect size
The effect size was determined using the mean difference and standard deviation of the two RCT articles.
Results and Discussion
Study characteristics
The initial search yielded 277 articles. Articles were screened using the titles, abstracts, and year (after 2016), leaving ten full-text articles for review [16,17]. Inclusion criteria were met by four articles [4,16,18,19]. Articles were excluded if they used different populations other than PD, incorrect interventions, and included exclusion criteria.
Based on Tomlin and Borgetto, two of the four articles were Level I Randomized Control Trials (RCTs), and two were Level III outcome research studies [16]. The studies were analyzed according to outcome measures, how MI was applied as an intervention and its effect on motor functioning for individuals with PD. The sample size ranged from 11 to 44 people, with a total of 103 participants (35 females and 67 males). The average age was 62, with a range of 45 to 79.
Outcome measures
The Movement Disorders Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS) was used in three of the four articles as part of their initial evaluation of the participants' disease severity and determining the stage of PD [16,18,19]. In addition, the researchers from these three articles utilized Magnetic Resonance Imaging (MRI) to analyze neurofeedback in different brain locations. The Parkinson's Disease Questionnaire-39 (PDQ-39) was another outcome measure from two studies that examined various aspects of PD [16,18].
Tinaz et al. assessed the MI skills of the participants as preliminary measures using the Movement Imagery Questionnaire-3 (MIQ-3) [18,19]. Fischer et al. contrasted with the other three articles, as the researchers applied Electromyographic (EMG) electrodes to record patients' abilities to perform motor imagery [4].
Intervention
Three out of four articles utilized MI as an intervention to improve motor outcomes in participants with PD. The MI intervention Tinaz et al. utilized was kinesthetic MI training [17]. Researchers in this study educated the Motor Imagery-Neurofeedback group (MI-NF) on the main principles of MI and the benefits of improving overall function. This same group participated in a mindfulness body scan through an audio recording. Participants practiced the kinesthetic MI basic movements (i.e, raise your knee, tap your foot) and imagined themselves performing these movements. Following these MI movements, participants practiced complex whole-body movements associated with everyday tasks (such as walking, balancing, and workouts), concentrating on body sensations and emotions observed during MI movements [17].
Using kinesthetic MI as an intervention for 10-15 minutes each day to determine if training strengthened the connection between the right insula and dorsomedial frontal cortex, Tinaz et al. discovered that participants had positive results [19]. Participants tracked their responses in a diary, noting if they engaged in mindfulness practice, how long they engaged in MI practice, the environment and subject of MI, and the justifications for choosing certain content.
In a study by Subramanian et al., researchers utilized MI with the fMRI-based NF (experimental group) for three sessions during the intervention's 2nd, 6th, and 12th weeks. Homework was required to practice MI for the first four weeks of intervention. This same group also received six weeks of Motor Training (MOT) with a Nintendo Wii. The second group of this study only received MOT through the Nintendo Wii for ten weeks. The fMRI-NF with MI and MOT was deemed a safe intervention that showed improvement in the motor symptoms of participants [16].
Fisher et al.was the only study out of the four that implemented MI as an intervention with PD participants to activate the Subthalamic Nucleus (STN) and Local Field Potential (LEP). Researchers asked participants to set aside the dynamometers following actual gripping to perform imagined gripping (for 2.5 s), which was assessed through EMG. This intervention had a positive effect as the STN LEP activity was noted without muscle activity [4].
Impact of motor imagery on motor functions
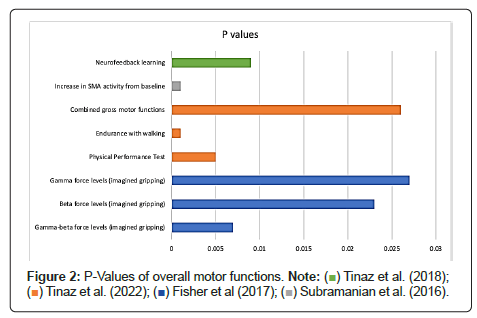
MI positively improved participants’ overall motor functions [4,16,18,19]. Fisher et al. found significant differences in the gamma-beta power changes based on real vs. imagined gripping, contralateral and ipsilateral gripping, and the amount of force for both early and late window gripping [4]. Figure 2 provides the p values regarding the gamma-beta, beta, and gamma data for the early window grip onset.
According to Tinaz et al., [18] after training for the Physical Performance Test, endurance walking, the gross motor combination, Motor Imagery Neurofeedback (MI-NF), and the active control visual imagery (VI) groups dramatically increased their gross motor performance and imaging skills (Figure 2). The primary outcome measure (MDS-UPDRS part III) pre/post scores were not significantly affected (p=0.279) for the MI-NF group. All participants in the MI-NF group reported subjective improvements in kinesthetic body awareness and increased mindfulness and sense of presence during everyday activities. The sense of progress led participants to correct their limb symmetry and coordination, improving arm swing and stride length while walking and more coordinated upper and lower body movements during exercises [18].
Tinaz et al. [19] found that participants who utilized kinesthetic MI as an intervention for 10-15 minutes each day had positive results based on subjective data. The complex movements during MI training could provide either positive or negative neurofeedback, thus strengthening the connection between the right insula and the dorsomedial frontal cortex. Forty-nine percent reported their reason for using MI of choice was to improve motor outcomes. Positive neurofeedback was determined by participants who reported more vivid body sensations during MI, such as “feeling the movement, stretch, and weight in joints and muscles” [19]. When MI was implemented, 72% of individuals reported “pleasant” body sensations, and 57% were “comfortable.” Three participants provided descriptive feedback that showed using MI strategies improved their overall motor performance throughout their daily tasks, such as having more control and the ability to relax and focus. The metric for results of MDS-UPDRS part III motor examination was used as a baseline and for neurofeedback training. After pre/post testing, the difference was not statistically significant (p=0.871) [19].
Subramanian et al. found the difference between the first group (neurofeedback with motor training) and the second group (motor training only) was not statistically significant (p=0.11) regarding the primary outcome measure (MDS-UPDRS-MS). However, functional magnetic resonance imaging analysis indicated an increase in the Supplementary Motor Area (SMA) during Neurofeedback (NF) sessions in the first group. The whole brain analysis reflected activation of the SMA across all sessions with significant activity compared to baseline in the subthalamic nucleus, cerebellum, frontal areas, insula, putamen, and anterior cingulate (Figure 2) [16].
Effect size
The effect size was analyzed with the available data from the two RCTs, see appendix B. Effect size (ES) was calculated by comparing the mean difference and standard deviation, demonstrating no to low practical significance when comparing the intervention to the control (Table 4) [16,18] .
| Article | Outcome Measure | Effect Size (ES) |
|---|---|---|
| Tinaz et al. (2022) | Physical Performance Test | 0.21 |
| Tinaz et al. (2022) | Endurance (walking) | 0.18 |
| Subramanian et al. (2016) | MDS-UPDRS-M-DL | 0.04 |
| Subramanian et al. (2016) | GaitRite-Walking cadence | 0.14 |
Table 4: Effect size for different performed.
Risk of bias
The GRADE tool was used to assess the risk of bias for each article and consists of five categories: imprecision, inconsistency, indirectness, publication bias, and study limitations. The articles were assessed and rated as either high, moderate, low, or very low [14]. A rating of high means the authors are confident that the quality of evidence is certain and effective; a very low rating means there is little to no confidence in the quality of evidence. [14]. See Table 3 for further details. Imprecision was rated high in Fisher et al. and Subramanian et al. [4,16]. Both studies had high confidence intervals (95%). Three out of four studies rated low inconsistency due to unexplained heterogeneity of the results [4,16,18]. Tinaz et al. had a very low rating due to the researchers using a mixed methods design, no control group, and a large variation in the results [19]. Two articles had high levels of indirectness as they did not have varying populations, interventions, or outcomes [16,18]. All of the articles were published and peer-reviewed, and levels of evidence were higher than observational studies; thus, a high rating was indicated for publication bias. Three out of four articles were rated high for study limitations, as the team of researchers perceived the disclosed limitations as minor (Table 5) [4,16,18].
| Study | Imprecision | Inconsistency | Indirectness | Publication bias | Study Limitations |
|---|---|---|---|---|---|
| Fischer et al. (2017) | ++++ | ++ | +++ | ++++ | ++++ |
| Tinaz et al. (2022) | ++ | ++ | ++++ | ++++ | ++++ |
| Tinaz et al. (2018) | ++ | + | +++ | ++++ | +++ |
| Subramanian et al. (2016) | ++++ | ++ | ++++ | ++++ | ++++ |
Note: Categories are as follows: +: low risk of bias, ++: low risk of bias, +++: moderate risk, ++++: high risk of bias. Risk of bias table adapted from “Grade Handbook” by H. Schünemann, J. Brożek, G. Guyatt, and A. Oxman, 2013, GradePro.
Table 5: Risk of bias adapted.
The use of MI as an intervention for individuals with PD led to a variety of positive outcomes, including improvement in gait, coordination, body awareness, gross motor performance, imagery skills, grip strength, significant differences in gamma-beta power changes, SMA brain region activation, and brain regulation for motor symptoms [4,16,18,19]. These findings are consistent with previous research investigating MI as a PD intervention. For example, in 2021, a research study was completed by Huang et al., and it supported the hypothesis that MI helps to compensate for neurological deficits in PD to improve gait performance [18]. Similarly, in 2021, Shapiro reported that when MI was used as a cointervention for individuals with PD along with physical therapy and virtual reality, participants had significantly decreased motor symptoms, including tremors, bradykinesia, and postural instability [19].
Researchers deemed it necessary to investigate all motor function outcomes of MI rather than focus on a specific outcome such as gait. Research demonstrates that MI is influential in multiple aspects of motor performance and should be recognized for its broad treatment potential for individuals with PD [20].
The findings of this systematic review can be generalized to other neurodegenerative disease populations, such as multiple sclerosis and ALS, along with conditions such as stroke, as reported in the literature [13,21]. One must consider that the results of MI on motor performance are subjective to the timeline of a progressive disease. However, that does not diminish the generalizability these findings have for a positive outcome on motor performance.
When analyzing the effect size, it is important to consider the nature of PD as an incurable, neurodegenerative disease with rehabilitation treatment goals focusing on sustainability and overall QOL. Although MI did not report a significant effect size in the two randomized control trials, multiple positive impacts were captured in various ways. MI is noninvasive, safe, user-friendly, and cost-effective, with no considerable risks or adverse effects that would determine MI to be an unsuitable intervention in a treatment setting for individuals with neurodegenerative diseases.
Limitations
One Level III study had a small sample size and lacked a control group [18]. In contrast, another study had a four-week intervention period with no follow-up, limiting the ability to analyze the longevity of the results [19]. The limitations of the evidence in this systematic review included the risk of imprecision, which was found to be low in two articles [18,19]. In addition, three out of four studies rated a low inconsistency due to unexplained heterogeneity of the result [4,16,18].
Implications
Synthesis of current research demonstrating MI as an effective intervention provides sufficient support for utilizing MI for individuals with PD to address motor limitations. This systematic review offers data that supports integrating motor imagery into clinical practice as a co-intervention to manage motor performance [9]. MI has shown to be an effective intervention with PD. Future studies should explore the use of PD for extended durations and the optimal time to administer MI interventions within the progression of mild to moderate PD.
Conclusion
The evidence demonstrates that MI is effective as an intervention to improve motor performance in PD patients and other neurological conditions such as stroke. The patient-reported outcomes of using MI are also positive and promising. With innovative and cost-effective intervention options such as MI, the motor performance of individuals with PD will improve. It’s imperative to continue producing high levels of evidence on the use of MI in various settings with various diagnoses to understand the impact on motor performance further.
References
- Parkinson's Foundation (2022) What is Parkinson's? Retrieved.
- Parkinson's Foundation (2022) Movement symptoms. Retrieved.
- Mulder T. (2007) Motor imagery and action observation: cognitive tools for rehabilitation. J Neural Transm 114(10): 1265–1278.
- Fischer P, Pogosyan A, Cheeran B, Green AL, Aziz TZ, et al. (2017) Subthalamic nucleus beta and gamma activity is modulated depending on the level of imagined grip force. Exp Neurol 293:53-61.
- Subramanian L, Morris MB, Brosnan M, Turner DL, Morris HR et al. (2016) Functional magnetic resonance imaging neuro feedback-guided motor imagery training and motor training for Parkinson’s disease: Randomized trial.
- Tinaz S, Kamel S, Aravala SS, Elfil M, Bayoumi A, et al. (2022) Neurofeedback-guided kinesthetic motor imagery training in Parkinson’s disease: Randomized trial. Neuroimage Clin 34:102980.
- Tinaz S, Para K, Vives-Rodriguez A, Martinez-Kaigi V, Nalamada K, et al. (2018) Insula as the interface between body awareness and movement: A neurofeedback-guided kinesthetic motor imagery study in Parkinson’s disease.
- Parkinson's Foundation (2022) Statistics. Retrieved.
- Parkinson’s Foundation (2022) Non-Movement symptoms. Retrieved.
- Abbruzzese G, Avanzino L, Marchese R, Pelosin E (2015) Action Observation and Motor Imagery: Innovative Cognitive Tools in the Rehabilitation of Parkinson's Disease. Parkinsons Dis 124214.
- Rajesh T. (2015) Effects of Motor Imagery on Upper Extremity Functional Task Performance and Quality of Life among Stroke Survivors. Disability, CBR & Inclusive Development 26(1): 109–124.
- Caligiore D, Mustile M, Spalletta G, Baldassarre G (2017) Action observation and motor imagery for rehabilitation in parkinson's disease: A systematic review and an integrative hypothesis. Neurosci Biobehav Rev 72:221-222.
- Sen EI (2021) Is motor imagery effective for gait rehabilitation after stroke? A Cochrane review summary with commentary. NeuroRehabilitation 49(2):329-331.
- Yin XJ, Wang YJ, Ding XD, Shi TM (2021) Effects of motor imagery training on lower limb motor function of patients with chronic stroke: A pilot single‐blind Randomized Controlled trial. Int J Nurs Pract 28(3):e12933.
- Schünemann H, Brożek J, Guyatt G, Oxman A (2013) Grade Handbook. GradePro.
- Tomlin G, Borgetto B (2011) Research Pyramid: A new evidence-based practice model for occupational therapy. Am J Occup Ther 65(2):189-196.
- Huang HC, Chen CM, Lu MK, Liu BL, Li CI, et al. (2021) Gait-related brain activation during motor imagery of complex and simple ambulation in parkinson's disease with freezing of gait. Front Aging Neurosci 22;13:731332
- Shapiro L (2022) Virtual reality, motor imagery plus PT ease Parkinson’s motor symptoms.
- Abraham A, Hart A, Andrade I, Hackney ME (2018) Dynamic neuro-cognitive imagery improves mental imagery ability, disease severity, and motor and cognitive functions in people with Parkinson's disease. Neural Plast 6168507.
- Nascimento IAP da S, Santiago LM de M, de Souza AA, Pegado C de L, Ribeiro TS, et al. (2019) Effects of motor imagery training of Parkinson's disease: A protocol for a randomized clinical trial. BioMed Central Trials 20(1):626.
- Tamir R, Dickstein R, Huberman M (2007) Integration of Motor Imagery and Physical Practice in Group Treatment Applied to Subjects With Parkinson’s Disease. Neurorehabil Neural Repair 21(1):68-75.
Citation: Schoellig S, Forsyth E, Parker A, Mortimer C, O’Neill E (2023) The Effectiveness of Motor Imagery on Motor Performance in Individuals with Parkinson's Disease: A Systematic Review. J Alzheimers Dis Parkinsonism 13: 559. DOI: 10.4172/2161-0460.1000559
Copyright: © 2023 Schoellig S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2404
- [From(publication date): 0-2023 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 2137
- PDF downloads: 267