The Effect of Tonsillectomy on the Salivary Immune Factors: A Systematic Review and Meta-Analysis
Received: 21-Jan-2015 / Accepted Date: 16-Feb-2015 / Published Date: 25-Feb-2015 DOI: 10.4172/2161-119X.1000187
Abstract
Background & objectives: The effect of tonsillectomy on the immune system is a controversial issue. The debate is largely based on contradictory findings in the literature. However, it is unlikely for tonsils to produce a negative systemic effect and it is more logical to think about a local effect that can be transient or long lasting. The aim of this systematic review is to analyze the observed changes in salivary immune factors following tonsillectomy and understand their clinical significance.
Materials & methods: Systematic review of the English literature was performed using Medline, Embase and Cochrane. We used the terms tonsillectomy, adenotonsillectomy, humoral immunity, immune system, saliva in various combination to look for pertinent studies. We excluded duplicate publications, reviews and studies that did not analyze salivary immune factors.
Results: Thirty four manuscripts studied the effect of tonsillectomy on the immune system. Only 9 of them (including 585 patients) looked at the effect on salivary immune factors. All studies analyzed the effect on salivary Immunoglobulin (Ig) A, four of them studied additional factors such as other salivary Ig’s, anti-microbial proteins (lactoferrin, peroxidases, lysozyme), anti-viral and anti-bacterial Ig’s. One study showed a significant decrease in salivary IgA, another showed a decrease in salivary IgG and a third showed a decrease in salivary IgM, lactoferrin and antimicrobial salivary IgG. Two studies had non-conclusive concerns regarding the observed changes in salivary immune factors, the third study recommended measuring salivary IgA pre and postoperatively. Only 11.1% of the studied patients had on the short term, a significant decrease in SecIgA level that can be attributed to tonsillectomy.
Conclusion: Tonsillectomy does not seem in general to negatively affect the host’s salivary immune defenses. The concerns raised are based on a partial apparent down-regulation of the some of the salivary immune components. More longitudinal studies are needed to really understand the clinical effect of any observed change in the salivary immune system.
Introduction
In their critical position at the entrance of the aerodigestive tract, the palatine tonsils play an important role in sampling antigens directly from the epithelial surfaces. The number of bacteria shed into the saliva reaches 100 billion per day in healthy individuals [1]. A healthy environment is maintained in the oral cavity by adequate salivary flow to clear the microbes and by the various local immune factors, in which the tonsils are believed to play an important role [2]. The architecture of the tonsils resembles that of a lymph node in having antigen-presenting cells, T cells, B cells [3]. The palatine tonsils produce antibodies locally and distally through their migrating B cells. They are quite active in the pediatric age group when their size is most prominent knowing that the size is directly proportional to the number of tonsillar B and T cells [4].
Though the tonsils’ role in local immunity cannot be underestimated, they are not the only source of salivary immune factors. These factors are synthesized in the salivary glands (e.g. secretory IgA, salivary peroxidase) or originate from both the glands and the blood stream (lysozyme, lactoferrin, and IgM). In addition oral polymorphonuclear leukocytes can release significant amounts of myeloperoxidase, lysozyme, and lactoferrin [2]. On the other hand, there is limited data on the role of tonsils in the immune and non-immune protection of the oropharyngeal area [3].
To better understand the role of tonsils in local oropharyngeal immunity, we underwent a systematic review of the English literature regarding the effect of tonsillectomy on the salivary immune factors
Methods
A systematic review of the English literature was performed using Medline, Embase and Cochrane. We used the terms tonsillectomy, adenotonsillectomy, humoral immunity, immune system, immunity, saliva to look for pertinent studies. We reviewed the abstract of all articles that studied the effect of tonsillectomy on the immune system. We included in the review only the studies that included salivary immune factors among the studied parameters. We only analyzed the salivary components of the immune system in the reviewed articles. We excluded duplicate publications, reviews and studies that did not include actual measurements of the salivary immune factors (e.g. descriptive studies or those using only questionnaires). We looked at the age of the studied patient, the presence of a control group and or preoperative testing, and the timing of postoperative testing. We attempted to perform a meta-analysis where feasible.
Results
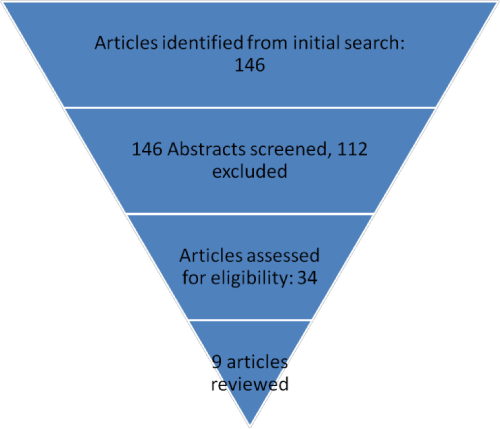
Our search identified 34 manuscripts that studied the effect of tonsillectomy on the immune system (Figure 1). Of these only 9 (including 585 patients) looked at the effect of tonsillectomy on the salivary immune factors [5-13]. All studies analyzed the effect on salivary Immunoglobulin (Ig) A, four of them studied additional factors such as other salivary Ig’s, anti-microbial proteins (lactoferrin, peroxidases, lysozyme), anti-viral and anti-bacterial Ig’s (Table 1). One study showed a significant decrease in salivary IgA, another showed a decrease in salivary IgG and a third showed a decrease in salivary IgM, lactoferrin and antimicrobial salivary IgG (Table 2). Two studies had non-conclusive concerns regarding the observed changes in salivary immune factors, the third study recommended measuring salivary IgA pre and postoperatively (Table 1).
| Authors / Year | Studied immune factors | Conclusion |
|---|---|---|
| 1)Veltri RW et al (1972) | Salivary IgA | Tonsillectomy does not modify host's salivary IgA |
| 2) Ostergaard PA (1977) | Salivary IgA, IgG, IgM | No effect of tonsillectomy on salivary IgA level Significant decrease in salivary IgG |
| 3)D'Amelio R et al (1982) | Salivary IgA | No negative effect of tonsillectomy on salivary IgA |
| 4)Cantani A et al(1986) | Salivary IgA | Recommend measuring salivary IgA level before and after tonsillectomy |
| 5)Lenander-Lumikari M et al (1992) | Salivary IgA, IgG, and IgM; salivary anti-microbial proteins lactoferrin, salivary peroxidase, myelo-peroxidase; antibodies against viral antigens and streptococcus mutans | Tonsillectomy does not impair the antimicrobial capacity of human saliva |
| 6)Del Rio-Navarro BE et al (1995) | Salivary IgA | No negative effect of tonsillectomy on salivary IgA |
| 7) Kirstila V et al (1996) | Salivary Ig’s (IgA, IgG, and IgM),anti-Streptococcus mutans,anti-viral Ig’s, lysozyme, lactoferrin, peroxidases, thiocyanate,hypothiocyanate, agglutinin | Tonsillectomy does not notably impair the saliva-mediated host defence mechanisms. Some concerns were raised regarding some decrease in some immune factors. |
| 8) Jung KY et al (1996) | Salivary IgA | No significant changes in the local immune system after tonsillectomy |
| 9) Childers NK et al (2001) | Salivary IgA, Ag-specific salivary IgA in whole and parotid specific saliva | Tonsillectomy does not decrease the salivary IgA level. The increase in parotid saliva specific IgA needs to be explored |
Table 1: Reviewed studies and their conclusions.
| Study | No | Age(y) | Control | Postop testing (months) | Findings |
|---|---|---|---|---|---|
| 1) Veltri RW et al | 17 | 2-10 | Preop levels | 1, 3, 9 to 12 | Preop Normal Postop SIgA was not affected |
| 2) Ostergaard PA | 27 | 6-11 | 27 Controls Preop levels | 30 | Preop: SIgA significantly lower than controls Postop:SIgA increased but level was no significantly different from preop SIgG decreased significantly compared to preop level |
| 3) D'Amelio R et al | 274 | 16-24 | 726 controls | NS | Postop: SIgA levels were non- significantly different |
| 4) Cantani A et al | 65 | 2-11 | Preop levels | 1 | Preop: Normal SIgA levels Postop:SIgA levels decreased significantly beyond normal. |
| 5) Lenander-Lumikari M et al | 53 | 5-8 | Normal children | 48 | Postop: •Higher levels of SIg’s, lactoferrin and myeloperoxidase •No difference in anti S mutans IgA and IgG •Higher antibodies level against viruses |
| 6) Del Rio-Navarro BE et al | 33 | 3-13 | Preop levels | 1-4, 5-12, > 12 weeks | Postop: SIgA significantly increased |
| 7) Kirstila V et al | 25 | 15-34 | Preop levels | 1 and 6 | Postop: At 1month: SIg’s levels decreased, but only significantly for IgM. At 6months: -SIgA returned to normal,SIgG increased but SIgM remained low -Non-Ig salivary defensefactors remained normal except for lactoferrin -No change in antiviralSIg’s except for SIgG against EBV which decreased significantly. -No change of anti Streptococcus mutans SIg’s except for SIgG which decreased |
| 8) Jung KY et al | 66 | <4->19 | Preop levels60 controls | 1 | Postop: SIgA decreased to control levels |
| 9) Childers NK et al | 25 | 4.4-12.8 | 25 controls | 6-14 | Postop: •No significant difference in total and specific whole saliva SIgA from controls •Significantly higherlevels of specific SIgA in parotid specific saliva |
Preop, preoperative; postop, postoperative; SIg, salivary immunoglobulin;
Table 2: Details and results of the reviewed studies.
As SIgA was the most commonly studied salivary immune factor, it was analyzed by pooling all the results (Table 3). Because there was heterogeneity in the normal reference value for SIgA and some compared postoperative values to controls while others to preoperative values, it was decided to report the values as normal (when normal or above normal) or abnormal (when considered below normal). Only 15.7% of the studied patients had an abnormally low SIgA level. However, in one of the studies that abnormality was present preoperatively which confound the real effect of tonsillectomy. Omitting that study in the analysis will result in having only 11.1% of the patients experiencing a significant decrease in SecIgA level secondary to tonsillectomy.
| Study | No | Control | Preop level | Postop level | Time of testing |
|---|---|---|---|---|---|
| 1) Veltri RW et al | 17 | - | Normal | Normal | Up to 12 months |
| 2) Ostergaard PA | 27 | Yes | Abnormal | Abnormal | 30 |
| 3) D'Amelio R et al | 274 | Yes | - | Normal | Not specified |
| 4) Cantani A et al | 65 | - | Normal | Abnormal | 1 |
| 5) Lenander-Lumikari M et al | 53 | Yes | - | Normal | 48 |
| 6) Del Rio-Navarro BE et al | 33 | - | Not specified | Normal | Up to > 12 weeks |
| 7) Kirstila V et al | 25 | - | Not specified | Normal | Up to 6 months |
| 8) Jung KY et al | 66 | Yes | Not specified | Normal | 1 |
| 9) Childers NK et al | 25 | Yes | - | Normal | 6-14 months |
Preop, preoperative; postop, postoperative; SIg, salivary immunoglobulin;
Table 3: Meta-analysis of SIgA levels.
Discussion
The saliva is a rich medium of microbial and a mixture of anti microbial and immune factors. These factors are supplied via the surrounding blood vessels, the salivary glands and lymphoid tissues. The role of the palatine tonsils in the immune system in general and in the local oropharyngeal immunity in particular has long raised concerns about the immunological sequalae of tonsillectomy. There is no doubt that the tonsils play an important role in the immune defense mechanism of the upper aero-digestive tract.
The tonsils’ crypts, which increase the tonsillar surface area up to 300cm2 are populated by lymphocytes (50-65% B cells, most of which are memory B- cells) forming the lymphoepithelium area. Other cells present include T cells, macrophages, and dendritic cells [4,14]. Unlike lymph nodes, tonsils do not possess afferent lymph vessels; instead antigens are captured directly by epithelial cells known as M cells. M cells capture antigens via endocytosis, translocate them to the basal membrane, then exocytose them intact in intraepithelial spaces [4,15]. This will induce a cascade to activate the primary immune response. This immune reaction is transmitted to the extrafollicular area where T-B lymphocytes interaction generate a long lasting immune response by stimulating differentiation and proliferation of B lymphocytes that move to the lymphoid follicles and act as founder cells in germinal centers [16].
Tonsillar secondary immune response is characterized by a small germinal center reaction and a large extrafollicular plasma cell reaction. Memory B cells in the crypt epithelium play an important role in generating a rapid secondary immune response. In fact, they have a strong antigen presenting ability enabling them to activate memory T helper cells in the crypt epithelium rapidly [4,14,16-18].
Though the main immune role of the tonsils is local, the tonsils are connected to the body immune system through trafficking of immune cells. The naïve T and B cells are transported into the tonsils via the high endothelial venules present in the extrafollicular regions, while plasmablasts migrate from the tonsils via efferent lymphatic vessels that drain in the cervical lymph nodes [4,16]. They then can join the circulation and disseminate to the upper airway mucosa, regional exocrine glands (e.g. lacrimal and salivary glands) and to a lesser extent to the gut mucosa [17].
The effect of tonsillectomy on the systemic immune system is controversial though recent studies showed no significant effect [19-21]. However, it is unclear if tonsillectomy would result in compromising the local immune defenses.
During this review, it was interesting to find that most of the studies that looked at the effect of tonsillectomy on the immune system focused primarily on the systemic humoral and cellular immunity. Only quarter of the published studies dealt with salivary immune factors, especially SIgA; meaning that we need more studies in that field. Though the results of the reviewed studies are reassuring, one should be cautious about considering that this issue has been answered. That is mainly due to the heterogeneity in the way these studies were conducted in terms of timing of postoperative testing, the presence or absence of controls and sometimes considering the preoperative values as a control reference though it was not always clear if these preoperative values were actually normal compared to control levels.
Despite that, it is reassuring to see that only 2 out of the 9 studies (Cantani et al. [9] and Kirstila et al. [12]) raised some concerns about a negative effect of tonsillectomy on some salivary immune factors with Cantani A et al advising measuring SIgA levels prior and following tonsillectomy [9]. This recommendation can not be adopted without further longitudinal studies in that field to clarify what really happens months to years after tonsillectomy. In fact, Kirstila et al. [12] actually demonstrated a rise in SIgA level 6 months after tonsillectomy following an initial drop at 1 month (similar to Cantani et al. findings). This means that the salivary immune system can compensate for the loss of the tonsillar tissues several months postoperatively.
The most commonly studied salivary immune factor is SIgA which was shown to remain within normal levels in 84.3%. Mind you that in Ostergaard PA study [7], the level of SIgA was already abnormally low preoperatively; which make us doubt that tonsillectomy caused that.
Conclusion
Tonsillectomy does not seem in general to negatively affect the host’s salivary immune defenses. The concerns raised are based on a partial apparent down-regulation of the some of the salivary immune components. More longitudinal controlled studies are needed to really understand the clinical effect of any observed change in the salivary immune system.
References
- Loesche WJ (1988) Ecology of the oral flora in Oral Microbiology and Immunology. MG Newman, R Nisengard eds. Philadephia: WB Saunders Co 351-66.
- Tenovuo J (1989) Non-immunoglobulin defense factors in human saliva, in Human Saliva: Clinical Chemistry and Microbiology, Vol II, J Tenovuo ed. Boca Raton, FL: CRC Pres Inc 55-91.
- Brandtzaeg P. Immune functions of human nasal mucosa and tonsils in healthand disease. In: Bienenstock J, ed. Immunology of the lung and upper respiratorytract. New York: McGraw-Hill,1984: 28–95
- Nave H, Gebert A, Pabst R (2001) Morphology and immunology of the human palatinetonsil. AnatEmbryol (Berl) 204:367-373.
- Veltri RW, Sprinkle PM, Keller SA, Chicklo JM (1972) Immunoglobulin changes in a pediatric otolaryngic patient sample subsequent to T & A. J LaryngolOtol 86:905-916
- Ostergaard, PA (1977) IgA levels and carrier rate of pathogenic bacteria in 27 children previously tonsillectomized. Actapathol. microbiol. scand., C, Immunol. 85: 178-186
- D'Amelio R, Palmisano L, Le Moli S, Seminara R, Aiuti F (1982) Serum and salivary IgA levels in normal subjects: comparison between tonsillectomized and non-tonsillectomized subjects. Int Arch Allergy ApplImmunol 68:256-259.
- Cantani A, Bellioni P, Salvinelli F, Businco L (1986) Serum immunoglobulins and secretory IgA deficiency in tonsillectomized children. Ann Allergy 57:413-416.
- Lenander-Lumikari M, Tenovuo J, Puhakka HJ et al. (1992) Salivary antimicrobial proteins and mutans streptococci in tonsillectomized children. Pediatr Dent 14:86-91.
- Del Rio-Navarro BE, Torres S, Barragan-Tame L et al. (1995) Immunological effects of tonsillectomy/adenectomy in children. AdvExp Med Biol 371B:737-739.
- Kirstila V, Tenovuo J, Ruuskanen O, Suonpaa J, Meurman O, et al. (1996) Longitudinal analysis of human salivary immunoglobulins, nonimmune antimicrobial agents, and microflora after tonsillectomy. ClinImmunolImmunopathol 80:110-115.
- Jung KY, Lim HH, Choi G, Choi JO (1996) Age-related changes of IgA immunocytes and serum and salivary IgA after tonsillectomy. ActaOtolaryngolSuppl 523:115-119.
- Childers NK, Powell WD, Tong G, Kirk K, Wiatrak B, et al.(2001) Human salivary immunoglobulin and antigen-specific antibody activity after tonsillectomy. Oral MicrobiolImmunol16:265-269.
- Brandtzaeg P (2003) Mucosal immunity in infectious disease and allergy.International Congress Series 1257:11-20.
- Casselbrant ML (1999) what is wrong in chronic adenoitis/ tonsillitis anatomical considerations. Journal of Pediatric Otorhinolaryngology 49: S133- S135.
- Van Kempen MJ, Rijkers GT, Van Cauwenberge PB (2000) The immune response in adenoids and tonsils. Int Arch Allergy Immunol 122:8-19
- Brandtzaeg P, Baekkevold ES, Farstad IN, Jahnsen FL, Johansen FE, et al. (1999) Regional specialization in the mucosal immune system: What happens in the microcompartments? Immunology Today 20: 141-151.
- Brandtzaeg P (1996) The B-cell development in tonsillar lymphoid follicles.ActaOto-laryngologica.Supplementum 523:55-59.
- Kaygusuz I, Alpay HC, Godekmerdan A et al. (2009) Evaluation of long-term impacts of tonsillectomy on immune functions of children: a follow-up study. Int J PediatrOtorhinolaryngol. 73:445-449.
- Nasrin M, Miah MR, Datta PG et al. (2012) Effect of tonsillectomy on humoral immunity. Bangladesh Med Res Counc Bull. 38:59-61.
- Santos FP, Weber R, Fortes BC et al. (2013) Short and long term impact of adenotonsillectomy on the immune system. Braz J Otorhinolaryngol. 79:28-34.
Citation: Bitar MA (2015) The Effect of Tonsillectomy on the Salivary Immune Factors: A Systematic Review and Meta-Analysis. Otolaryngology 5:187. DOI: 10.4172/2161-119X.1000187
Copyright: © 2015 Bitar MA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 18534
- [From(publication date): 3-2015 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 13927
- PDF downloads: 4607